Abstract
Human CD317 (BST-2/tetherin) is an intrinsic immunity factor that blocks the release of retroviruses, filoviruses, herpesviruses, and arenaviruses. It is unclear whether CD317 expressed endogenously in rodent cells has the capacity to interfere with the replication of the retroviral rodent pathogen murine leukemia virus (MLV) or, in the context of small-animal model development, contributes to the well-established late-phase restriction of human immunodeficiency virus type 1 (HIV-1). Here, we show that small interfering RNA (siRNA)-mediated knockdown of CD317 relieved a virion release restriction and markedly enhanced the egress of HIV-1, HIV-2, and simian immunodeficiency virus (SIV) in rat cells, including primary macrophages. Moreover, rodent CD317 potently inhibited MLV release, and siRNA-mediated depletion of CD317 in a mouse T-cell line resulted in the accelerated spread of MLV. Several virus-encoded antagonists have recently been reported to overcome the restriction imposed by human or monkey CD317, including HIV-1 Vpu, envelope glycoproteins of HIV-2 and Ebola virus, Kaposi's sarcoma-associated herpesvirus K5, and SIV Nef. In contrast, both rat and mouse CD317 showed a high degree of resistance to these viral antagonists. These data suggest that CD317 is a broadly acting and conserved mediator of innate control of retroviral infection and pathogenesis that restricts the release of retroviruses and lentiviruses in rodents. The high degree of resistance of the rodent CD317 restriction factors to antagonists from primate viruses has implications for HIV-1 small-animal model development and may guide the design of novel antiviral interventions.
Human CD317 (BST-2/tetherin/HM1.24) is an antiviral cellular factor that impairs the release of particles of many enveloped viruses, including human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus (SIV), simple retroviruses (23, 38), Lassa virus-like particles (VLPs) (46), Marburg and Ebola VLPs (23, 24, 46), and Kaposi's sarcoma-associated herpesvirus (KSHV) (32, 40), from human cells. CD317 causes mature virus particles to be retained at the surface of infected cells (38, 53). A current model suggests that CD317 connects the virion and plasma membrane via the molecule's N- and C-terminal membrane-anchoring domains and this “tether” is stabilized by disulfide bond-mediated dimers of the restriction factor (18, 41).
All CD317 restriction factors cloned from humans, nonhuman primates, and rodents are capable of restricting Vpu-deficient HIV-1 (10, 36, 47), with the notable exception of an owl monkey (Aotus lemurinus griseimembra)-derived ortholog (56). The mouse and rat orthologs of CD317 potently inhibited HIV-1 release upon expression in 293T cells but were completely resistant to counteraction by HIV-1 Vpu. Human-rodent interspecies chimeras of CD317 showed that the rodent-specific resistance to Vpu antagonism had a complex genetic basis involving all three major structural domains of the restriction factor (10). Besides Vpu from HIV-1 group M viruses, proteins encoded by diverse viruses have recently been reported to overcome the virus release restriction imposed by human CD317 (hCD317), including the envelope glycoproteins from Ebola virus (Ebola GP) and from some HIV-2 strains (e.g., HIV-2 ROD10), and the K5 protein of KSHV (24, 29, 32). Recently, Nef alleles encoded by SIV strains that lack a vpu gene were shown to be potent antagonists of the rhesus macaque and sooty mangabey CD317 restriction factors, but not of hCD317 (22, 47, 58). This indicates that the antagonistic activity of virus-encoded proteins may depend on the species origin of the CD317 restriction factor.
The host range and cell tropism of HIV-1 are highly restricted to primary and immortalized T cells and macrophages of human origin. Cells from rats and mice do not support or only inefficiently support various steps of the HIV-1 replication cycle (2, 3, 14, 28, 48, 54, 59). Molecular characterization of some of these species-specific barriers has revealed the inability of rodent orthologs of cellular factors, essential for HIV-1 replication in human cells, to support distinct steps of the viral life cycle. Specifically, expression of the HIV-1 receptor complex, as well as of the HIV-1 Tat-interacting protein hCyclin T1, has overcome barriers in the early phase of the HIV-1 replication cycle at the levels of entry and viral transcription, respectively (3, 14, 27, 50, 54). Corresponding transgenesis in laboratory rats has resulted in considerable permissivity for HIV-1 in vivo, characterized by high proviral loads in lymphatic organs (27). However, viremia was low in level and only transient (11, 27). In mouse and rat cells, additional less-defined late-phase blocks add up to a profound drop in the yield of infectious HIV-1 progeny, up to 104-fold or 102-fold, respectively, from a single round of replication (2, 19, 28, 34, 59). While some of these quantitative limitations in the HIV-1 late phase may be due to nonfunctional cellular cofactors (2, 6, 33), the role of species-specific restriction factors such as CD317 that may act at this stage of the replication cycle has not been elucidated.
Murine leukemia virus (MLV), a simple retrovirus that infects mice and rats, is also a target of the intrinsic antiviral host cell defense. While pathogenesis of adult animals is apparently prevented by an effective humoral immune response, neonates are susceptible to MLV infection and develop T-cell lymphoma following a relatively long phase of latency (for a review, see reference 8). It has been estimated that the pathogen and host have coexisted for around two million years (31). MLV replication can apparently be affected by several restriction factors, including tripartite motif (TRIM) and APOBEC family members (17, 45, 52, 55, 57). Remarkably, knockout of APOBEC3 in laboratory mice enhanced the replication and pathogenesis of Moloney MLV (MoMLV) in vivo (30). The potential role of endogenous levels of CD317 in MLV replication is unknown.
In the current study, we investigated whether CD317 expressed in mouse cells has the capacity to interfere with the replication of MLV or, in the context of small-animal model development, contributes to the late-phase restriction of HIV-1 in rat cells. Furthermore, we explored whether any of the known viral antagonists of hCD317 is capable of counteracting the rodent orthologs of the restriction factor.
MATERIALS AND METHODS
Plasmids.
pcDNA3.1/neo-based expression vectors for CD317 from all three species with or without an amino-terminal hemagglutinin (HA) epitope tag have been reported (10). pcDNA-Vphu, expressing a codon-optimized, Rev-independent HIV-1NL4-3 Vpu protein, was obtained from Klaus Strebel. The expression plasmid pEbola GP (5) was from Mark Goldsmith, pTRACER-K5-GFP (where GFP stands for green fluorescent protein) (20) was from Jae Jung, and pHIV-2 EnvRod10 and pHIV-2 Env Rod10 6ct (1) were from Paula Cannon. A vector encoding a truncated form of glycosylated MoMLV Gag (HA-gg189) was provided by Massimo Pizzato (43). Expression constructs for MoMLV GagPol (M57 DAW) (49) and Friend MLV Env were gifts from Hans-Georg Kräusslich and Maik Lehmann, respectively. Sequences of small interfering RNAs (siRNAs) targeting rat CD317 (rCD317) were siRNA1 (5′-ACACCACGCACCUGU UGAATT-3′), siRNA2 (5′-UAAGAUCGAGAGGCUGAACTT-3′), and siRNA3 (5′-GGAACUUGAGAAUAAGAUCTT-3′). Control siRNA sequences were siRNA targeting firefly luciferase (5′-CGUACGCGGAAUACUUCGA-3′) and nontargeting siRNA (5′-AGGUAGUGUAAUCGCCUUGTT-3′) (all from MWG). We obtained 29-mer short hairpin RNA (shRNA) plasmids targeting mouse CD317 (mCD317) and a negative-control shRNA plasmid, all with a neomycin resistance gene, from SuperArray (KM35747N).
Viruses.
Proviral plasmids pHIV-1NL4-3wt (BH10 Env) and pHIV-1NL4-3Δvpu (BH10 Env) (42) were from Valerie Bosch, pHIV-1Ada-M was from Mario Stevenson (51), pSIVmac-1A11 (35) and pHIV-21153 were from Emil Palacios, and pNL4-3 E− GFP was from Nathanial Landau. pMoMLV-GFP was constructed by introducing the egfp gene driven from an internal ribosomal entry site (IRES) into the untranslated region between the env gene and the 3′ long terminal repeat (LTR) of MoMLV (GenBank accession no. AF033811.1) at unique NotI/MluI sites, and viral stocks (MLV-GFP) were generated as previously reported (14). Both the virus titers and reverse transcriptase activities of MLV-GFP and the wild-type virus produced in BOSC cells were comparable (V. Hermann and H.-G. Kräusslich, unpublished data). Of note, this MoMLV carries the upstream initiation site in the Gag reading frame for translation of glycosylated Gag. Supernatants from provirus-transfected cells were harvested on day 2 posttransfection, and virions were pelleted through a 20% sucrose cushion by ultracentrifugation (44,000 × g, 4°C, 60 min) and resuspended in phosphate-buffered saline (PBS) (HIV-1 infectivity analysis) or 1% Triton X-100-PBS (immunoblotting or p24CA enzyme-linked immunosorbent assay [ELISA]). Pellets of transfected cells were washed in PBS and lysed in sodium dodecyl sulfate (SDS) lysis buffer for immunoblotting or 1% Triton X-100-PBS for p24CA antigen ELISA (p24CA ELISA). The virion- and cell-associated amounts of HIV-1 p24 antigen were determined by p24CA ELISA.
Cells and transfections.
All cell lines were obtained from the American Type Culture Collection and cultivated under standard conditions in Dulbecco's modified Eagle medium or RPMI 1640 supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% l-glutamine (all from GIBCO). Adherent cell lines were transfected by calcium phosphate DNA precipitation. The T-cell lines A3.01 (human) and S1A.TB.4.8.2 (S1A.TB; mouse) were transfected by Amaxa nucleofection, as reported previously (13). For selection of low mCD317 expression S1A.TB cells, shRNA vectors targeting mCD317 were introduced by nucleofection, and following neomycin selection of bulk cultures at 1 mg/ml, single-cell clones were obtained by fluorescence-activated cell sorter (FACS) sorting and characterized for surface expression of mCD317 and MoMLV-GFP susceptibility.
Primary macrophage cultures were derived from spleens of Sprague-Dawley rats and cultivated as reported previously (27). For CD317 knockdown experiments, rat macrophages were transfected with siRNAs targeting rCD317 or a control siRNA (both at 50 nM) using the Lipofectamine RNAiMAX transfection reagent (Invitrogen). Where indicated, macrophages were pretreated with efavirenz (10 μM; Bristol-Myers Squibb) prior to vesicular stomatitis virus G protein (VSV-G) HIV-1 infection.
Virion infectivity assays.
The infectivity of HIV and SIV was determined 48 h after infection in a standardized 96-well titration assay on TZM-bl cells by analysis of either firefly luciferase activity (9) or β-galactosidase activity (blue cell assay) (25).
Flow cytometry.
Analyses were carried out on a FACScalibur with BD CellQuest Pro 4.0.2 software, in principle as reported previously (26). Briefly, pelleted cells were stained with either unconjugated or biotin-conjugated rat anti-mCD317 (clone eBio927; eBioscience) or mouse anti-hCD317 (gift from Chugai Pharmaceuticals), followed by appropriate fluorochrome-labeled secondary reagents.
rCD317 mRNA quantification.
Total RNA was extracted by a standard Trizol-chloroform-methanol wash and precipitated with isopropyl alcohol. RNA pellets were washed with 75% ethanol, dissolved in water, and stored at −80°C until use. Following treatment with DNA-free DNase (Ambion) and cDNA synthesis (NEB), relative quantitative PCR analyses were performed on the ABI Prism 7500 sequence detection system (Applied Biosystems). rCD317 mRNA expression levels (assay no. Rn01419798_m1) were quantified by using the ΔΔCt method with rat glyceraldehyde-3-phosphate dehydrogenase mRNA as an endogenous reference control (catalog no. 4352338E; both from Applied Biosystems). All samples were run in duplicate or triplicate. Data analysis was conducted using the 7500 System Software (Applied Biosystems).
Immunoblotting.
Cell pellets were directly resuspended in SDS lysis buffer. Proteins were subjected to 10% or 12.5% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto nitrocellulose. Blocked membranes were probed with the following primary antisera/antibodies: rabbit polyclonal serum anti-Vpu (from Eric Cohen and Klaus Strebel), anti-BST2 (from Klaus Strebel), anti-HIV-1 p24CA (from Hans-Georg Kräusslich), anti-MLV p30CA (from Carol Stocking), anti-Ebola GP (from Stefan Becker), anti-GFP, anti-mitogen-activated protein kinase (MAPK; Santa Cruz), goat anti-Rauscher MLV gp70 with known cross-reactivity to MLV Env (from Christian Buchholz), mouse anti-HA monoclonal antibody (MAb) HA.11 (Covance), and mouse anti-α-tubulin MAb B-5-1-2 (Sigma). Secondary antibodies were conjugated to horseradish peroxidase for enhanced-chemiluminescence-based detection.
RESULTS
CD317 depletion in rat cells enhances the release of HIV-1, HIV-2, and SIV.
In light of (i) apparent, yet poorly understood, limitations in the late phase of the HIV-1 replication cycle in rodent cells that impair the yield of viral progeny (2, 27, 28, 34, 59) and (ii) the resistance of ectopically expressed rodent CD317 to HIV-1 Vpu (10), we explored whether endogenous rCD317 restricts HIV release from rat cells.
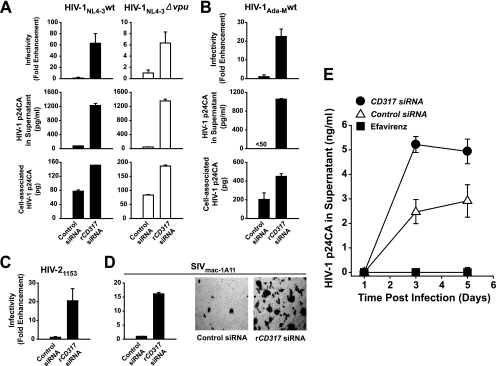
Rat2 cells, which constitutively express hCycT1 (Rat2hCycT1), were transfected twice with rCD317-targeted siRNAs or control siRNAs with the addition of proviral plasmid DNA during the second transfection. Depletion of endogenous rCD317 with any one of three different rCD317-targeting siRNAs enhanced HIV-1NL4-3 release from Rat2hCycT1 cells, with siRNA2 displaying the most robust knockdown (90 to 93% mRNA reduction) and the most pronounced virological phenotype (Fig. 1 and data not shown). Virion yield and infectivity in culture supernatants were enhanced by up to 63-fold relative to those in control siRNA-treated cells (Fig. 1A). The Vpu status of HIV-1 was irrelevant for this effect (Fig. 1A), consistent with the recently reported Vpu resistance of rCD317 expressed in human cells (10). Cell-associated p24CA levels differed by no more than 2-fold between control and rCD317-depleted samples (Fig. 1A, bottom), indicating that CD317 primarily affects particle release in rat cells. Similar results were obtained with parental Rat2 cells (data not shown). Knockdown of CD317 in Rat2hCycT1 cells also resulted in the enhancement of the yield of infectious HIV-1Ada-M (Fig. 1B), HIV-21153 (Fig. 1C), and SIVmac-1A11 (Fig. 1D) by a factor of 17- to 23-fold.
FIG. 1.
siRNA knockdown of endogenous CD317 in rat cells enhances lentiviral release. Rat2hCycT1 cells were transfected twice with rCD317-targeting siRNAs (siRNA2) or control siRNAs, and during the second transfection, the indicated proviral DNAs were added. At 2 days posttransfection, supernatants were concentrated and sucrose-purified virions were analyzed for infectivity in a TZM-bl blue cell assay (top histograms and microscopic images in panel D) and virion-associated p24CA antigen (middle parts of panels A and B). In panels A and B, cellular levels of p24CA levels and rCD317 mRNA were quantified by antigen ELISA and real-time PCR, respectively, at the end of the experiment. Histograms show the arithmetic means ± standard deviations from triplicate samples. The data shown are representative of six (NL4-3 wt) and two (NL4-3 Δvpu) experiments conducted. siRNA-mediated rCD317 depletion led to 6- to 63-fold and 5- to 20-fold enhancement of virus release in the context of wild-type and Δvpu mutant NL4-3, respectively. (E) Rat macrophages were first infected (in the presence or absence of the reverse transcriptase inhibitor efavirenz) with VSV-G-pseudotyped HIV-1YU-2 and 1 day later transfected with a siRNA targeting endogenous rCD317 (filled circle) or a control siRNA (open triangle). Release of HIV-1 virions into the supernatant was monitored by p24CA antigen ELISA. The results shown are representative of four experiments conducted. The value of <50 in panel B indicates that the signal was below the limit of detection of the ELISA (<50 pg p24CA/ml).
We next tested whether depletion of endogenous rCD317 in primary HIV-1 target rat cells following infection also increased the release of HIV-1. To this end, spleen-derived rat macrophages were first challenged with VSV-G-pseudotyped HIV-1NL4-3 and transfected 1 day later with either rCD317 or control siRNAs. rCD317-depleted rat macrophages (∼85% CD317 mRNA reduction) released 2- to 3-fold larger amounts of HIV-1 virions than control siRNA-treated reference cells (Fig. 1E). Collectively, endogenously expressed CD317 limits lentivirus release in rat cells and RNA interference can relieve this replication barrier. This identifies CD317 as one relevant factor in the inefficient late phase of HIV-1 replication in rodent cells.
The rodent CD317-imposed restriction of HIV-1 release is resistant to counteraction by antagonists from primate viruses.
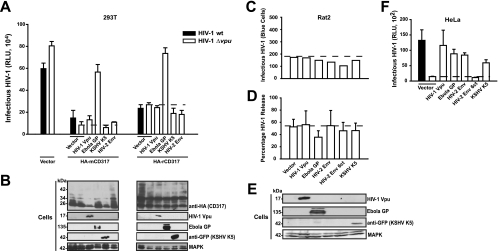
Proteins encoded by diverse viruses have recently been reported to overcome the virus release restriction imposed by hCD317 (24, 29, 32). We and others previously established the inability of HIV-1 Vpu to counteract rodent and monkey CD317 (10, 15, 36, 47). To gain further insight into the potential species specificity of virus-encoded antagonists, we first quantified the release of infectious HIV-1 from human 293T cells cotransfected with either wild-type or Δvpu mutant HIV-1 provirus, expression plasmids encoding N-terminally HA-tagged rodent CD317 proteins, together with one of these virus-encoded antagonists (HIV-2ROD-10 Env, Ebola GP, KSHV K5, or HIV-1 Vpu). Expression of viral antagonists was confirmed in lysates of transfected cells by Western blotting (Fig. 2 B; for HIV-2 Env, no antiserum was available). In the absence of CD317 expression, levels of infectious virions in the supernatant were in the same range for wild-type and Δvpu mutant HIV-1 (Fig. 2A, vector). As demonstrated previously (10, 36), ectopically expressed mCD317 or rCD317 potently reduced HIV-1 release. Coexpression of HIV-1 Vpu, KSHV K5, or HIV-2ROD-10 Env did not rescue the release of Vpu-defective HIV-1 in the context of the HA-tagged (Fig. 2A) or untagged (data not shown) rodent CD317 proteins. Of note, despite this lack of functional antagonism, HA-mCD317 levels in cell lysates were found to be reduced in the presence of Vpu or K5 (Fig. 2B). Interestingly, Ebola GP expression markedly enhanced levels of HIV-1 in the supernatant of mCD317- or rCD317-expressing 293T cells (Fig. 2A) without affecting steady-state levels of the restriction factor (Fig. 2B).
FIG. 2.
Antagonism of rodent CD317-mediated restriction of HIV-1 particle release is cell type, species, and/or context specific. (A) 293T cells were cotransfected with pHIV-1wt (filled histogram bars) or pHIV-1Δvpu (open histogram bars), expression plasmids encoding HA-CD317 of mouse or rat origin, or the vector only and potential virus-encoded antagonists of CD317. At 2 days posttransfection, cells were analyzed for the yield of infectious HIV-1 in the culture supernatant in a luminometric TZM-bl reporter assay. The infectious HIV-1 titer is presented as relative light units (RLU), and arithmetic means of triplicate results from one experiment are given. (B) Western blot detection of HA-CD317 and viral antagonists in cell lysates, plotted onto a single nitrocellulose membrane, by consecutive antibody reprobing (Cells). (A, B) The results shown are representative of three experiments conducted. (C) Rat2 cells were cotransfected with pHIV-1Δvpu and plasmids encoding potential antagonists. Three days later, secretion of infectious HIV-1 into the supernatant was monitored using (C) a TZM-bl reporter blue cell assay and (D) the percentage of virion-associated p24CA in supernatants relative to the total p24CA (in cells and supernatants), in principle as reported previously (10). (E) Western blot detection of Vpu, Ebola GP, K5-GFP, and mitogen-activated protein kinase (MAPK) in Rat2 cell lysates, plotted onto a single nitrocellulose membrane, by consecutive antibody reprobing. The results shown are representative of three independent experiments conducted. (F) HeLa cells were cotransfected with pHIV-1wt or pHIV-1Δvpu and expression plasmids encoding the indicated viral antagonists. Two days later, the HIV-1 titer in the culture supernatants was quantified on TZM-bl reporter cells. The HIV-1 titer is presented as RLU, and arithmetic means of triplicate results from one of four similar experiments are given.
We extended these antagonist studies to cells endogenously expressing rCD317. To this end, Rat2 cells were transfected with HIV-1Δvpu proviral DNA and expression constructs encoding individual viral antagonists. Two days later, concentrated supernatants were analyzed for the yield of infectious HIV-1 (Fig. 2C) and HIV-1 release was expressed as the percentage of total p24CA (in cells and supernatant) that was secreted as virion-associated p24CA (Fig. 2D). Remarkably, none of the viral antagonists, including Ebola GP, had a discernible impact on the release of Δvpu mutant HIV-1 from Rat2 cells (Fig. 2C to E). As a control for antagonist functionality, coexpression of all four viral antagonists in HeLa cells, which express abundant endogenous levels of hCD317 (53), promoted the release of Vpu-defective HIV-1 (Fig. 1F). Expectedly, the ability of HIV-2 Env to augment virus release in HeLa cells was dependent on its C terminus (HIV-2 Env 6ct mutant) (Fig. 1F), which contains the YXXφ motif (1). We conclude that these viral proteins can effectively antagonize the hCD317-imposed HIV-1 release restriction, yet this capacity is highly species dependent and for Ebola GP, partly cell type and/or context dependent. Our results demonstrate that the HIV-1 release restriction imposed by endogenous rCD317 is highly resistant to counteraction by these four antagonists from primate viruses.
CD317 is a regulator of MLV replication in a mouse T-cell line.
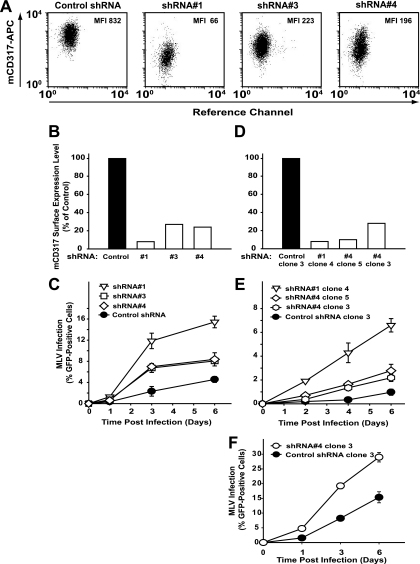
Next, we analyzed the ability of rodent CD317 to affect the replication of the natural rodent pathogen MLV. To test whether endogenous mCD317 restricts MLV replication, we modulated its expression in natural target cells. We first screened several mouse T-cell lines for surface expression of mCD317 (data not shown). S1A.TB cells were found to harbor high levels of mCD317 and were selected for subsequent studies. Using neomycin-selectable mCD317-targeting shRNAs, several S1A.TB sublines and clones were established. As depicted in Fig. 3 A and B, flow cytometric quantification of cell surface-exposed mCD317 demonstrated potent (shRNA1, mean fluorescence intensity [MFI] of CD317 surface expression of 66) or intermediate (shRNA3, MFI of CD317 surface expression of 223; shRNA4, MFI of CD317 surface expression of 196) depletion of the restriction factor relative to that obtained with a control shRNA (MFI of CD317 surface expression of 832) in neomycin-selected S1A.TB cultures. To monitor the effect of variable mCD317 levels on S1A.TB cells on MLV replication, we performed infection studies with MLV-GFP, a replication-competent MoMLV which encodes an egfp gene driven from an IRES in the untranslated region between the env gene and the 3′ LTR (14). Following infection with MLV-GFP at a low multiplicity of infection (MOI), moderate viral spread was detected in control shRNA-expressing (Fig. 3C) or parental cells (data not shown) over the 6-day observation period. In contrast, the dynamics of MLV-GFP spread were markedly accelerated in efficiently mCD317-silenced S1A.TB cells (shRNA1), reaching up to 6-fold higher infection levels (Fig. 3C). Consistent with CD317 surface level-dependent virion release restriction, S1A.TB shRNA cultures with intermediate mCD317 expression levels (shRNA3 and shRNA4) supported moderately enhanced MLV-GFP replication (Fig. 3C). Moreover, in a set of shRNA-expressing S1A.TB single-cell clones (Fig. 3D), this inverse correlation between mCD317 expression levels and the ability to support MLV-GFP spread was largely confirmed (Fig. 3E and F). These findings suggest that CD317 expression can partly control MLV spread in mouse T cells.
FIG. 3.
Depletion of endogenous CD317 in mouse T cells accelerates MLV spread. Neomycin-selected bulk cultures of mouse S1A.TB T cells expressing mCD317-targeting shRNAs (open columns, symbols) or a control shRNA (filled columns, symbols) were characterized for (A, B) steady-state levels of surface-exposed mCD317, assessed by the MFI in flow cytometric analyses, with values for control shRNA-expressing cells set to 100%. (C) Cells' MLV-GFP susceptibility (MLV infection) was assessed by the percentage of GFP-positive cells at various time points postinoculation with a low MOI. Shown are the arithmetic means ± standard deviations (n = 3) from one representative experiment out of three. (D) Cell surface expression of mCD317 in individual S1A.TB clones. The value for the control shRNA-expressing clone was set to 100%. (E, F) Cell clones were infected with MLV-GFP (E, low MOI; F, high MOI) and monitored for viral spread as described for panel C.
Glycosylated Gag and the envelope of Friend MLV cannot counteract the mCD317-imposed restriction of murine leukemia VLP release.
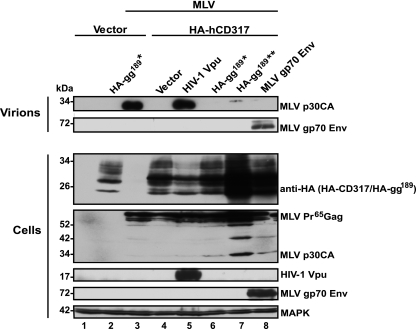
We wondered whether MLV may encode a partially active CD317 antagonist. In this context, it is of note that MLV produces a unique glycosylated form of the Gag polyprotein (glycogag [gg]), which was recently shown to facilitate MLV release in interferon-stimulated cells (39) and enhanced particle infectivity of Nef-deficient HIV-1 (43). To directly address whether MLV encodes a CD317 antagonist, we tested the ability of overexpressed HA-gg189 or Friend MLV Env to overcome the CD317-induced release restriction for murine leukemia VLPs in 293T cells. As expected, HA-hCD317 strongly impaired VLP release and HIV-1 Vpu overcame this release block (Fig. 4). In contrast, trans-expression of neither HA-gg189 nor Env enhanced levels of murine leukemia VLPs in the supernatant (Fig. 4). These results provide no evidence for functional CD317 antagonism of these MLV proteins.
FIG. 4.
CD317-mediated restriction of MLV particle release cannot be antagonized by HA-gg189 or Env. 293T cells were cotransfected with pMLV-GagPol, the empty vector, or a vector encoding HA-hCD317 and expression plasmids for a known viral antagonist (HIV-1 Vpu) or potential MLV-encoded antagonists (HA-gg189 [*, 200 ng per well; **, 2 μg per well] or Friend MLV Env). At 2 days posttransfection, supernatants were analyzed for the release of sucrose-pelletable murine leukemia VLPs by Western blotting using consecutive antibody reprobing (Virions). Cell lysates were processed for Western blot assay detection of anti-HA (HA-hCD317 and HA-gg189), MLV Gag, HIV-1 Vpu, and MAPK in 293T cell lysates, plotted onto a single nitrocellulose membrane, by consecutive antibody reprobing (Cells). For the detection of MLV gp70 Env, the identical cell lysates were run on a separate gel and analyzed by Western blotting. The anti-HA blot in lane 2 shows the band pattern of HA-gg189 alone, and the anti-HA blot in lane 4 shows the band pattern of HA-hCD317 alone.
Only Ebola GP overcomes the MLV release block imposed by rodent and human CD317.
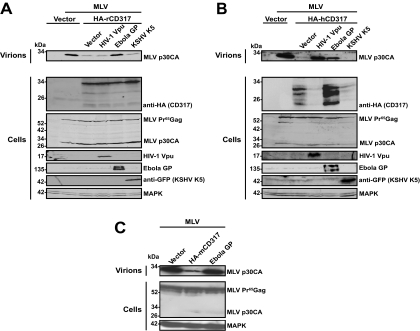
We then tested the ability of the viral antagonists to augment the release of MLV from cells expressing rodent or human orthologs of CD317. To this end, 293T cells were transfected with MLV proviral DNA and expression constructs encoding HA-tagged CD317 and viral antagonists. MLV release was potently restricted by rCD317 (Fig. 5 A), hCD317 (Fig. 5B), and mCD317 (data not shown). Coexpression of Ebola GP enhanced the release of MLV particles into the supernatants of 293T cells expressing any of the three restriction factors (Fig. 5A and B and data not shown). For the human ortholog, Ebola GP's antagonism was somewhat less pronounced, as seen for the rat (Fig. 5A) and mouse (data not shown) restriction factors. Interestingly, cell-associated levels of HA-CD317 were either unchanged or even enhanced by Ebola GP relative to those of the vector control. Of note, coexpression of Ebola GP in the absence of mCD317 did not enhance MLV release (Fig. 5C). K5 expression did not augment MLV release (Fig. 5A and B), despite its ability to deplete the human restriction factor (Fig. 5B). Consistent with the species-specific antagonism of Vpu observed for HIV-1 (Fig. 2 and 3) (10), coexpression of Vpu with the human (Fig. 5B; see Fig. S1C in the supplemental material), but not the rat (Fig. 5A) or mouse (not shown), ortholog resulted in an increase of MLV particle release. This functional outcome was paralleled by a marked depletion of the human, but not of the rodent, restriction factor in 293T producer cells (Fig. 5A and B and data not shown). In contrast to the results obtained for HIV-1 (see Fig. S1A and B in the supplemental material), coexpression of HIV-2ROD-10 Env in 293 cells did not augment the release of MLV in the context of human or rodent CD317 (see Fig. S1C in the supplemental material) (data not shown). Technical limitations due to low MLV production in transfected rodent cell lines precluded analysis of the viral antagonists in the context of endogenous mCD317 or rCD317. In summary, rodent CD317 potently restricts MLV release and, with the exception of Ebola GP, cannot be antagonized by the tested viral antagonist encoded by primate viruses.
FIG. 5.
Rodent and human CD317-mediated restriction of MLV particle release can be antagonized by Ebola GP. 293T cells were cotransfected with pMLV-GFP, expression plasmids encoding HA-CD317 of (A) rat or (B) human origin, or the vector only and plasmids encoding potential antagonists of CD317. At 3 days posttransfection, supernatants were analyzed for the presence of sucrose-pelletable MLV particles by Western blotting (Virions). (A, B) Cell lysates were separated by SDS-PAGE in three gels run in parallel and probed for (i) HA-hCD317 and MAPK, (ii) MLV Gag and Ebola GP, or (iii) HIV-1 Vpu and KSHV K5-GFP (Cells). The results shown are representative of three experiments conducted. (C) 293 cells were cotransfected with pMLV-GFP and expression plasmids encoding the vector only, HA-mCD317, or Ebola GP. Supernatants and cell lysates were processed as described for panels A and B.
Species- and antagonist-specific downregulation of endogenous CD317 from the cell surface.
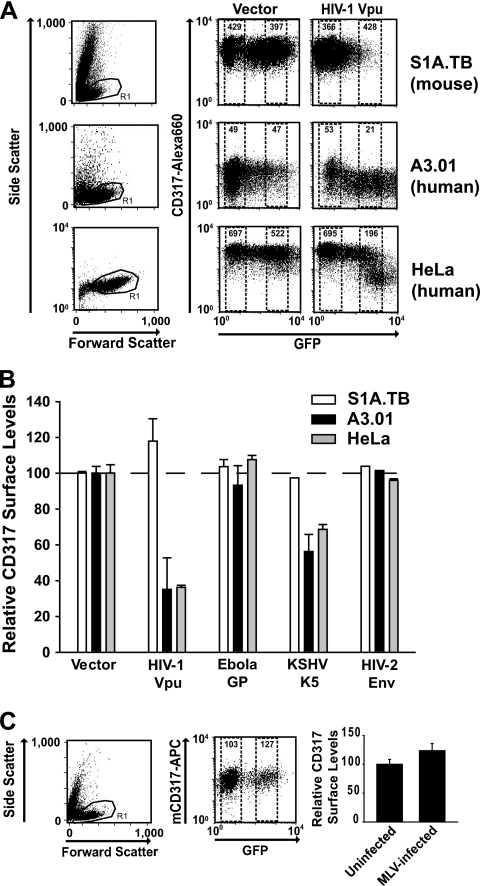
According to the mechanistic model of a direct physical involvement of CD317 in the process of virion tethering (18, 41), its surface levels may be key to its restrictive capacity (12, 16, 53). Here, we examined the ability of the four viral antagonists to modulate levels of endogenous CD317 on human and mouse cells. The A3.01, HeLa (human), and S1A.TB (mouse) cell lines were cotransfected with plasmids encoding one of the antagonists and pEGFP. One day later, cells were stained with species-specific antibodies, which bind to the extracellular domain of CD317, and analyzed by flow cytometry for surface exposure on cells expressing high levels of GFP/antagonist. Expression of Vpu resulted in a reduction of hCD317 from the surface of A3.01 T cells and adherent HeLa cells (Fig. 6 A and B), consistent with previous studies with human cells (10, 53). In contrast, levels of CD317 remained unaltered in mouse S1A.TB T cells upon Vpu expression (Fig. 6A and B). The same species-specific pattern of receptor level changes was observed in K5-expressing T cells (Fig. 6B). Interestingly, neither Ebola GP nor HIV-2 Env expression influenced surface levels of hCD317 or mCD317 (Fig. 6B). Thus, two of four viral antagonists downregulated hCD317, while none was able to affect endogenous CD317 levels on the surface of mouse cells. Finally, we assessed mCD317 surface levels on MLV-GFP-infected S1A.TB cells. Endogenous levels of the restriction factor did not differ between productively infected, GFP-positive T cells and uninfected cells in the same culture (Fig. 6C). This indicates that MLV does not downregulate tetherin levels at the plasma membrane of infected cells.
FIG. 6.
Species- and antagonist-specific regulation of cell surface levels of CD317 in the context of HIV-1 and MLV. Mouse S1A.TB T cells and human A3.01 T and HeLa cells were transfected with the empty vector (pcDNA) or pHIV-1 Vphu together with pEGFP-N1 and analyzed 1 day later for CD317 surface exposure relative to GFP expression by flow cytometry. (A) Representative FACS dot plots. Values within the gates (GFP negative and highly GFP positive) indicate the MFI of CD317 expression. (B) Relative surface expression of CD317 in the context of the indicated viral antagonists. CD317 levels for empty-vector-transfected cells were set to 100%. Indicated are the arithmetic means ± the standard errors of the means from two or three independent experiments. (C) S1A.TB T cells were infected with MLV-GFP, and 3 days later, mCD317 surface levels were quantified on infected and uninfected cells by flow cytometry.
DISCUSSION
Infection and pathogenesis of retroviruses are characterized by a high degree of species specificity. The control of retroviral replication in foreign hosts and sometimes even in cognate hosts can underlie the action of cellular restriction factors that inhibit distinct steps of the viral replication cycle and are thus an important arm of the innate immune system. In an apparent evolutionary adaptation, some retroviruses encode gene products that can overcome these intrinsic antiviral barriers by diverse, so far poorly understood, strategies.
In the context of efforts to establish fully permissive, immunocompetent rat (27, 37) and mouse (3, 59) models of HIV infection, and given the ability of ectopically expressed rodent CD317 to inhibit HIV-1 release in a Vpu-resistant manner (10), we explored whether endogenous CD317 contributes to HIV-1 late-phase restriction in rodent cells. Depletion of CD317 by RNA interference markedly enhanced titers of wild-type HIV-1 secreted from Rat2 cells and also augmented the release of HIV-1 in infected primary rat macrophages. Moreover, this beneficial effect of rCD317 depletion extended to other, related, primate lentiviruses, including HIV-2 and SIVmac. Endogenous CD317 therefore imposes a barrier to the release of wild-type HIV-1 in rat cells and, in part, contributes to the long-appreciated inefficiency of the late phase of HIV-1 replication in rodent cells (2, 27, 28, 34, 59). Levels of HIV-1 production in rCD317-depleted Rat2 cells are, however, still ca. 10- to 100-fold lower than in human HeLa cells, indicating that additional limitations, including the nuclear export of viral RNA (2, 34), hamper the overall late-phase efficiency for HIV-1 in rodent cells. Based on the current findings, we envision two independent strategies to enhance the permissivity of the hCD4/CCR5/cyclin T1-transgenic rat model (27, 37). First, a knockout of CD317 in rats (21) provides the most direct approach to interfere with the activity of this antiviral restriction factor in vivo. However, the physiological consequences of such genetic interference with CD317 expression are unknown. Activities of CD317, apart from virion tethering, are just starting to be uncovered and include immunomodulation and organization of the actin cytoskeleton (4, 44).
Second, identification or evolution of a viral antagonist that can interfere with the HIV-1-restrictive activity of rCD317 may allow a genetic modification of the virus to overcome the release block. However, none of the thus-far-known hCD317 antagonists, HIV-1 Vpu, HIV-2 Rod Env, KSHV K5, or Ebola GP, overcame the rCD317-imposed restriction of HIV-1 release in its endogenous context. Notably, Ebola GP displayed antagonistic activity against rodent CD317 expressed ectopically in human 293T cells (this study and reference 24), suggesting a strong cell type and/or context dependence. Preliminary results indicate that also Nef proteins encoded by SIVmac, which is a potent antagonist of the rhesus macaque and sooty mangabey CD317 restriction factors (22, 47, 58), cannot augment HIV-1 release in rat cells (data not shown). This is not surprising, since the specificity of SIV Nef for rhesus CD317 maps to a four-amino-acid sequence stretch in the N terminus of the restriction factor that is not present in hCD317, rCD317, or mCD317. We conclude that endogenous rCD317 is highly resistant to the tested antagonists from primate viruses. Conceivably, introduction of one of these viral genes into the nef locus of HIV-1 may allow an adaptation or forced evolution to rCD317-expressing cells in tissue culture. It is of note that Ebola GP was a functional rCD317 antagonist in 293T cells (Fig. 2A), indicating a context dependence of its release-enhancing activity. This notion is also underscored by the cell type-specific antiviral activity of HIV-2 Env and KSHV K5. These viral proteins were unable to enhance Δvpu mutant HIV-1 release in 293T cells that ectopically express hCD317, while both augmented virus release from HeLa cells. The reasons for such species- and/or cell type-specific activities of viral CD317 antagonists are currently unknown. A deeper understanding of their specific modes of antagonism, involved cellular pathways, and cofactors is necessary to unravel this complexity. For the prototypic CD317 antagonist Vpu, downregulation of hCD317 from the cell surface and accelerated degradation have been proposed as key mechanisms (10, 15, 53). Recent reports (7, 12, 24) and the current study demonstrate that a reduction of cell-associated CD317 levels is neither sufficient (Fig. 2A and B) nor required (Fig. 2A and B; see Fig. S1A and B in the supplemental material) for HIV-1 release enhancement by Vpu and several other CD317 antagonists. Moreover, downregulation of CD317 from the cell surface is not a universal property of a successful antagonist, as exemplified by the activities of Ebola GP in the current study.
Analysis of a second, rodent, retroviral pathogen, MLV, yielded insight with respect to the breadth, specificity, and biological importance of CD317's antiviral activity in a virus-host pair with considerable time for coevolution. Ectopic expression of human, as well as rodent, CD317 protein potently restricted MLV release, providing first evidence that also the host species-derived restriction factors are functional antagonists of wild-type MLV replication. In line with this observation, shRNA-mediated depletion of endogenous CD317 in a mouse T-cell line accelerated the dynamics of MLV spread. It will be exciting to explore the consequences of genetic interference with CD317 expression for MLV spread and pathogenesis in neonatal rodents. From a different perspective, it is noteworthy that despite the apparent antiviral potency of rodent CD317 restriction factors, MLV has evolved its genetically simple replication machinery to evade the restriction factor by an as-yet-unknown mechanism. Trans-complementation studies provided no evidence of functional CD317 antagonism of either HA-gg189 or MLV Env. Nevertheless, the degree of MLV evasion is apparently sufficient for replication, persistence, and induction of leukemia in the rodent host. None of the viral antagonists tested, with the notable exception of Ebola GP, was able to rescue MLV virion release in the context of rodent CD317. The similarity of the antagonist pattern with HIV-1 indicates that success or failure of CD317 antagonism may mechanistically not depend on the restricted retrovirus.
Collectively, our findings suggest that CD317 is a broadly acting and conserved mediator of innate control of retroviral infection in mammals and unmask a complex, partly species-specific and partly context-dependent pattern of resistance to this antiviral tethering factor with implications for HIV/AIDS drug design and small-animal model development.
Supplementary Material
Acknowledgments
We thank Stefan Becker, Jens Bohne, Valerie Bosch, Christian Buchholz, Paula Cannon, Eric Cohen, Jae Jung, Hans-Georg Kräusslich, Nathaniel Landau, Maik Lehmann, Barbara Müller, Emil Palacios, Massimo Pizzato, Mario Stevenson, Carol Stocking, Klaus Strebel, and Chugai Pharmaceuticals for the generous gift of reagents. We thank Volker Hermann and Hans-Georg Kräusslich for providing unpublished data, Dieter Stefan for FACS sorting, and Oliver T. Fackler for discussion and comments on the manuscript.
C.G. was supported by the Peter and Traudl Engelhorn-Stiftung and is now a recipient of a fellowship of the Medical Faculty of the University of Heidelberg. This work was in part funded by the Deutsche Forschungsgemeinschaft (KE742/4-1) and by the HIV-ACE research network (HEALTH-F3-2008-201095), which is supported by a grant from the European Commission within the Priority 1 Health work program of the 7th Framework Program of the European Union.
Footnotes
Published ahead of print on 11 August 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Abada, P., B. Noble, and P. M. Cannon. 2005. Functional domains within the human immunodeficiency virus type 2 envelope protein required to enhance virus production. J. Virol. 79:3627-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieniasz, P. D., and B. R. Cullen. 2000. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 74:9868-9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browning, J., J. W. Horner, M. Pettoello-Mantovani, C. Raker, S. Yurasov, R. A. DePinho, and H. Goldstein. 1997. Mice transgenic for human CD4 and CCR5 are susceptible to HIV infection. Proc. Natl. Acad. Sci. U. S. A. 94:14637-14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao, W., L. Bover, M. Cho, X. Wen, S. Hanabuchi, M. Bao, D. B. Rosen, Y. H. Wang, J. L. Shaw, Q. Du, C. Li, N. Arai, Z. Yao, L. L. Lanier, and Y. J. Liu. 2009. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J. Exp. Med. 206:1603-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, S. Y., R. F. Speck, M. C. Ma, and M. A. Goldsmith. 2000. Distinct mechanisms of entry by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J. Virol. 74:4933-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coskun, A. K., M. van Maanen, V. Nguyen, and R. E. Sutton. 2006. Human chromosome 2 carries a gene required for production of infectious human immunodeficiency virus type 1. J. Virol. 80:3406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubé, M., B. B. Roy, P. Guiot-Guillain, J. Binette, J. Mercier, A. Chiasson, and E. A. Cohen. 2010. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS Pathog. 6:e1000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan, H. 1997. Leukemogenesis by Moloney murine leukemia virus: a multistep process. Trends Microbiol. 5:74-82. [DOI] [PubMed] [Google Scholar]
- 9.Geuenich, S., C. Goffinet, S. Venzke, S. Nolkemper, I. Baumann, P. Plinkert, J. Reichling, and O. T. Keppler. 2008. Aqueous extracts from peppermint, sage and lemon balm leaves display potent anti-HIV-1 activity by increasing the virion density. Retrovirology 5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goffinet, C., I. Allespach, S. Homann, H. M. Tervo, A. Habermann, D. Rupp, L. Oberbremer, C. Kern, N. Tibroni, S. Welsch, J. Krijnse-Locker, G. Banting, H.-G. Kräusslich, O. T. Fackler, and O. T. Keppler. 2009. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe 5:285-297. [DOI] [PubMed] [Google Scholar]
- 11.Goffinet, C., I. Allespach, and O. T. Keppler. 2007. HIV-susceptible transgenic rats allow rapid preclinical testing of antiviral compounds targeting virus entry or reverse transcription. Proc. Natl. Acad. Sci. U. S. A. 104:1015-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goffinet, C., S. Homann, I. Ambiel, N. Tibroni, D. Rupp, O. T. Keppler, and O. T. Fackler. 2010. Antagonism of CD317 restriction of human immunodeficiency virus type 1 (HIV-1) particle release and depletion of CD317 are separable activities of HIV-1 Vpu. J. Virol. 84:4089-4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goffinet, C., and O. T. Keppler. 2006. Efficient nonviral gene delivery into primary lymphocytes from rats and mice. FASEB J. 20:500-502. [DOI] [PubMed] [Google Scholar]
- 14.Goffinet, C., N. Michel, I. Allespach, H. M. Tervo, V. Hermann, H.-G. Kräusslich, W. C. Greene, and O. T. Keppler. 2007. Primary T-cells from human CD4/CCR5-transgenic rats support all early steps of HIV-1 replication including integration, but display impaired viral gene expression. Retrovirology 4:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta, R. K., S. Hue, T. Schaller, E. Verschoor, D. Pillay, and G. J. Towers. 2009. Mutation of a single residue renders human tetherin resistant to HIV-1 Vpu-mediated depletion. PLoS Pathog. 5:e1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Habermann, A., J. Krijnse-Locker, H. Oberwinkler, M. Eckhardt, S. Homann, A. Andrew, K. Strebel, and H.-G. Kräusslich. 2010. CD317/tetherin is enriched in the HIV-1 envelope and downregulated from the plasma membrane upon virus infection. J. Virol. 84:4646-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatziioannou, T., D. Perez-Caballero, A. Yang, S. Cowan, and P. D. Bieniasz. 2004. Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5alpha. Proc. Natl. Acad. Sci. U. S. A. 101:10774-10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinz, A., N. Miguet, G. Natrajan, Y. Usami, H. Yamanaka, P. Renesto, B. Hartlieb, A. A. McCarthy, J. P. Simorre, H. Gottlinger, and W. Weissenhorn. 2010. Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain. Cell Host Microbe 7:314-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iida, S., T. Fukumori, Y. Oshima, H. Akari, A. H. Koyama, and A. Adachi. 1999. Compatibility of Vpu-like activity in the four groups of primate immunodeficiency viruses. Virus Genes 18:183-187. [DOI] [PubMed] [Google Scholar]
- 20.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74:5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izsvák, Z., J. Frohlich, I. Grabundzija, J. R. Shirley, H. M. Powell, K. M. Chapman, Z. Ivics, and F. K. Hamra. 2010. Generating knockout rats by transposon mutagenesis in spermatogonial stem cells. Nat. Methods 7:443-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia, B., R. Serra-Moreno, W. Neidermyer, A. Rahmberg, J. Mackey, I. B. Fofana, W. E. Johnson, S. Westmoreland, and D. T. Evans. 2009. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5:e1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jouvenet, N., S. J. Neil, M. Zhadina, T. Zang, Z. Kratovac, Y. Lee, M. McNatt, T. Hatziioannou, and P. D. Bieniasz. 2009. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 83:1837-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaletsky, R. L., J. R. Francica, C. Agrawal-Gamse, and P. Bates. 2009. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 106:2886-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keppler, O. T., I. Allespach, L. Schuller, D. Fenard, W. C. Greene, and O. T. Fackler. 2005. Rodent cells support key functions of the human immunodeficiency virus type 1 pathogenicity factor Nef. J. Virol. 79:1655-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keppler, O. T., N. Tibroni, S. Venzke, S. Rauch, and O. T. Fackler. 2006. Modulation of specific surface receptors and activation sensitization in primary resting CD4+ T lymphocytes by the Nef protein of HIV-1. J. Leukoc. Biol. 79:616-627. [DOI] [PubMed] [Google Scholar]
- 27.Keppler, O. T., F. J. Welte, T. A. Ngo, P. S. Chin, K. S. Patton, C. L. Tsou, N. W. Abbey, M. E. Sharkey, R. M. Grant, Y. You, J. D. Scarborough, W. Ellmeier, D. R. Littman, M. Stevenson, I. F. Charo, B. G. Herndier, R. F. Speck, and M. A. Goldsmith. 2002. Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J. Exp. Med. 195:719-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keppler, O. T., W. Yonemoto, F. J. Welte, K. S. Patton, D. Iacovides, R. E. Atchison, T. Ngo, D. L. Hirschberg, R. F. Speck, and M. A. Goldsmith. 2001. Susceptibility of rat-derived cells to replication by human immunodeficiency virus type 1. J. Virol. 75:8063-8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Tortorec, A., and S. J. Neil. 2009. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 83:11966-11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Low, A., C. M. Okeoma, N. Lovsin, M. de Las Heras, T. H. Taylor, B. M. Peterlin, S. R. Ross, and H. Fan. 2009. Enhanced replication and pathogenesis of Moloney murine leukemia virus in mice defective in the murine APOBEC3 gene. Virology 385:455-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maksakova, I. A., M. T. Romanish, L. Gagnier, C. A. Dunn, L. N. van de Lagemaat, and D. L. Mager. 2006. Retroviral elements and their hosts: insertional mutagenesis in the mouse germ line. PLoS Genet. 2:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansouri, M., K. Viswanathan, J. L. Douglas, J. Hines, J. Gustin, A. V. Moses, and K. Fruh. 2009. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83:9672-9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariani, R., B. A. Rasala, G. Rutter, K. Wiegers, S. M. Brandt, H.-G. Kräusslich, and N. R. Landau. 2001. Mouse-human heterokaryons support efficient human immunodeficiency virus type 1 assembly. J. Virol. 75:3141-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mariani, R., G. Rutter, M. E. Harris, T. J. Hope, H.-G. Kräusslich, and N. R. Landau. 2000. A block to human immunodeficiency virus type 1 assembly in murine cells. J. Virol. 74:3859-3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marthas, M. L., B. Banapour, S. Sutjipto, M. E. Siegel, P. A. Marx, M. B. Gardner, N. C. Pedersen, and P. A. Luciw. 1989. Rhesus macaques inoculated with molecularly cloned simian immunodeficiency virus. J. Med. Primatol. 18:311-319. [PubMed] [Google Scholar]
- 36.McNatt, M. W., T. Zang, T. Hatziioannou, M. Bartlett, I. B. Fofana, W. E. Johnson, S. J. Neil, and P. D. Bieniasz. 2009. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 5:e1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michel, N., C. Goffinet, K. Ganter, I. Allespach, V. N. Kewalramani, M. Saifuddin, D. R. Littman, W. C. Greene, M. A. Goldsmith, and O. T. Keppler. 2009. Human cyclin T1 expression ameliorates a T-cell-specific transcriptional limitation for HIV in transgenic rats, but is not sufficient for a spreading infection of prototypic R5 HIV-1 strains ex vivo. Retrovirology 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neil, S. J., T. Zang, and P. D. Bieniasz. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425-430. [DOI] [PubMed] [Google Scholar]
- 39.Nitta, T., Y. Kuznetsov, A. McPherson, and H. Fan. 2010. Murine leukemia virus glycosylated Gag (gPr80gag) facilitates interferon-sensitive virus release through lipid rafts. Proc. Natl. Acad. Sci. U. S. A. 107:1190-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pardieu, C., R. Vigan, S. J. Wilson, A. Calvi, T. Zang, P. Bieniasz, P. Kellam, G. J. Towers, and S. J. Neil. 2010. The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathog. 6:e1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perez-Caballero, D., T. Zang, A. Ebrahimi, M. W. McNatt, D. A. Gregory, M. C. Johnson, and P. D. Bieniasz. 2009. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139:499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfeiffer, T., T. Pisch, G. Devitt, D. Holtkotte, and V. Bosch. 2006. Effects of signal peptide exchange on HIV-1 glycoprotein expression and viral infectivity in mammalian cells. FEBS Lett. 580:3775-3778. [DOI] [PubMed] [Google Scholar]
- 43.Pizzato, M. 2010. MLV glycosylated-Gag is an infectivity factor that rescues Nef-deficient HIV-1. Proc. Natl. Acad. Sci. U. S. A. 107:9364-9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rollason, R., V. Korolchuk, C. Hamilton, M. Jepson, and G. Banting. 2009. A CD317/tetherin-RICH2 complex plays a critical role in the organization of the subapical actin cytoskeleton in polarized epithelial cells. J. Cell Biol. 184:721-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rulli, S. J., Jr., J. Mirro, S. A. Hill, P. Lloyd, R. J. Gorelick, J. M. Coffin, D. Derse, and A. Rein. 2008. Interactions of murine APOBEC3 and human APOBEC3G with murine leukemia viruses. J. Virol. 82:6566-6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakuma, T., T. Noda, S. Urata, Y. Kawaoka, and J. Yasuda. 2009. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 83:2382-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sauter, D., M. Schindler, A. Specht, W. N. Landford, J. Munch, K. A. Kim, J. Votteler, U. Schubert, F. Bibollet-Ruche, B. F. Keele, J. Takehisa, Y. Ogando, C. Ochsenbauer, J. C. Kappes, A. Ayouba, M. Peeters, G. H. Learn, G. Shaw, P. M. Sharp, P. Bieniasz, B. H. Hahn, T. Hatziioannou, and F. Kirchhoff. 2009. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6:409-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawada, S., K. Gowrishankar, R. Kitamura, M. Suzuki, G. Suzuki, S. Tahara, and A. Koito. 1998. Disturbed CD4+ T cell homeostasis and in vitro HIV-1 susceptibility in transgenic mice expressing T cell line-tropic HIV-1 receptors. J. Exp. Med. 187:1439-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schambach, A., J. Bohne, S. Chandra, E. Will, G. P. Margison, D. A. Williams, and C. Baum. 2006. Equal potency of gammaretroviral and lentiviral SIN vectors for expression of O6-methylguanine-DNA methyltransferase in hematopoietic cells. Mol. Ther. 13:391-400. [DOI] [PubMed] [Google Scholar]
- 50.Sun, J., T. Soos, V. N. Kewalramani, K. Osiecki, J. H. Zheng, L. Falkin, L. Santambrogio, D. R. Littman, and H. Goldstein. 2006. CD4-specific transgenic expression of human cyclin T1 markedly increases human immunodeficiency virus type 1 (HIV-1) production by CD4+ T lymphocytes and myeloid cells in mice transgenic for a provirus encoding a monocyte-tropic HIV-1 isolate. J. Virol. 80:1850-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swingler, S., A. Mann, J. Jacque, B. Brichacek, V. G. Sasseville, K. Williams, A. A. Lackner, E. N. Janoff, R. Wang, D. Fisher, and M. Stevenson. 1999. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat. Med. 5:997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uchil, P. D., B. D. Quinlan, W. T. Chan, J. M. Luna, and W. Mothes. 2008. TRIM E3 ligases interfere with early and late stages of the retroviral life cycle. PLoS Pathog. 4:e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Damme, N., D. Goff, C. Katsura, R. L. Jorgenson, R. Mitchell, M. C. Johnson, E. B. Stephens, and J. Guatelli. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei, P., M. E. Garber, S. M. Fang, W. H. Fischer, and K. A. Jones. 1998. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92:451-462. [DOI] [PubMed] [Google Scholar]
- 55.Wolf, D., and S. P. Goff. 2007. TRIM28 mediates primer binding site-targeted silencing of murine leukemia virus in embryonic cells. Cell 131:46-57. [DOI] [PubMed] [Google Scholar]
- 56.Wong, S. K., M. Connole, J. S. Sullivan, H. Choe, A. Carville, and M. Farzan. 2009. A New World primate deficient in tetherin-mediated restriction of human immunodeficiency virus type 1. J. Virol. 83:8771-8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yap, M. W., S. Nisole, C. Lynch, and J. P. Stoye. 2004. Trim5alpha protein restricts both HIV-1 and murine leukemia virus. Proc. Natl. Acad. Sci. U. S. A. 101:10786-10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, F., S. J. Wilson, W. C. Landford, B. Virgen, D. Gregory, M. C. Johnson, J. Munch, F. Kirchhoff, P. D. Bieniasz, and T. Hatziioannou. 2009. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe 6:54-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, J. X., G. E. Diehl, and D. R. Littman. 2008. Relief of preintegration inhibition and characterization of additional blocks for HIV replication in primary mouse T cells. PLoS One 3:e2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.