Abstract
Arteria lusoria, an aberrant or anomalous right subclavian artery, is the most common anomaly of the aortic arch. It may be associated with other congenital anomalies of the heart and great vessels—including, rarely, truncus bicaroticus (a common trunk of both common carotid arteries), and, even more rarely, aneurysmal formation.
Herein, we report the case of a 72-year-old man who had both an atherosclerotic aneurysm of an aberrant right subclavian artery and truncus bicaroticus. We resected the aneurysm through a posterolateral thoracotomy and did not restore the distal pulsatile blood supply to the right arm. During long-term clinical follow-up, the patient experienced no arm ischemia or cerebrovascular insufficiency.
Aneurysm of arteria lusoria should be suspected in the presence of a right superior mediastinal mass on chest radiographs and should be considered as a cause of new-onset dyspnea, chest pain, or dysphagia. Symptomatic right arteria lusoria aneurysm should be removed promptly after diagnosis. Despite disagreement among investigators regarding the need to restore pulsatile blood flow to the right arm, we recommend reconstructing that flow, when possible.
Key words: Aneurysm/complications/diagnosis/surgery/therapy; arm/blood supply; cardiovascular abnormalities/complications/radiography/surgery; diagnosis, differential; dyspnea/etiology; ischemia/etiology; postoperative complications; subclavian artery/abnormalities/pathology/radiography/surgery; treatment outcome; vascular malformations/complications/surgery; vascular surgical procedures/methods
Arteria lusoria, an anomalous or aberrant right subclavian artery (RSA),1,2 is the most common anomaly of the aortic arch.3–5 Its incidence varies from 0.4% to 2%.3,6,7 It results from embryologic malformation of the aortic arch.5,8,9 Hunauld first described the anatomy of arteria lusoria, in 1735.10 The vascular aberrancy is always isolated but may be associated with other congenital anomalies of the heart, great vessels, and branches of the aortic arch.4,11 Arteria lusoria is usually asymptomatic,4,8,12–14 but it may cause dyspnea, stridor, dysphagia, and chest pain.2,12,15 Dysphagia due to esophageal compression by a retroesophageal RSA is known as dysphagia lusoria,6,8,12,14 or dysphagia from “freak of nature,” as described by Bayford in 1794.16 Hamburger and Hyrtl discerned no relationship between aberrant RSA and dysphagia and proposed changing “dysphagia lusoria” to “dysphagia illusoria.”12 The diagnosis of arteria lusoria was reported only on necropsy until 1936, when Kommerell described the clinical diagnosis of an aberrant RSA that originated from an aortic diverticulum (later known as Kommerell's diverticulum) in a 65-year-old man who was presumed to have stomach cancer.16 In 1946, Gross was the first to report the surgical treatment of dysphagia lusoria, in a 4-month-old infant.14
Aneurysmal formation in arteria lusoria is extremely rare.17–19 Herein, we report the case of a patient who had an aneurysm of the aberrant RSA in association with truncus bicaroticus (a common origin of both common carotid arteries), discuss the long-term clinical follow-up of the patient after the surgical resection of the aneurysm, and review the relevant literature.
Case Report
In July 1996, a 72-year-old man was admitted to our hospital with progressive chest discomfort, dyspnea, and cough. He reported dysphagia that was intermittent but not prominent. His medical history included ischemic heart disease, previous myocardial infarction, peripheral vascular disease, chronic obstructive pulmonary disease, and moderate renal insufficiency. He had undergone coronary artery bypass grafting (1981), thromboendarterectomy of the left internal carotid artery (1988), aorto-bifemoral bypass to correct an abdominal aortic aneurysm (1993), and pacemaker implantation because of bradyarrhythmia (1994). At the July 1996 presentation, coronary angiography revealed 3-vessel coronary disease, a patent venous bypass graft to the left anterior descending coronary artery, moderate left ventricular dysfunction, and trivial aortic and mitral valve insufficiency. Measurements of blood pressure by different members of the nursing staff varied from equal pressures in both arms to pressures 5 to 10 mmHg lower in the right arm. The radial pulse was present in both wrists but was weaker in the right.
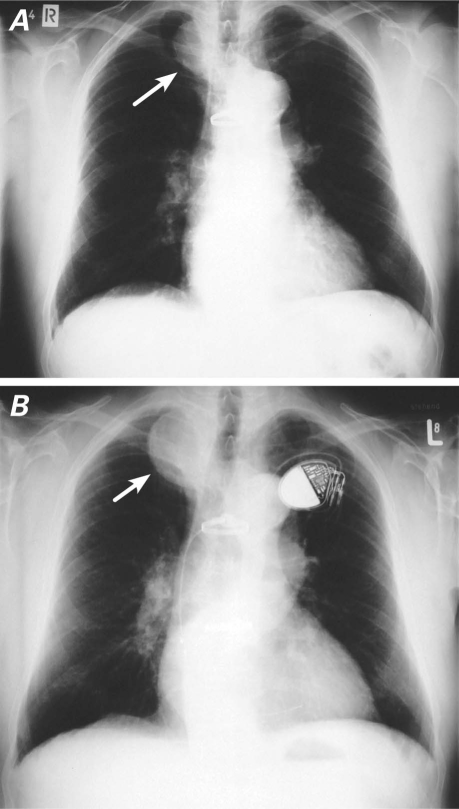
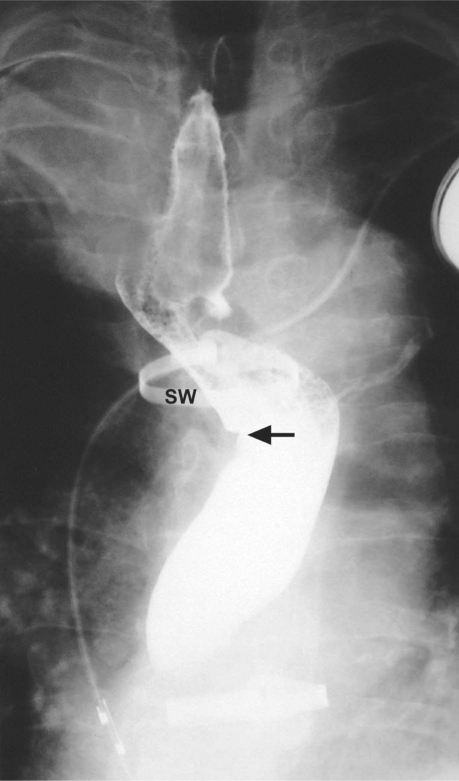
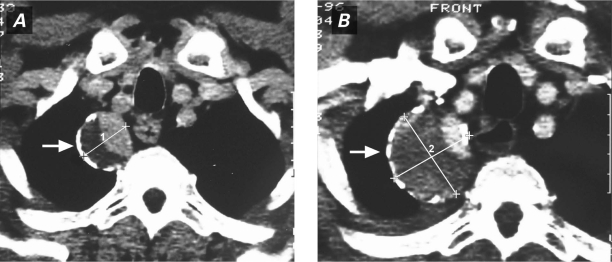
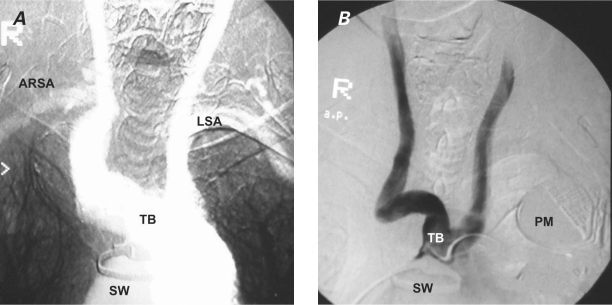
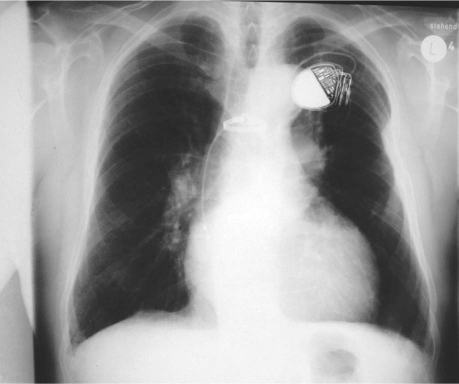
A chest radiograph in September 1996 showed a right superior mediastinal mass that had grown in comparison with its radiographic appearance in 1990 (Fig. 1). A barium esophagogram showed compression and displacement of the esophagus by a calcified aneurysm (Fig. 2). Distal dilation and indentation of the esophagus were also apparent. Chest computed tomograms from 1991 and 1996 revealed a calcified aneurysm of the aberrant RSA, originating from the aortic arch, that had grown slowly and become totally thrombosed (Fig. 3). A digital subtraction angiogram of the aortic arch showed 3 arterial branches that originated from the aortic arch: the truncus bicaroticus, the left subclavian artery, and the aberrant RSA. The RSA took a retroesophageal and retrotracheal course and crossed the mediastinum into the right arm (Fig. 4).
Fig. 1 Preoperative chest radiographs show the aberrant right subclavian artery aneurysm (arrow) and its growth from A) 1990 through B) 1996. A pacemaker was implanted in 1994.
Fig. 2 Barium esophagogram shows esophageal compression and displacement by the calcified aneurysm of the aberrant right subclavian artery. The esophageal indentation is visible (arrow).
SW = sternal wire
Fig. 3 Contrast computed tomograms of the chest show A) the calcified, partially thrombosed aberrant right subclavian artery aneurysmal sac in 1991 (arrow); and B) the enlarged, almost totally thrombosed aneurysm in 1996 (arrow).
Fig. 4 Digital subtraction angiograms show A) the aortic arch vascular anomaly with its 3 branches and the ARSA crossing the mediastinum to arrive in the right arm; and B) the truncus bicaroticus.
ARSA = aberrant right subclavian artery; LSA = left subclavian artery; PM = pacemaker; SW = sternal wire; TB = truncus bicaroticus
Because of the patient's progressive symptoms and the risk of embolization or rupture, it was decided to remove the aneurysm surgically. Due to the previous median sternotomy for coronary artery bypass grafting, the planned surgical approach was a left posterolateral thoracotomy.
Good exposure of the aneurysmal sac was achieved through a thoracotomy in the 4th intercostal space. The 5.5 × 6-cm RSA anuerysm was atherosclerotic, totally thrombosed, and completely atrophied. Care was taken to avoid damaging the left subclavian artery, the vagus nerve, and the recurrent laryngeal nerve. The aneurysmal sac was completely mobilized by releasing the surrounding adhesive bands and dividing the arterial ligament. The aneurysm's proximal origin from the aortic arch was controlled with the use of a wide partial occluding clamp. Resection and closure were achieved with the use of mattress sutures and over-and-over 4-0 Prolene sutures. After sufficient backflow was observed, the intrathoracic part of the right subclavian artery and its healthy section distal to the aneurysmal sac were ligated and oversewn. Because of the risk of atheroma embolization and the evident calcification of the ascending aorta and the right carotid artery, pulsatile flow to the right arm was not restored. Pathologic examination showed a completely atrophied, thrombosed, atherosclerotic aneurysmal sac. No malignancy was found. The patient experienced an uneventful postoperative course and was discharged from the hospital on the 18th postoperative day. His right arm and hand were warm and pink, and the radial pulse was weak but palpable. No sensorial or motor deficits were observed.
The patient was readmitted in 1997 for successful percutaneous transluminal angioplasty of the superficial femoral and popliteal arteries. In 1999, a chest radiograph showed that the upper right mediastinal mass had disappeared (Fig. 5). A year later, clinical follow-up revealed satisfactory blood supply to the right arm. No functional disability, arm ischemia, or subclavian steal syndrome were documented. Intermittent weakness and numbness of the patient's right arm were noted during subsequent long-term clinical follow-up; however, the symptoms—which evidently resulted from the relative underperfusion of the right arm in comparison with the left—did not disable him in his daily activities. The patient died in June 2007 of progressive heart failure and concomitant comorbidities.
Fig. 5 Chest radiograph 3 years after surgery shows the complete disappearance of the upper right mediastinal mass.
Discussion
Arteria lusoria results from abnormal embryologic development of the aortic arch. It can be categorized as a vascular ring.9 The abnormal arterial origin is explained by the involution of the right 4th vascular arch with the proximal right dorsal aorta and the persistence of the right 7th intersegmental artery, which remains attached to the dorsal aorta: after the rotation of the dorsal aorta, the right 7th intersegmental artery becomes the aberrant RSA.5,8 The vessel arises as the 4th branch of the aortic arch (after the left subclavian, the left common carotid, and the right common carotid arteries), passes behind the esophagus, and crosses the mediastinum to arrive in the right arm.16 Although its usual course is retroesophageal, the aberrant RSA can pass between the trachea and the esophagus.12 A pretracheal course of the RSA had been described, but the existence of this course is very doubtful.12 Arteria lusoria is usually asymptomatic.6,14,16,18 However, it can manifest itself as dyspnea, stridor, dysphagia, chest pain, or fever.5,15 Arteria lusoria may clinically mimic pericarditis, endocarditis, or aortic dissection.5 Gross noted 3 circumstances in which an aberrant RSA may cause or aggravate dysphagia: “bow-string” phenomenon, when the artery becomes taut and stretches across the esophagus; when the artery becomes sclerotic and less elastic, particularly in adulthood; or when the artery becomes aneurysmal.14
Arteria Lusoria and Truncus Bicaroticus. Klinkhamer stated that truncus bicaroticus is a precondition for tracheal–esophageal compression and the development of clinical symptoms.12 Under these circumstances, the truncus bicaroticus holds the trachea from the front and the aberrant RSA compresses the esophagus from behind.12,15,16 The association of aberrant RSA and truncus bicaroticus is somewhat rare.12,20,21 Klinkhamer reported that aberrant RSA was combined with truncus bicaroticus in 85 of 295 patients (29%).12 Hartyánszky and colleagues15 reported the cases of 8 symptomatic infants who had an aberrant RSA with truncus bicaroticus among 111 pediatric patients who had vascular rings. These 8 underwent surgical division and implantation of the RSA into the ascending aorta through a right thoracotomy.
Arteria Lusoria and Atherosclerotic Disease or Aortic Dissection. Aberrant RSA is rarely involved in atherosclerotic stenotic disease. Roland and Cherry described a case of symptomatic atherosclerotic disease of aberrant RSA that was not associated with an aneurysm. The patient presented with severe arm claudication and was treated by means of carotid–subclavian bypass surgery.10
Aberrant RSA may be involved in dissection of the descending aorta or the aortic arch.6 The 1st such case, which involved a patient who also had truncus bicaroticus, was described by DeBakey and colleagues in 1955.22
Aneurysm in Arteria Lusoria. Just as aneurysms of the subclavian arteries are rare, so too are aneurysmal formations in the aberrant RSA.1,13,17–19 On chest radiography, these aneurysms may appear as a right superior mediastinal mass13,18 and should be included in the differential diagnosis of substernal goiter, parathyroid adenoma, thymoma, lymphoma, bronchogenic cyst, and carcinoma.13 The 1st case of an aberrant RSA aneurysm was reported by McCallen and Schaff in 1956.23 In a review of the world literature from 1956 to 1991, Knight and Codd19 documented 49 aneurysms, most of which were atherosclerotic. The patients' ages ranged from 11 to 79 years. The youngest, an 11-year-old girl who had undergone heart catheterization 2 years earlier, developed a traumatic false aberrant RSA aneurysm as a sequela: it was surgically removed without reconstruction of the RSA. Arm ischemia was not observed in that patient during 3 years of follow-up.4
Treatment of Arteria Lusoria Aneurysms. Different surgical and endovascular approaches have been used to treat and manage aneurysms of the aberrant RSA, indicating a lack of agreement in regard to this rare clinical entity. The optimal treatment method will vary according to the anatomy of the aneurysm, its extent, and the presence of concomitant aortic thoracic aneurysm, aortic dissection, aneurysm of Kommerell's diverticulum, or comorbidities in high-risk patients.1,3,9,10,19,24–29
New endovascular techniques, such as intraluminal implantations of PTFE-covered stent-grafts and other devices with or without surgical restoration of the blood supply to the right arm, have been introduced as alternative, minimally invasive approaches to excluding aneurysms of the aberrant RSA.26–29 By such means, the proximal part of the aneurysm can be excluded in combination with carotid–subclavian artery bypass surgery that restores distal pulsatile blood flow to the right arm.27–29 In addition, endovascular stent-graft placement is an alternative to high-risk surgical intervention in concomitant aneurysm and thoracic aortic type B dissection.30 In all instances, long-term clinical follow-up is warranted in order to preclude any late complications such as endoleak or stent-graft migration. Patients of advanced age have been given conservative therapy for these aneurysms, without surgical intervention.13
Restoring Pulsatile Blood Flow to the Right Arm. The necessity of restoring distal pulsatile flow to the right arm has been debated.1,4,11,14 According to a published review, only 11 of 33 patients who had surgery to correct aberrant RSAs also underwent revascularization of the right arm, and only 3 of the 22 who did not undergo revascularization developed limb ischemia with fingertip necrosis.19 The intraoperative observation of sufficient backflow from the intrathoracic distal RSA has influenced surgical decisions not to restore the blood supply to the right arm.4,11 In 1 case, Elewaut and Rubens31 restricted their surgical procedure to the simple division of a nonaneurysmal aberrant RSA in a 68-year-old woman who had dysphagia lusoria.
Gross, who treated dysphagia lusoria in a 4-month-old infant by simple division of the aberrant RSA, reported that the intrathoracic segment of the RSA can be divided without risk, because the 2nd and 3rd portions of that artery have sufficient collateral vessels to provide satisfactory blood flow to the arm.14 A month after the operation, that patient had no arm ischemia or motor disability, and although the radial pulse could not be accurately counted, frequent beats could be felt.
Severe upper-extremity ischemia or gangrene has occurred in only 0.2% of cases as a sequela of subclavian artery division to accomplish systemic–pulmonary connection (such as in the creation of a Blalock-Taussig shunt to palliate cyanotic congenital heart disease).32 By surgically dividing and reestablishing flow to anomalous subclavian arteries through either graft insertion or the reimplantation of those arteries, Smith and colleagues treated children who had symptomatic manifestations that had resulted from compressed anomalous retroesophageal subclavian arteries.9
Cooley emphasized the need to restore pulsatile distal flow to the right arm via right carotid–subclavian artery bypass, especially in adults.25 This is achieved by means of further supraclavicular space incision after the resection of aberrant RSA aneurysms.
In the presence of adequate collateral vessels, the simple resection of an aberrant RSA aneurysm without restoring the arm's blood supply seems to be safe. However, it might not completely eliminate clinical symptoms, as was our patient's experience. We believe that these postoperative symptoms resulted from underperfusion of the right arm. The existing anastomotic branches between the 2 subclavian arteries and the network of collateral circulation around the shoulder (which had developed 5 years previously when the RSA aneurysm became thrombosed) may explain the viability of our patient's right arm. His need to use his arm did not depend upon a particular minimum threshold of blood flow that only revascularization could have supplied.
Rich collateral circulation around the shoulder may explain why serious ischemia is uncommon even after injuries to subclavian arteries.33 Even ligation of the brachial artery in the antecubital fossa in children with renal disease does not compromise the viability of the arm, or its blood supply.34 This is probably due to well-formed collateral vessels, such as the profunda brachii, that provide flow that is sufficient for limb growth in children who have chronic renal disease.34
Conclusion
Arteria lusoria aneurysm is a rare clinical entity. It should be suspected in the presence of a right superior mediastinal mass and should be considered in the differential diagnosis of new-onset dyspnea, chest pain, and dysphagia. In our opinion, a symptomatic aneurysm of the aberrant RSA should be removed promptly after its diagnosis, because of possible embolization or potential rupture due to the weakness and atrophy of the aneurysmal wall. We recommend reconstructing the pulsatile blood flow to the right arm, when possible, in order to maintain the arm's viability. Surgical, endovascular, or combined interventions can be used. The choice of treatment should be adapted to the nature of the aberrant RSA and to the patient's age, clinical condition, comorbidities, and any concomitant lesions.
Footnotes
Address for reprints: Giovanni Saeed, MD, Department of Cardiac Surgery, Klinikum Bayreuth GmbH, Preuschwitzer Str. 59a, D-95445 Bayreuth, Germany
E-mail: dr.gsaeed@web.de
Presented partially as a poster at the Aortic Surgery Symposium IX, 22–23 April 2004 (New York) and at the 16th World Society of Cardio-Thoracic Surgeons (WSCTS), 17–20 August 2006 (Ottawa, Canada).
References
- 1.Kamiya H, Knobloch K, Lotz J, Bog A, Lichtenberg A, Hagl C, et al. Surgical treatment of aberrant right subclavian artery (arteria lusoria) aneurysm using three different methods. Ann Thorac Surg 2006;82(1):187–90. [DOI] [PubMed]
- 2.Atay Y, Engin C, Posacioglu H, Ozyurek R, Ozcan C, Yagdi T, et al. Surgical approaches to the aberrant right subclavian artery. Tex Heart Inst J 2006;33(4):477–81. [PMC free article] [PubMed]
- 3.Frank MW, Blakeman BP. Two-stage elephant trunk reconstruction for aneurysm of an aberrant right subclavian artery in association with aneurysmal distal aortic arch and descending thoracic aorta. Tex Heart Inst J 2000;27(4):412–3. [PMC free article] [PubMed]
- 4.Rodgers BM, Tabert JL, Hollenbeck JI. Aneurysm of anomalous subclavian artery: an unusual cause of dysphagia lusoria in childhood. Ann Surg 1978;187(2):158–60. [DOI] [PMC free article] [PubMed]
- 5.Bisognano JD, Young B, Brown JM, Gill EA, Fang FC, Zisman LS. Diverse presentation of aberrant origin of the right subclavian artery: two case reports. Chest 1997;112(6):1693–7. [DOI] [PubMed]
- 6.Duff SB, Hicks GL. Aortic arch dissection with an aberrant right subclavian artery: surgical treatment. Tex Heart Inst J 1986;13(2):233–5. [PMC free article] [PubMed]
- 7.Alvarez JR, Quiroga SJ, Nazar AB, Comendador MJ, Carro GJ. Aberrant right subclavian artery and calcified aneurysm of Kommerell's diverticulum: an alternative approach. J Cardiothorac Surg 2008;3:43. [DOI] [PMC free article] [PubMed]
- 8.Carrizo GJ, Marjani MA. Dysphagia lusoria caused by an aberrant right subclavian artery. Tex Heart Inst J 2004;31(2): 168–71. [PMC free article] [PubMed]
- 9.Smith JM 3rd, Reul GJ Jr, Wukasch DC, Cooley DA. Retroesophageal subclavian arteries: surgical management of symptomatic children. Cardiovasc Dis 1979;6(3):331–4. [PMC free article] [PubMed]
- 10.Roland CF, Cherry KJ Jr. Symptomatic atherosclerotic stenotic disease of an aberrant right subclavian artery. Ann Vasc Surg 1991;5(2):196–8. [DOI] [PubMed]
- 11.Gomes MM, Bernatz PE, Forth RJ. Arteriosclerotic aneurysm of an aberrant right subclavian artery. Dis Chest 1968;54(6): 549–52. [DOI] [PubMed]
- 12.Klinkhamer AC. Aberrant right subclavian artery. Clinical and roentgenologic aspects. Am J Roentgenol Radium Ther Nucl Med 1966;97(2):438–46. [DOI] [PubMed]
- 13.Engelman RM, Madayag M. Aberrant right subclavian artery aneurysm: a rare cause of a superior mediastinal tumor. Chest 1972;62(1):45–7. [DOI] [PubMed]
- 14.Gross RE. Surgical treatment for dysphagia lusoria. Ann Surg 1946;124(3):532–4. [PMC free article] [PubMed]
- 15.Hartyanszky IL, Lozsadi K, Marcsek P, Huttl T, Sapi E, Kovacs AB. Congenital vascular rings: surgical management of 111 cases. Eur J Cardiothorac Surg 1989;3(3):250–4. [DOI] [PubMed]
- 16.van Son JA, Konstantinov IE. Burckhard F. Kommerell and Kommerell's diverticulum. Tex Heart Inst J 2002;29(2):109–12. [PMC free article] [PubMed]
- 17.Gordini V, Collice M, Fedriga E, Moreo A, Morello M, Porrini A, Donatelli F. Aneurysm of an aberrant right subclavian artery. Report of a surgically treated case. Tex Heart Inst J 1991;18(1):76–9. [PMC free article] [PubMed]
- 18.Cunningham GC. Radiologic case presentation. Cardiovasc Dis 1977;4(1):4–6. [PMC free article] [PubMed]
- 19.Knight GC, Codd JE. Anomalous right subclavian artery aneurysms. Report of 3 cases, with a review of the literature. Tex Heart Inst J 1991;18(3):209–18. [PMC free article] [PubMed]
- 20.Murzi M, Mariani M, Tiwari KK, Farneti P, Berti S, Karimov JH, Glauber M. Aberrant right subclavian artery aneurysm in coexistence with a common carotid trunk. Ann Thorac Surg 2009;88(1):e8. [DOI] [PubMed]
- 21.Epstein DA, Debord JR. Abnormalities associated with aberrant right subclavian arteries-a case report. Vasc Endovascular Surg 2002;36(4):297–303. [DOI] [PubMed]
- 22.De Bakey ME, Cooley DA, Creech O Jr. Surgical considerations of dissecting aneurysm of the aorta. Ann Surg 1955;142 (4):586–612. [DOI] [PMC free article] [PubMed]
- 23.McCallen AM, Schaff B. Aneurysm of an anomalous right subclavian artery. Radiology 1956;66(4):561–3. [DOI] [PubMed]
- 24.Kedora J, Grimsley B, Pearl G. Endovascular treatment of an aberrant right subclavian artery aneurysm with use of the Zenith iliac plug. Proc (Bayl Univ Med Cent) 2009;22(2):144–5. [DOI] [PMC free article] [PubMed]
- 25.Cooley DA. Congenital aortic aneurysms. In: Cooley DA, Kneipp M, Lawrence EP, editors. Surgical treatment of aortic aneurysms. Philadelphia: WB Saunders; 1986. p. 175–84.
- 26.Davidian M, Kee ST, Kato N, Semba CP, Razavi MK, Mitchell RS, Dake MD. Aneurysm of an aberrant right subclavian artery: treatment with PTFE covered stentgraft. J Vasc Surg 1998;28(2):335–9. [DOI] [PubMed]
- 27.Lacroix V, Astarci P, Philippe D, Goffette P, Hammer F, Verhelst R, Noirhomme P. Endovascular treatment of an aneurysmal aberrant right subclavian artery. J Endovasc Ther 2003; 10(2):190–4. [DOI] [PubMed]
- 28.Attmann T, Brandt M, Muller-Hulsbeck S, Cremer J. Two-stage surgical and endovascular treatment of an aneurysmal aberrant right subclavian (lusoria) artery. Eur J Cardiothorac Surg 2005;27(6):1125–7. [DOI] [PubMed]
- 29.Corral JS, Zuniga CG, Sanchez JB, Guaita JO, Basail AM, Gimeno CC. Treatment of aberrant right subclavian artery aneurysm with endovascular exclusion and adjunctive surgical bypass. J Vasc Interv Radiol 2003;14(6):789–92. [DOI] [PubMed]
- 30.Baccin CE, Montenegro MA, Mourao GS. Thoracic aorta dissection associated with aberrant right subclavian artery: treatment with endovascular stent-graft placement. Yale J Biol Med 2004;77(3–4):59–62. [PMC free article] [PubMed]
- 31.Elewaut D, Rubens R. An unusual case of dysphagia. Postgrad Med J 1994;70(828):768. [DOI] [PMC free article] [PubMed]
- 32.Geiss D, Williams WG, Lindsay WK, Rowe RD. Upper extremity gangrene: a complication of subclavian artery division. Ann Thorac Surg 1980;30(5):487–9. [DOI] [PubMed]
- 33.Holleman JH Jr, Hardy JD, Williamson JW, Raju S, Neely WA. Arterial surgery for arm ischemia. A survey of 136 patients. Ann Surg 1980;191(6):727–37. [DOI] [PMC free article] [PubMed]
- 34.Lally KP, Foster CE 3rd, Chwals WJ, Brennan LP, Atkinson JB. Long-term follow-up of brachial artery ligation in children. Ann Surg 1990;212(2):194–6. [DOI] [PMC free article] [PubMed]