Abstract
Nephrogenic systemic fibrosis is a recently recognized disease entity that is potentially debilitating. The exact pathogenesis of nephrogenic systemic fibrosis is unclear, but the disease has been linked with the use of gadolinium-based contrast agents, predominantly in patients with acute renal failure or end-stage renal disease. Consequent to increased physician awareness of this link, the incidence of nephrogenic systemic fibrosis has begun to decrease. The aims of this review are to provide a concise summary of the approved gadolinium-based contrast agents available in the United States, to discuss the postulated pathogenesis of nephrogenic systemic fibrosis, to describe the clinical features of the disease, and to provide broad recommendations for gadolinium-based contrast agent use.
Key words: Chelating agents/adverse effects, gadolinium/diagnostic use/toxicity, magnetic resonance imaging/methods, nephrogenic fibrosing dermopathy/chemically induced, nephrogenic systemic fibrosis
The 1st noninvasive tomographic images of the human body using magnetic resonance imaging (MRI) were published almost 3 decades ago.1 Since that time, MRI has undergone remarkable technological and methodological advances. Because of its ability to provide information on microscopic and macroscopic tissue structure, physiology, and metabolism, this technique is now widely used clinically to distinguish normal tissue from abnormal tissue. Compared with other noninvasive imaging techniques, MRI has a unique combination of advantages, including high spatial resolution, exquisite tissue contrast, reproducibility, lack of limitations with respect to acoustic windows, and lack of a need for ionizing radiation.
In cardiovascular cases, the exquisite soft-tissue contrast provided by MRI has been used to study cardiac structure and function. In fact, cardiac magnetic resonance imaging has emerged as the gold standard for evaluating ventricular function and characterizing tissue.2 The introduction of MRI contrast agents has further expanded the clinical usefulness of this method of evaluating cardiovascular disease. In particular, the introduction of gadolinium (Gd)-based contrast agents (GBCAs) has expanded the role of MRI for visualizing vascular structures within the body. Gadolinium-based contrast agents dramatically reduce the tissue's spin-lattice relaxation time (T1), and this reduction in T1 is used to visualize vascular space after the administration of these agents. Because of its high spatial and contrast resolution, such imaging of vascular space—called MR angiography—has gained widespread acceptance in the assessment of aortic and peripheral vascular disease.3–6
In addition to enabling visualization of the macrovascular space, the contrast-agent kinetics within tissue can be exploited to provide further information about the physiologic or metabolic state of the tissue. For example, the distribution volume for Gd is greater in necrotic myocardium than in normal myocardium, and this difference serves as a contrast mechanism in delayed-enhancement (DE) MRI, a recently introduced technique for identifying irreversibly injured myocardium. Several researchers have shown that DE-MRI can identify areas of myocardial fibrosis with a high degree of accuracy and reproducibility.7,8
Gadolinium has traditionally been deemed a relatively safe contrast agent. Since 1988, at least 7 GBCAs, with different structural, pharmacokinetic, and physicochemical properties, have been approved by the U.S. Food and Drug Administration (FDA) for clinical use during MRI. Millions of patients have been exposed to these agents, which were considered safe for use even in patients with renal insufficiency.9 Since 2007, however, a relatively rare but potentially fatal disease termed nephrogenic systemic fibrosis (NSF) has been linked to Gd exposure in patients with advanced renal disease. The specific mechanism associated with the induction of NSF in these patients is not yet known. As a result, the diagnostic imaging community has become concerned about the safe use of GBCAs for clinical MRI evaluation.
The aim of this review is to provide a concise summary of the currently used GBCAs, the pathogenesis and clinical characteristics of NSF, and clinical recommendations for using GBCAs now that NSF has been recognized.
Gadolinium-Based Contrast Agents
Metal ions with unpaired electrons are paramagnetic, and in an aqueous solution, the dipolar interaction between the paramagnetic ions and the water protons reduces both the spin-lattice (T1) and the spin-spin (T2) relaxation-time constants of the water protons. The paramagnetic ions gadolinium (Gd3+) and manganese (Mn2+) have 7 and 5 unpaired electrons, respectively, and are effective in reducing the T1, and T2 of protons. However, in its native ionic form, Gd3+ is highly toxic to the human body; this substance accumulates in the bones, liver, or spleen by displacing endogenous materials such as Fe3+, Zn2+, Cu3+, and calcium via a process known as transmetallation.10 Therefore, a metal ion complex or chelate with high thermodynamic and kinetic stability at a physiologic pH is necessary to ensure near-complete, safe excretion of a GBCA from the body.
Biodistribution of Gadolinium-Based Contrast Agents
After intravenous administration, GBCAs leak from the blood pool into the interstitial space. The size of the GBCA molecules determines the rate of this leakage. Almost all of the GBCAs approved for clinical use by the FDA are low-molecular-weight, extracellular fluid agents that have a distribution half-life of about 5 minutes and are cleared predominantly by the kidneys, with an elimination half-life of about 80 minutes.10 The FDA recently approved gadofosveset (Ablavar™, Lantheus Medical Imaging, Inc.; N. Billerica, Mass), a GBCA that binds strongly and reversibly to a much larger serum albumin molecule and that has a longer distribution half-life and higher relaxivity than those of extracellular fluid agents. Such high-molecular-weight GBCAs, which reside in the intravascular space for a relatively long time, are referred to as blood-pool agents.
In patients who have normal renal function, the elimination half-life of most of the Gd-chelate agents is approximately 1 to 2 hours.11 However, in 1 study that investigated patients who had severe renal insufficiency (defined as a creatinine clearance of 10–30 mL/min), the mean half-life of gadopentetate dimeglumine (Magnevist®, Bayer HealthCare Pharmaceuticals Inc.; Wayne, NJ) increased to 6.1 hours after administration.12 Moreover, in another study,13 the mean half-life of gadodiamide (Omniscan™, GE Healthcare; Chalfont St. Giles, UK) increased to 34.2 hours in patients with severe renal insufficiency (defined as a glomerular filtration rate [GFR] of 2–10 mL/min).
Physical Structure and Stability of Gadolinium-Based Contrast Agents
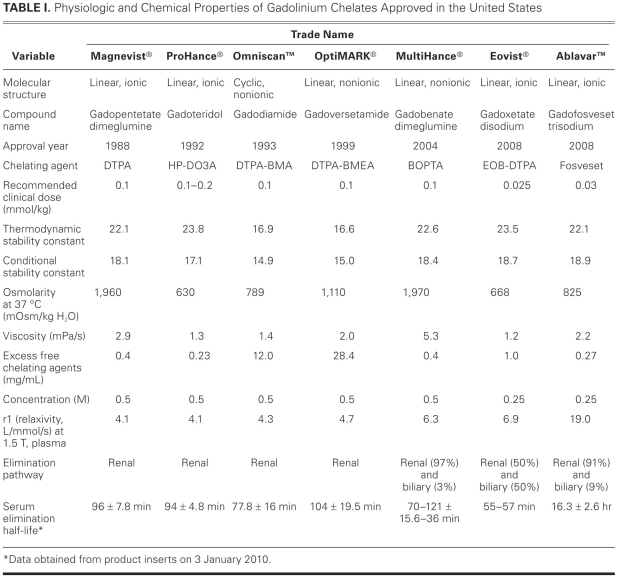
Table I lists the Gd chelates that have been approved by the FDA.11,14–16 The commercially available extracellular fluid agents are Gd3+ ions ligated with linear or cyclic polyaminocarboxylates.9 In aqueous solutions, the cyclic Gd chelates, because of their “ring” structure, have higher conditional and thermodynamic stability constants; compared with their linear counterparts, the cyclic Gd chelates require more energy to dissociate the Gd3+ ions from their chelated configuration. In addition, Gd chelates can be classified as ionic or nonionic. In the ionic compounds, which are meglumine salts of negatively charged chelates, the electrostatic interaction between the Gd and the chelate is stronger than that in the nonionic compounds. Researchers have shown that, in aqueous solutions, the ionic forms of Gd chelates commercially available in the United States, such as gadopentetate dimeglumine and gadobenate dimeglumine (MultiHance®, Bracco Diagnostics Inc.; Princeton, NJ), have higher conditional and thermodynamic stability constants than do nonionic gadolinium chelates such as gadodiamide and gadoversetamide (OptiMARK®, Covidien Imaging Solutions; Hazelwood, Mo). Some GBCAs (gadodiamide, for example) have a significant excess of chelates, and the presence of excess chelates is often considered an indirect measure of Gd-chelate stability in vivo. In general, Gd chelates with a macrocyclic structure are more stable than are those with a linear structure.
TABLE I. Physiologic and Chemical Properties of Gadolinium Chelates Approved in the United States
Nephrogenic Systemic Fibrosis
In 2000, Cowper and associates17 reported that 15 hemodialysis patients presented with “scleromyxoedema-like” skin lesions. The following year, the same author used the term nephrogenic fibrosing dermopathy to describe this clinical syndrome.18 In 2003, researchers first noted that the disease could also involve other systemic organs,19 and subsequently the term nephrogenic systemic fibrosis (NSF) was adopted in the literature.20 In 2006, reports started to surface suggesting that patients diagnosed with NSF had frequently been exposed to Gd chelates before developing clinical symptoms.21,22 Since then, the FDA and other regulatory authorities and professional societies have provided recommendations and continual updates regarding NSF and the administration of Gd chelates.23–26
Pathogenesis
Over the past few years, investigators have associated various clinical conditions with NSF, including metabolic acidosis,21 increased iron mobilization,27 coexisting proinflammatory conditions (such as concurrent infection, recent vascular surgery, and thrombosis),28 high serum calcium and phosphate levels,29 and the use of high-dose erythropoietin.30 However, most patients with NSF have advanced renal insufficiency in association with Gd exposure.22,31
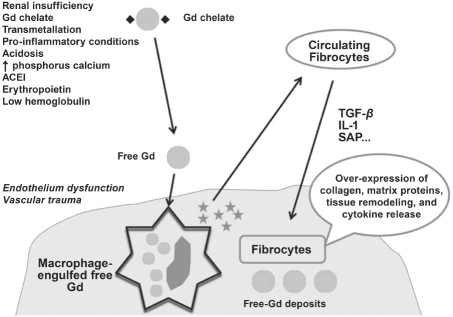
The exact pathogenesis of NSF is unknown and is a subject of intense research. On the basis of evidence that the half-life of the Gd chelate is prolonged in the setting of severe renal insufficiency and in the presence of other risk factors, researchers have suggested that the longer half-life increases the probability of dissociation of Gd ions (Fig. 1). Subsequent to dissociation, the free Gd3+ ion is absorbed in the body via transmetallation. In the setting of endothelial dysfunction, as well as vascular injury due to inflammation, Gd3+ ions can enter tissues more easily. After these ions are taken up by macrophages, profibrotic cytokines are produced; in the presence of locally produced mediators, circulating fibrocytes are attracted to tissue, thereby inducing fibrosis.32
Fig. 1 Proposed pathogenesis of nephrogenic systemic fibrosis. See text for details.
* = cytokine; ACEI = angiotensin-converting enzyme inhibitor; Gd = gadolinium; IL-1 = interleukin-1; SAP = serum amyloid protein; TGF-β = transforming growth factor-β
Association with Gadolinium Chelates
In analyzing 229 cases of NSF reported in the peer-reviewed literature, Shellock and Spinazzi33 recently found that 194 patients (85%) had a GFR of <15 mL/min/1.73 m2 and that another 14 of the patients had a GFR of <30 mL/min/1.73 m2 (the renal status of the remaining 21 patients was unknown). The same authors found 148 unconfounded cases associated with gadodiamide and 20 cases associated with gadopentetate dimeglumine; in the remaining cases, they did not report the name of the Gd agent. In the original 2006 reports,21,22 most of the NSF cases were also related to gadodiamide administration.
In a recent animal study,34 7 groups, consisting of 6 subjects in each group, received a different commercially available Gd chelate (cumulative dose, 50 mmol/kg) over a 4-week period, which resulted in prolonged exposure to the Gd-containing compound. Concurrently, an 8th (control) group received saline solution. Fibrosis of the dermis, similar to that found in NSF, was observed only in the group that received high doses of gadodiamide. Furthermore, the animals that received gadodiamide and gadoversetamide (the 2 agents with the lowest conditional and thermodynamic stability constants) had the highest Gd concentration in their skins. The authors postulated that the stability of the Gd chelates probably played a major role in producing NSF-like findings experimentally.
Clinical Features
The term NSF indicates that almost all patients with this condition have advanced renal disease (nephrogenic), that the condition can affect multiple organ systems (systemic), and that fibrotic changes are noted on histopathologic examination (fibrosis).
Neither race nor sex has shown a predilection for NSF.35 According to different investigators, the incidence of this condition varies in accordance with the Gd-chelate dose and the severity of the underlying renal insufficiency. In a recent study,36 none of 74,124 patients developed NSF after the administration of a single dose of Gd chelate (0.1 mmol/kg), but 15 of 8,997 patients (0.017%) developed NSF after the administration of a high dose (≥0.2 mmol/kg). Of 265 patients undergoing chronic hemodialysis, 1 (0.4%) developed NSF (all received high-dose Gd chelate); and of 131 patients in acute renal failure, 11 (8.4%) developed NSF.36
The organ most commonly affected by NSF is the skin, predominantly from the ankle to the mid thigh and from the wrist to the mid upper arm37; the face is typically spared, although white-yellow scleral plaques may be present.38 The skin is thickened and hard, with a “woody” appearance. Papules, plaques, subcutaneous nodules, erythema, and peau d'orange may also be present.18 Some patients will have pruritus. If the musculoskeletal system is affected, the patient will experience muscle and limb pain; in more severe cases, there could be contractures and a reduction in mobility.38 Potential constitutional symptoms include fever and general malaise. The disease can extend to the lungs, diaphragm, esophagus, myocardium, and dura mater.37 Patients can experience hypercoagulability and thrombotic events.35 Laboratory findings are nonspecific, and increased acute-phase reactants are commonly found.39
The diagnosis of NSF is confirmed by means of biopsy, which shows an abundance of spindle cells that are positive for CD34 immunostaining, with fibroblast-like proliferation in the dermis, a thickened collagen bundle with a lack of inflammation, and increased numbers of macrophages and dendritic cells that are positive for CD68 and factor XIIIA.40–42
Clinical Course
After exposure to a Gd chelate, the time of onset of NSF is reported to range from a few days to 6 months.21,22,26 In most patients, NSF is chronic and unremitting. Joint contractures can be debilitating; patients can become wheelchair-bound within days to weeks after onset of the disease.35 In a series of 12 patients reported by Mendoza and colleagues,38 the disease had a progressive course in 6 cases: 3 of the patients were wheelchair-bound, and 3 others died (the cause of death was not reported). In a cohort of 186 hemodialysis patients, those who had cutaneous changes diagnostic for NSF had a higher mortality rate than patients who did not have such changes (P = 0.003; odds ratio, 3.6; 95% confidence interval, 1.5–8.6) over a 2-year follow-up period.24 However, more than half of the patients with cutaneous changes of NSF died of cardiovascular disease (rather than of NSF per se), and 1 patient died of an unspecified debility.
Treatment
To date, there is no proven treatment for NSF. Most therapeutic studies have had small numbers of subjects and limited follow-up periods. Therapy designed to improve renal function appears to be the best option for slowing, arresting, or reversing the effects of NSF. Physical therapy is aimed at improving mobility.
The following approaches have been used to treat NSF with variable degrees of success: renal transplantation,26 sodium thiosulfate therapy,25 extracorporeal photophoresis,23 imatinib mesylate therapy,43 plasmapheresis, pentoxifylline therapy, intravenous immunoglobulin therapy, steroid therapy, and cyclophosphamide therapy.38
General Recommendations
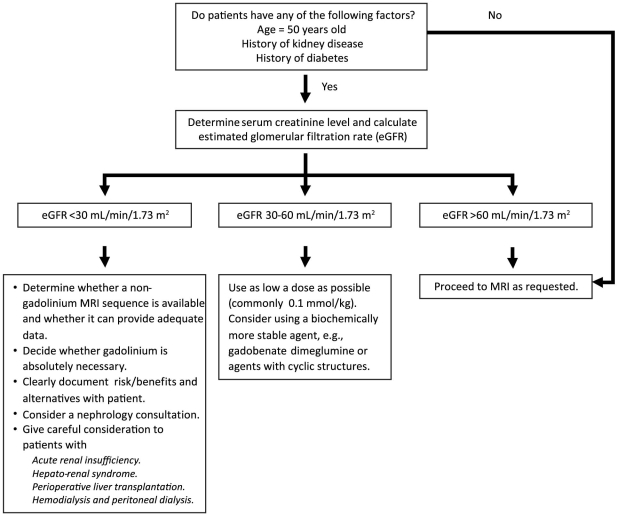
On the basis of the guidelines and recommendations of various professional societies,23–26 as well as our own experience, we believe the following general recommendations (summarized in Fig. 2) to be reasonable:
Fig. 2 Guidelines for gadolinium administration at the Texas Heart Institute at St. Luke's Episcopal Hospital.
MRI = magnetic resonance imaging
Patients at risk of renal insufficiency should undergo renal function tests. At our institution, all patients who have known risk factors and all patients older than 50 years (regardless of risk-factor status) have their GFR estimated.
Administration of Gd chelates—especially those that are linear, are nonionic, and contain excess chelating agents (gadodiamide and gadoversetamide)—should be avoided in patients who have severe renal insufficiency (GFR, <30 mL/min/1.73 m2), acute renal failure, or hepato-renal syndrome, and also should be avoided during the perioperative liver-transplant period.
Some researchers have suggested that the risk of contrast-induced nephropathy might be lower with intravenous than intra-arterial administration of iodine contrast agents.44 However, an increased mortality rate due to iodine contrast exposure has been well documented in patients with acute renal insufficiency.45 Therefore, careful consideration is necessary before administering either Gd or iodine contrast agents to an at-risk population.
If the clinical benefit of Gd-enhanced MRI outweighs the risk of NSF and if no other imaging method can provide the necessary information, a more stable agent (for example, gadobenate dimeglumine) or agents with cyclic structures should be considered. The dose of the Gd chelate should be as low as possible, and repeat dosing within a short period should be avoided. The risks, benefits, and alternatives should be documented clearly and discussed with the patient and the referring physician.
If non-contrast-enhanced MRI can provide clinical information similar to that provided by Gd-enhanced MRI, the non-contrast option should be chosen for high-risk patients.
Okada and associates46 have suggested that 95% of Gd will be excreted after the 2nd hemodialysis session. However, the limitations of their study and of several other studies have been detailed by Penfield and Reilly.47 In hemodialysis patients, it is reasonable to proceed with dialysis as soon as possible after Gd administration in order to facilitate Gd elimination—yet there is insufficient evidence to justify the initiation of hemodialysis simply to eliminate Gd. In fact, to date, there has been no clear-cut evidence to support the hypothesis that hemodialysis can prevent NSF in an at-risk population.
Few data are available regarding peritoneal dialysis and Gd removal.47 At our institution, we avoid using Gd in patients who are undergoing peritoneal dialysis.
In a recent report concerning more than 25,000 patients at 2 centers, use of a strict protocol and substitution of gadobenate dimeglumine for gadodiamide resulted in no new NSF cases during a 1-year follow-up period; the series included 147 patients who had risk factors for NSF and 402 patients who were on hemodialysis.48 Similarly, another center had 24 documented cases of NSF as of March 200629; since then, the hospital has switched from gadodiamide to another biochemical (but more stable) agent, and no further cases of NSF have been encountered, despite the fact that a Gd chelate other than gadodiamide is still being administered, when clinically indicated, to patients with renal insufficiency.
Non-Gadolinium-Enhanced Cardiovascular Magnetic Resonance Imaging
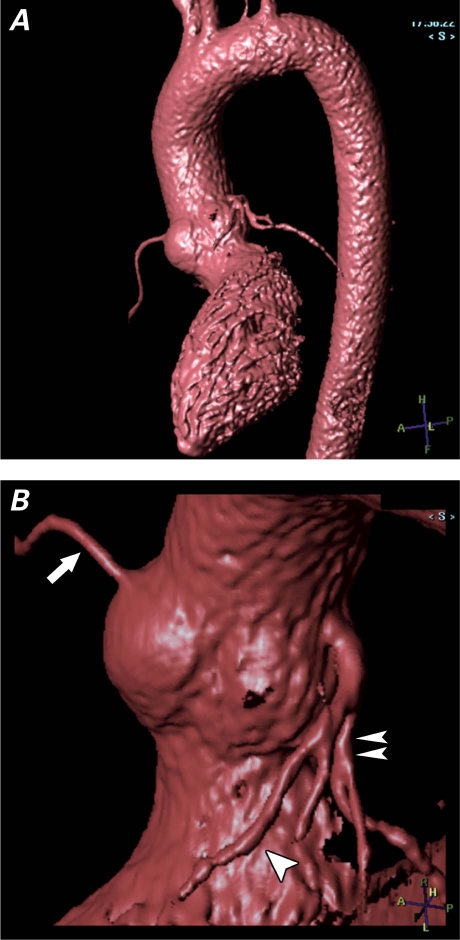
In cardiovascular patients, alternative imaging sequences can be used to evaluate the vascular structures without the use of gadolinium. Conventionally, the head and neck vessels have been imaged by means of the time-of-flight technique, using the inflow effect of the moving spins. The thoracoabdominal aorta and the renal arteries can be visualized at high spatial resolution by using a 3-dimensional, electrocardiographically triggered, steady-state free-precession sequence (Figs. 3 and 4) that is commercially available from all major vendors of MRI equipment. Myocardial viability can be evaluated by use of low-dose dobutamine. However, these techniques depend heavily on the expertise and experience of the cardiovascular MRI personnel. Furthermore, the non-Gd sequence is usually more time-consuming, and it relies greatly on the presence of a regular electrocardiographic rhythm, as well as on the patient's ability to remain still inside the MRI gantry.
Fig. 3 Non-gadolinium-enhanced, electrographic-gated 3-dimensional magnetic resonance angiogram with an isotropic resolution of 1 × 1 × 1 mm and a total imaging time of 5 minutes, using a 32-channel cardiac coil. A) Volume-rendered image of the entire thoracic aorta; no pulsation artifact is seen in the aortic root. B) Magnified view (orig. ×2) of the volume-rendered image of the aortic root clearly shows the origins of the left anterior descending coronary artery (arrowhead), the left circumflex coronary artery (double arrowhead), and the right coronary artery (arrow).
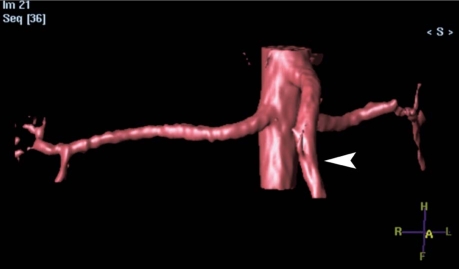
Fig. 4 Volume-rendered image from a non-gadolinium-enhanced electrographic-gated 3-dimensional magnetic resonance angiogram using a commercially available, balanced-TRiggered Angiography Non-Contrast-Enhanced (b-TRANCE) sequence. The superior mesenteric artery is shown (arrowhead).
Summary
Nephrogenic systemic fibrosis is a potentially debilitating systemic disorder. Although its exact pathogenesis remains to be determined, NSF occurs predominantly in patients with advanced renal insufficiency who have been exposed to Gd chelates. For this reason, one should use extreme caution in administering Gd chelates to patients with a GFR of <30 mL/min/1.73 m2 or to those with acute renal failure. Although there is currently no effective treatment for NSF, its incidence appears to be declining, likely due to increased precaution on the part of physicians.
Acknowledgment
We are grateful for the editorial assistance of Virginia Fairchild, of the Department of Scientific Publications, Texas Heart Institute at St. Luke's Episcopal Hospital, Houston.
Footnotes
Address for reprints: Benjamin Y.C. Cheong, MD, FACC, Department of Diagnostic & Interventional Radiology, Texas Heart Institute at St. Luke's Episcopal Hospital, 6720 Bertner Ave., MC 2–270, Houston, TX 77030
E-mail: bcheong@sleh.com
References
- 1.Hinshaw WS, Bottomley PA, Holland GN. Radiographic thin-section image of the human wrist by nuclear magnetic resonance. Nature 1977;270(5639):722–3. [DOI] [PubMed]
- 2.Shan K, Constantine G, Sivananthan M, Flamm SD. Role of cardiac magnetic resonance imaging in the assessment of myocardial viability. Circulation 2004;109(11):1328–34. [DOI] [PubMed]
- 3.Neimatallah MA, Ho VB, Dong Q, Williams D, Patel S, Song JH, Prince MR. Gadolinium-enhanced 3D magnetic resonance angiography of the thoracic vessels. J Magn Reson Imaging 1999;10(5):758–70. [DOI] [PubMed]
- 4.Ersoy H, Zhang H, Prince MR. Peripheral MR angiography. J Cardiovasc Magn Reson 2006;8(3):517–28. [DOI] [PubMed]
- 5.Brasch RC, Weinmann HJ, Wesbey GE. Contrast-enhanced NMR imaging: animal studies using gadolinium-DTPA complex. AJR Am J Roentgenol 1984;142(3):625–30. [DOI] [PubMed]
- 6.Wolf GL, Fobben ES. The tissue proton T1 and T2 response to gadolinium DTPA injection in rabbits. A potential renal contrast agent for NMR imaging. Invest Radiol 1984;19(4): 324–8. [DOI] [PubMed]
- 7.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100(19):1992–2002. [DOI] [PubMed]
- 8.Mahrholdt H, Wagner A, Holly TA, Elliott MD, Bonow RO, Kim RJ, Judd RM. Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation 2002;106(18):2322–7. [DOI] [PubMed]
- 9.Runge VM. Safety of magnetic resonance contrast media. Top Magn Reson Imaging 2001;12(4):309–14. [DOI] [PubMed]
- 10.Bellin MF, Vasile M, Morel-Precetti S. Currently used non-specific extracellular MR contrast media. Eur Radiol 2003;13 (12):2688–98. [DOI] [PubMed]
- 11.Kirchin MA, Runge VM. Contrast agents for magnetic resonance imaging: safety update. Top Magn Reson Imaging 2003;14(5):426–35. [DOI] [PubMed]
- 12.Swan SK, Lambrecht LJ, Townsend R, Davies BE, McCloud S, Parker JR, et al. Safety and pharmacokinetic profile of gadobenate dimeglumine in subjects with renal impairment. Invest Radiol 1999;34(7):443–8. [DOI] [PubMed]
- 13.Joffe P, Thomsen HS, Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad Radiol 1998;5(7):491–502. [DOI] [PubMed]
- 14.Dawson P. Nephrogenic systemic fibrosis: possible mechanisms and imaging management strategies. J Magn Reson Imaging 2008;28(4):797–804. [DOI] [PubMed]
- 15.Port M, Idee JM, Medina C, Robic C, Sabatou M, Corot C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review. Biometals 2008;21(4):469–90. [DOI] [PubMed]
- 16.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol 2005;40(11):715–24. [DOI] [PubMed]
- 17.Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet 2000;356(9234):1000–1. [DOI] [PubMed]
- 18.Cowper SE, Su LD, Bhawan J, Robin HS, LeBoit PE. Nephrogenic fibrosing dermopathy. Am J Dermatopathol 2001;23 (5):383–93. [DOI] [PubMed]
- 19.Ting WW, Stone MS, Madison KC, Kurtz K. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol 2003;139(7):903–6. [DOI] [PubMed]
- 20.Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. Am J Kidney Dis 2005;46(4):754–9. [DOI] [PubMed]
- 21.Grobner T. Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? [published erratum appears in Nephrol Dial Transplant 2006;21(6):1745] Nephrol Dial Transplant 2006; 21(4):1104–8. [DOI] [PubMed]
- 22.Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006; 17(9):2359–62. [DOI] [PubMed]
- 23.Linfert DR, Schell JO, Fine DM. Treatment of nephrogenic systemic fibrosis: limited options but hope for the future. Semin Dial 2008;21(2):155–9. [DOI] [PubMed]
- 24.Todd DJ, Kagan A, Chibnik LB, Kay J. Cutaneous changes of nephrogenic systemic fibrosis: predictor of early mortality and association with gadolinium exposure. Arthritis Rheum 2007;56(10):3433–41. [DOI] [PubMed]
- 25.Yerram P, Saab G, Karuparthi PR, Hayden MR, Khanna R. Nephrogenic systemic fibrosis: a mysterious disease in patients with renal failure–role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin J Am Soc Nephrol 2007;2(2):258–63. [DOI] [PubMed]
- 26.Panesar M, Banerjee S, Barone GW. Clinical improvement of nephrogenic systemic fibrosis after kidney transplantation. Clin Transplant 2008;22(6):803–8. [DOI] [PubMed]
- 27.Swaminathan S, Horn TD, Pellowski D, Abul-Ezz S, Bornhorst JA, Viswamitra S, Shah SV. Nephrogenic systemic fibrosis, gadolinium, and iron mobilization. N Engl J Med 2007; 357(7):720–2. [DOI] [PubMed]
- 28.Sadowski EA, Bennett LK, Chan MR, Wentland AL, Garrett AL, Garrett RW, Djamali A. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 2007;243(1): 148–57. [DOI] [PubMed]
- 29.Marckmann P, Skov L, Rossen K, Heaf JG, Thomsen HS. Case-control study of gadodiamide-related nephrogenic systemic fibrosis. Nephrol Dial Transplant 2007;22(11):3174–8. [DOI] [PubMed]
- 30.Swaminathan S, Ahmed I, McCarthy JT, Albright RC, Pittelkow MR, Caplice NM, et al. Nephrogenic fibrosing dermopathy and high-dose erythropoietin therapy. Ann Intern Med 2006;145(3):234–5. [DOI] [PubMed]
- 31.Agarwal R, Brunelli SM, Williams K, Mitchell MD, Feldman HI, Umscheid CA. Gadolinium-based contrast agents and nephrogenic systemic fibrosis: a systematic review and meta-analysis. Nephrol Dial Transplant 2009;24(3):856–63. [DOI] [PubMed]
- 32.Perazella MA. Nephrogenic systemic fibrosis, kidney disease, and gadolinium: is there a link? Clin J Am Soc Nephrol 2007; 2(2):200–2. [DOI] [PubMed]
- 33.Shellock FG, Spinazzi A. MRI safety update 2008: part 1, MRI contrast agents and nephrogenic systemic fibrosis. AJR Am J Roentgenol 2008;191(4):1129–39. [DOI] [PubMed]
- 34.Sieber MA, Lengsfeld P, Frenzel T, Golfier S, Schmitt-Willich H, Siegmund F, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol 2008;18(10): 2164–73. [DOI] [PubMed]
- 35.Cowper SE. Nephrogenic fibrosing dermopathy: the first 6 years. Curr Opin Rheumatol 2003;15(6):785–90. [DOI] [PubMed]
- 36.Prince MR, Zhang H, Morris M, MacGregor JL, Grossman ME, Silberzweig J, et al. Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology 2008;248(3): 807–16. [DOI] [PubMed]
- 37.Gibson SE, Farver CF, Prayson RA. Multiorgan involvement in nephrogenic fibrosing dermopathy: an autopsy case and review of the literature. Arch Pathol Lab Med 2006;130(2):209–12. [DOI] [PubMed]
- 38.Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum 2006;35(4):238–49. [DOI] [PMC free article] [PubMed]
- 39.Swaminathan S, Shah SV. New insights into nephrogenic systemic fibrosis. J Am Soc Nephrol 2007;18(10):2636–43. [DOI] [PubMed]
- 40.Ortonne N, Lipsker D, Chantrel F, Boehm N, Grosshans E, Cribier B. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol 2004;150(5):1050–2. [DOI] [PubMed]
- 41.Cowper SE, Bucala R. Nephrogenic fibrosing dermopathy: suspect identified, motive unclear. Am J Dermatopathol 2003; 25(4):358. [DOI] [PubMed]
- 42.Cowper SE, Boyer PJ. Nephrogenic systemic fibrosis: an update. Curr Rheumatol Rep 2006;8(2):151–7. [DOI] [PubMed]
- 43.Kay J, High WA. Imatinib mesylate treatment of nephrogenic systemic fibrosis. Arthritis Rheum 2008;58(8):2543–8. [DOI] [PubMed]
- 44.Rao QA, Newhouse JH. Risk of nephropathy after intravenous administration of contrast material: a critical literature analysis. Radiology 2006;239(2):392–7. [DOI] [PubMed]
- 45.McCullough PA. Contrast-induced acute kidney injury [published erratum appears in J Am Coll Cardiol 2008;51(22): 2197]. J Am Coll Cardiol 2008;51(15):1419–28. [DOI] [PubMed]
- 46.Okada S, Katagiri K, Kumazaki T, Yokoyama H. Safety of gadolinium contrast agent in hemodialysis patients. Acta Radiol 2001;42(3):339–41. [DOI] [PubMed]
- 47.Penfield JG, Reilly RF Jr. What nephrologists need to know about gadolinium. Nat Clin Pract Nephrol 2007;3(12):654–68. [DOI] [PubMed]
- 48.Altun E, Martin DR, Wertman R, Lugo-Somolinos A, Fuller ER 3rd, Semelka RC. Nephrogenic systemic fibrosis: change in incidence following a switch in gadolinium agents and adoption of a gadolinium policy–report from two U.S. universities. Radiology 2009;253(3):689–96. [DOI] [PubMed]