Abstract
Cardiovascular involvement is the leading cause of morbidity and death in Churg-Strauss syndrome. Herein, we describe the case of a 47-year-old man with Churg-Strauss syndrome, in whom the use of novel echocardiographic techniques revealed segmental cardiomyopathy. Tissue Doppler and speckle-tracking imaging showed that both longitudinal and radial strain were impaired at the septal level and that the impairment of circumferential strain affected left ventricular torsion. Our case shows that advanced echocardiography with myocardial strain imaging in multiple vectors can identify systolic-diastolic abnormalities in a patient with myocardial infiltration and a normal left ventricular ejection fraction.
Key words: Cardiomyopathies/diagnosis/etiology; Churg-Strauss syndrome/complications/diagnosis/physiopathology/ultrasonography; echocardiography/methods; echocardiography, Doppler, color/methods; fibrosis; heart/physiopathology; heart diseases/etiology; myocardium/pathology; torsion abnormality/ultrasonography; ventricular dysfunction, left/ultrasonography
Cardiovascular involvement is the leading cause of morbidity and death in patients who have Churg-Strauss syndrome.1 The cardiac manifestations of Churg-Strauss syndrome may be silent or overt and can include eosinophilic vasculitis, myocarditis, pericarditis, fibrosis, valvular heart disease, conduction disorders, intracavitary thrombi, or cardiomyopathies. Specific clinical and histopathologic features distinguish Churg-Strauss syndrome from other causes of hypereosinophilia. Although many clinical features are similar to those of hypereosinophilic syndrome, particularly in the early disease stages, Churg-Strauss syndrome is defined by the presence of systemic vasculitis.1–3 Herein, we present the case and discuss the diagnosis of a patient with Churg-Strauss syndrome who had segmental cardiomyopathy.
Case Report
In September 2008, a 47-year-old man with Churg-Strauss syndrome and a medical history of atopy, nasal polyposis, and asthma was admitted to the hospital with worsening dyspnea. Laboratory tests showed hypereosinophilia (eosinophil count, 4.6 ×109/L) but were negative for antineutrophil cytoplasmic antibodies and auto-antibodies. A computed tomographic scan of the chest revealed bilateral pulmonary infiltrates, and cranial computed tomography showed paranasal sinusitis. Biochemical markers of cardiac injury were within normal ranges. An electrocardiogram showed sinus tachycardia and nonspecific T-wave abnormalities. With the patient at rest, an echocardiogram showed increased septal thickness and echogenic deposits in the basal and middle septum despite normal left ventricular (LV) size and ejection fraction. Tissue-Doppler echocardiographic imaging showed decreased velocity and strain patterns in the basal and middle ventricular septum. Ten to 20 minutes after the injection of gadolinium-DTPA (0.1 mmol/kg body weight), contrast-enhanced cardiac magnetic resonance showed a diffuse subendocardial delayed hyperenhancement at the septal level, with the involvement of no other segments. This distribution of gadolinium-DTPA indicated both fibrosis and inflammation of the myocardium, most likely resulting from myocardial infiltration and from vasculitis of the small myocardial vasculature. An endomyocardial biopsy was not performed. The patient's symptoms and eosinophil counts responded well to 6 weeks of steroid therapy.
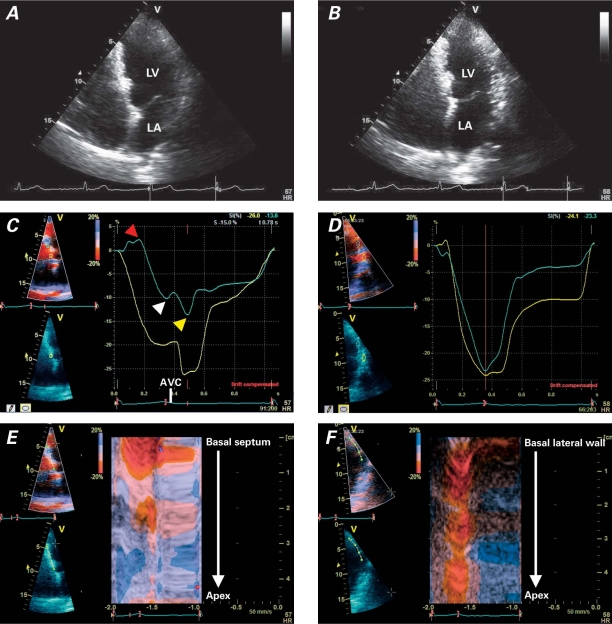
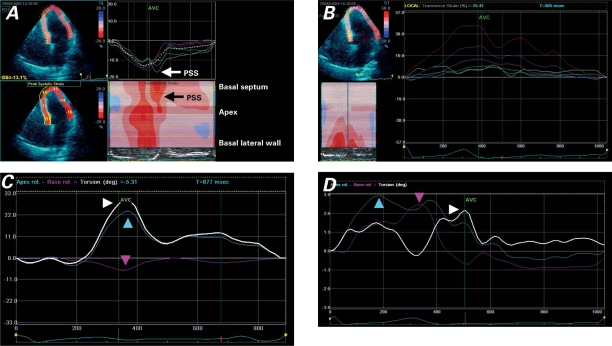
Follow-up cardiac magnetic resonance revealed a persistent hyperenhancement in the septum, suggesting fibrosis. The patient underwent rest and stress echocardiography so that myocardial involvement and inducible myocardial ischemia could be evaluated. The resting echocardiogram showed normal LV size and a reduction of septal thickness in the presence of persistent regional hyperechogenicity (Figs. 1A and 1B). Color-Doppler echocardiography revealed mild mitral regurgitation. The stress echocardiogram revealed no inducible ischemia. Echocardiography with the use of myocardial imaging methods (tissue Doppler and speckle-tracking imaging) was performed. Segmental impairment of septal strain was shown (Figs. 1C, 1D, 1E, 1F, and 2). Both longitudinal (Fig. 1) and radial strain (Fig. 2B) were impaired at the septal level. The impairment of circumferential septal strain affected LV torsion (Fig. 2D). Increased strain during isovolumic relaxation (Figs. 1C and 2A) indicated post-systolic shortening. The presumptive diagnosis was myocardial fibrosis, with particular involvement of the ventricular septum. The echocardiographic findings strongly suggested myocardial septal infiltration by an inflammatory process and progression toward a healed phase.
Fig. 1 Two-dimensional echocardiography (apical 4-chamber view) shows scattered hyperechogenicity of the ventricular septum in A) diastole and B) systole, with normal left ventricular (LV) ejection fraction. Tissue Doppler echocardiographic tracings of the LV septal and lateral walls (apical views) show C) reduction of longitudinal peak strain values in the mid-septal segments compared with D) normal values in the lateral wall; in C, pre-stretch (red arrowhead), reduced peak systolic value (white arrowhead), and post-systolic shortening (yellow arrowhead) are shown. Color tissue Doppler imaging (M-mode) shows E) the same reduction of strain values in the mid-septal segments (broken red line) compared with F) normal values in the lateral wall (straight red line).
AVC = aortic valve closure; LA = left atrium
Fig. 2 Speckle-tracking images of the left ventricular septal and lateral walls (multiple views). A) Tracing of longitudinal peak systolic strain shows post-systolic shortening (PSS) in the mid-septal segments. B) Tracing of transverse (radial) strain shows peak systolic strain impairment in the septal segments (yellow, turquoise, and green lines). C) Left ventricular torsion in a healthy patient shows apex rotation (blue arrowhead), base rotation (red arrowhead), and net ventricular torsion (white arrowhead). D) In our patient, net ventricular torsion (white arrowhead) is reduced because of a reduction of apex rotation (blue arrowhead) and a reversal of base rotation (red arrowhead) in early-to-mid-systole; diastolic untwisting is reduced and delayed.
AVC = aortic valve closure
Afterwards, the patient was monitored by immunologists. He remained in stable cardiac condition, without recurrence of symptoms.
Discussion
The effects of fibrosis on LV function in Churg-Strauss syndrome1–4 and in other myocardial diseases5–9 have been described; however, myocardial changes in longitudinal, as well as radial and circumferential, strain in Churg-Strauss syndrome have not been reported.
One echocardiographic goal—studied for decades but not yet established as a clinically useful technique—is to identify changes in myocardial tissue. One technique that has been used by different laboratories is cyclical variation in integrated backscatter of the myocardium,10 on the basis that cardiac cycle-dependent variation of integrated backscatter is negatively correlated with myocardial collagen deposition. This technique has been used to try to identify ischemic myocardium, scarred myocardium, and infiltrative cardiomyopathy. Ultrasonic tissue characterization uses myocardial integrated backscatter analysis to determine contractile performance and myocardial viability, independent of wall motion.
Other studies have shown that early abnormalities in regional myocardial function can be detected with the use of tissue Doppler imaging and ultrasound-based deformation (strain) in patients with various myocardial abnormalities (myocarditis, myocardial ischemia, and cardiomyopathies) who have preserved LV ejection fractions or fractional shortening.11 Strain and strain-rate imaging enables a more precise characterization of the mechanics of myocardial contraction and relaxation (deformation imaging). The ability to detect abnormalities and establish a diagnosis early in the course of the disease can influence specific treatments and provide important prognostic information.
The relationship between blood eosinophilia and heart disease is well recognized. Endomyocardial fibrosis is prominent in hypereosinophilic syndrome and leads to a characteristic clinical picture of restrictive cardiomyopathy that is accompanied by atrioventricular valve regurgitation when the papillary muscles are affected. Myocardial fibrosis of generalized distribution is a notable although usually subclinical feature of the Churg-Strauss syndrome.3 In our patient, we found increased strain during isovolumic relaxation, which indicated post-systolic shortening. Post-systolic shortening, or contraction after aortic valve closure, has been described in ischemia, myocardial infarction, and cardiomyopathies: its presence may affect diastolic filling.12,13 The mechanism could be inhomogeneity of contraction, with prolonged contraction in 1 area that results in a 2nd shortening when adjoining segments relax. This may be due to ischemia (because of hypertrophy or true ischemia) or to muscular disarrangement, as we supposed in our patient's case. Post-systolic shortening also appears to contribute to abnormal LV relaxation in cardiomyopathies; however, further investigations are needed in order to confirm this relationship.
In our patient, longitudinal and radial strain were impaired at the septal level. The impairment of circumferential septal strain affected LV torsion. Left ventricular torsion—the mean longitudinal gradient of the net difference in clockwise and counterclockwise rotation of the LV apex and base—is expressed in degrees per centimeter or radians per meter14; untwisting in the reverse direction during early diastole follows this torsion. Both LV torsion and diastolic untwisting were impaired in our patient. It has been shown that LV torsion is increased in patients who have mild diastolic dysfunction and is normalized in those who have more advanced diastolic dysfunction. In patients with severe diastolic dysfunction, torsion and untwisting are markedly reduced.14 When both longitudinal diastolic motion and torsion of the heart are decreased, LV diastolic filling is markedly compromised, with severe elevation in filling pressures. Diastolic dysfunction contributes substantially to the clinical syndrome of congestive heart failure in patients with either preserved or impaired LV systolic function; however, further studies are needed to understand the mechanism of LV torsion and untwisting in patients who are experiencing various stages of diastolic dysfunction.
Footnotes
Address for reprints: Antonio Vitarelli, MD, FACC, Cardiac Department, Sapienza University, via Lima 35, 00198 Rome, Italy
E-mail: vitar@tiscali.it
References
- 1.Pela G, Tirabassi G, Pattoneri P, Pavone L, Garini G, Bruschi G. Cardiac involvement in the Churg-Strauss syndrome. Am J Cardiol 2006;97(10):1519–24. [DOI] [PubMed]
- 2.Cohen A, Johnson N, Prier A, Zerbib E, Chauvel C, Kaplan G, Valty J. Segmental myocarditis in Churg-Strauss syndrome. Review of the literature apropos of a case [in French]. Rev Med Interne 1995;16(1):58–62. [DOI] [PubMed]
- 3.Morgan JM, Raposo L, Gibson DG. Cardiac involvement in Churg-Strauss syndrome shown by echocardiography. Br Heart J 1989;62(6):462–6. [DOI] [PMC free article] [PubMed]
- 4.Baccouche H, Yilmaz A, Alscher D, Klingel K, Val-Bernal JF, Mahrholdt H. Images in cardiovascular medicine. Magnetic resonance assessment and therapy monitoring of cardiac involvement in Churg-Strauss syndrome. Circulation 2008;117 (13):1745–9. [DOI] [PubMed]
- 5.Weidemann F, Niemann M, Herrmann S, Kung M, Stork S, Waller C, et al. A new echocardiographic approach for the detection of non-ischaemic fibrosis in hypertrophic myocardium. Eur Heart J 2007;28(24):3020–6. [DOI] [PubMed]
- 6.Park TH, Nagueh SF, Khoury DS, Kopelen HA, Akrivakis S, Nasser K, et al. Impact of myocardial structure and function postinfarction on diastolic strain measurements: implications for assessment of myocardial viability. Am J Physiol Heart Circ Physiol 2006;290(2):H724–31. [DOI] [PubMed]
- 7.Kang SJ, Lim HS, Choi BJ, Choi SY, Hwang GS, Yoon MH, et al. Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr 2008;21(8):907–11. [DOI] [PubMed]
- 8.Mertens L, Ganame J, Claus P, Goemans N, Thijs D, Eyskens B, et al. Early regional myocardial dysfunction in young patients with Duchenne muscular dystrophy. J Am Soc Echocardiogr 2008;21(9):1049–54. [DOI] [PubMed]
- 9.Hor KN, Wansapura J, Markham LW, Mazur W, Cripe LH, Fleck R, et al. Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance tagging study. J Am Coll Cardiol 2009;53(14):1204–10. [DOI] [PMC free article] [PubMed]
- 10.Mizuta Y, Kai H, Mizoguchi M, Osada K, Tahara N, Nakaura H, et al. Long-term treatment with valsartan improved cyclic variation of the myocardial integral backscatter signal and diastolic dysfunction in hypertensive patients: the echocardiographic assessment. Hypertens Res 2008;31(10):1835–42. [DOI] [PubMed]
- 11.Nesbitt GC, Mankad S, Oh JK. Strain imaging in echocardiography: methods and clinical applications. Int J Cardiovasc Imaging 2009;25 Suppl 1:9–22. [DOI] [PubMed]
- 12.Mastouri R, Mahenthiran J, Kamalesh M, Gradus-Pizlo I, Feigenbaum H, Sawada SG. Prediction of ischemic events by anatomic M-mode strain rate stress echocardiography. J Am Soc Echocardiogr 2008;21(4):299–306. [DOI] [PubMed]
- 13.Ganame J, Mertens L, Eidem BW, Claus P, D'hooge J, Havemann LM. Regional myocardial deformation in children with hypertrophic cardiomyopathy: morphological and clinical correlations. Eur Heart J 2007;28(23):2886–94. [DOI] [PubMed]
- 14.Park SJ, Miyazaki C, Bruce CJ, Ommen S, Miller FA, Oh JK. Left ventricular torsion by two-dimensional speckle tracking echocardiography in patients with diastolic dysfunction and normal ejection fraction. J Am Soc Echocardiogr 2008;21 (10):1129–37. [DOI] [PubMed]