Abstract
Severe bacterial aortitis without an aneurysmal component is a rare but life-threatening problem that requires aggressive treatment to eliminate the infection and prevent recurrence. Herein, we present the case of a 58-year-old man who underwent patch repair of a nonaneurysmal aorta that had ruptured due to Staphylococcus aureus infection. Postoperatively, he experienced a recurrent rupture that required reoperation. We successfully performed wide-margin débridement followed by aortic arch replacement with a prosthetic vascular graft and omental flap.
Key words: Anti-bacterial agents/therapeutic use; aorta, thoracic/microbiology/pathology; aortic diseases/complications/immunology/microbiology; disease progression; inflammation/pathology; omentum/transplantation; prosthesis-related infections/diagnosis/prevention & control/surgery; risk factors; staphylococcal infections/drug therapy/prevention & control/surgery; treatment outcome
Bacterial aortitis is thought to be caused by bacterial seeding onto the luminal surface of the aorta.1 Chronic infection caused by bacteria may contribute to the pathogenesis of atherosclerosis,2 which is believed to be the key factor in aortic wall degeneration, although the mechanism is uncertain. Acute and extensive infection may lead to nonaneurysmal aortic rupture.
Herein, we report the case of a patient who experienced a rare recurrent rupture of a nonaneurysmal infected aorta.
Case Report
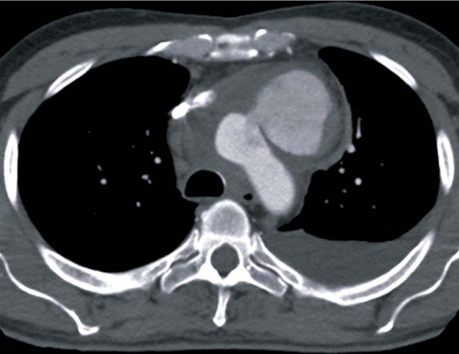
In October 2008, a 58-year-old man presented at a local hospital with chest pain. Computed tomography (CT) performed at admission showed no findings to account for the pain. Because of nonspecific but elevated inflammatory markers and low-grade fever, the patient was started on intravenous cephalosporin. Twelve days later, he became acutely dyspneic and underwent repeat CT, which showed a contained rupture of the aortic arch (Fig. 1). The patient was then transferred to our hospital.
Fig. 1 Preoperative computed tomogram shows a contained rupture of the anterior wall of the proximal aortic arch.
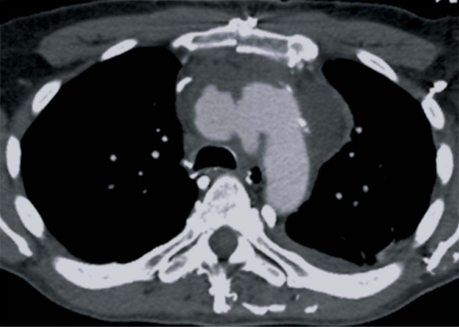
At operation, the anterior mediastinum was found to be severely inflamed and solid, and exposure of the aortic arch required decortication. After the patient was placed under deep hypothermic circulatory arrest with retrograde cerebral perfusion at 18 °C, the pseudoaneurysm was incised. When the inside wall of the aortic arch was examined through the defect, no other abnormality was obvious. The defect in the anterior wall of the proximal aortic arch was débrided and repaired with an expanded polytetrafluoroethylene patch. Cardiopulmonary bypass and circulatory arrest times were 265 and 26 minutes, respectively. The resected aortic and surrounding tissues were positive for Staphylococcus aureus. The patient was started on intravenous vancomycin and was extubated the next day. On the 3rd postoperative day, he lost consciousness and collapsed. Because we suspected cardiac tamponade, we emergently reopened the chest. However, there was no pericardial effusion or bleeding from any suture lines. Periaortic tissue samples were still positive for S. aureus. The inflammation subsided after antibiotic treatment, but repeat CT showed another contained rupture of the aortic arch (Fig. 2).
Fig. 2 Computed tomogram after the 1st operation shows a recurrent rupture of the posterior wall of the aortic arch opposite the patch.
At reoperation, the patient was placed under deep hypothermic circulatory arrest with retrograde cerebral perfusion at 18 °C. The transverse aorta was incised, and a posterior 3-cm hole was found. After thorough débridement, the aorta, the right brachiocephalic artery, and the left carotid artery were replaced with a branched Dacron graft that formed the distal aortic anastomosis between the left carotid artery and the left subclavian artery. Interrupted 4-0 monofilament polypropylene mattress stitches with fresh autologous pericardial pledgets were used for the distal aortic anastomosis. Cardiopulmonary bypass and circulatory arrest times were 318 and 60 minutes, respectively. The omentum was pedicled on the right gastroepiploic artery and was wrapped around the Dacron graft. Postoperatively, the patient resumed vancomycin therapy and recovered steadily.
Histopathologic findings of the resected aorta confirmed active inflammation. Granulated tissue exhibited a mixed inflammatory cell infiltrate of plasma cells, lymphocytes, and polymorphonuclear neutrophils. Further review of the patient's medical history revealed no clue about the infection source. Due to the patient's sensitivity to vancomycin, the vancomycin was changed to flucloxacillin and rifampicin.
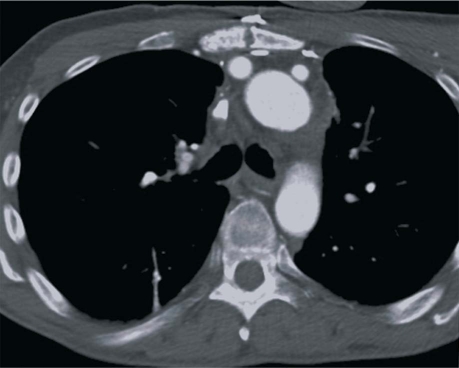
The patient was discharged from the hospital on the 24th postoperative day with a peripheral central venous catheter and an infusion pump. Six weeks postoperatively, the antibiotic therapy was changed to lifelong oral dicloxacillin. Twelve months postoperatively, the patient was doing well, without any recurrence of infection apparent on CT (Fig. 3).
Fig. 3 One-year postoperative computed tomogram shows the branched vascular graft wrapped with omentum.
Discussion
Severe infection is typically controlled by means of wide-margin débridement and appropriate antibiotic treatment. However, controlling infection is difficult when major vascular structures are involved, because foreign materials are necessary to construct them. An infected prosthetic graft can cause catastrophe; due to its avascularity, no antibiotics can be delivered.3
In our patient's 1st operation, patch repair was selected to reduce the risk of operative complications; however, a recurrent rupture developed at a different site, probably due to existing bacterial seeding. This required further resection of the infected aorta. Although no other obvious abnormality was found on the inside wall of the aortic arch during the 1st operation, retrospective study of the preoperative CT scan revealed dilation of the origin of the brachiocephalic artery, with minor irregularity of the posterior wall of the aortic arch. In severe aortic infection, morphologic examination of the aorta during surgery is often difficult because of severe periaortic inflammation, including abscess. Therefore, the importance of meticulously evaluating preoperative aortic images cannot be overemphasized.
Aortic arch infection necessitates in situ prosthetic graft replacement because of the region's complex anatomy; however, no graft material is optimal. Cryopreserved allografts seem to be more resistant to infection than are prosthetic grafts,4 but the allografts are not always available. Soaking prosthetic grafts in rifampicin reportedly increases the chance of a favorable outcome against staphylococcus5; nonetheless, it is uncertain how long the bond will last in the presence of graft ooze resulting from hypothermic coagulopathy. In our opinion, it is extremely important to wrap the graft in a vascularized tissue flap to enable adequate postoperative delivery of antibiotics and cytokines.6 Various muscles and omentum have been used in the surgical treatment of aortic infection.7 Harvesting the greater omentum is relatively simple,8 and the flap can even reach the arch vessels if it is pedicled on the right gastroepiploic artery.
To prevent late failure of the distal aortic anastomosis because of inflammation or infection, we used interrupted monofilament polypropylene mattress stitches with fresh autologous pericardial pledgets. This technique is also useful for ensuring hemostasis, because it creates a completely everting anastomosis.
In summary, we successfully treated a recurrent rupture of an infected aortic arch by using a branched Dacron graft and and wrapping it with an omental flap. From this experience, we believe that 2 steps were necessary to successfully treat this rare but devastating case of severe bacterial aortitis: first, a precise diagnosis on the basis of repeat aortic imaging at short intervals; and second, surgical intervention, including the extensive débridement of the infected aorta and the creation of a well-vascularized tissue flap to enable adequate delivery of antibiotics and cytokines.
Footnotes
Address for reprints: Tadashi Kitamura, MD, Cardiothoracic Surgical Unit, Level 4, East Wing, Royal Adelaide Hospital, North Terrace, Adelaide, SA 5000, Australia
E-mail: funcorogash@hotmail.com
References
- 1.Marques da Silva R, Lingaas PS, Geiran O, Tronstad L, Olsen I. Multiple bacteria in aortic aneurysms. J Vasc Surg 2003;38 (6):1384–9. [DOI] [PubMed]
- 2.Gois J, Higuchi M, Reis M, Diament J, Sousa J, Ramires J, Oliveira S. Infectious agents, inflammation, and growth factors: how do they interact in the progression or stabilization of mild human atherosclerotic lesions? Ann Vasc Surg 2006;20 (5):638–45. [DOI] [PubMed]
- 3.Kitamura T, Morota T, Motomura N, Ono M, Shibata K, Ueno K, et al. Management of infected grafts and aneurysms of the aorta. Ann Vasc Surg 2005;19(3):335–42. [DOI] [PubMed]
- 4.Koskas F, Goeau-Brissonniere O, Nicolas MH, Bacourt F, Kieffer E. Arteries from human beings are less infectible by Staphylococcus aureus than polytetrafluoroethylene in an aortic dog model. J Vasc Surg 1996;23(3):472–6. [DOI] [PubMed]
- 5.Goeau-Brissonniere OA, Fabre D, Leflon-Guibout V, Di Centa I, Nicolas-Chanoine MH, Coggia M. Comparison of the resistance to infection of rifampin-bonded gelatin-sealed and silver/collagen-coated polyester prostheses. J Vasc Surg 2002;35(6):1260–3. [PubMed]
- 6.van der Meer JWM, Kullberg BJ. Immunomodulation. In: Cohen J, Powderly WG, editors. Infectious diseases. 2nd ed. St. Louis: Mosby; 2004. Chapter 210.
- 7.Coselli JS, Crawford ES, Williams TW Jr, Bradshaw MW, Wiemer DR, Harris RL, Safi HJ. Treatment of postoperative infection of ascending aorta and transverse aortic arch, including use of viable omentum and muscle flaps. Ann Thorac Surg 1990;50(6):868–81. [DOI] [PubMed]
- 8.Shrager JB, Wain JC, Wright CD, Donahue DM, Vlahakes GJ, Moncure AC, et al. Omentum is highly effective in the management of complex cardiothoracic surgical problems. J Thorac Cardiovasc Surg 2003;125(3):526–32. [DOI] [PubMed]