Abstract
Although low levels of free triiodothyronine and high levels of brain natriuretic peptide have been shown as independent predictors of death in chronic heart failure patients, few studies have compared their prognostic values. The aim of this prospective study was to measure free triiodothyronine and brain natriuretic peptide levels and to compare their prognostic values among such patients.
A total of 334 patients (mean age, 62 ± 13 yr; 218 men) with ischemic and nonischemic dilated cardiomyopathy were included in the study. The primary endpoint was a major cardiac event.
During the follow-up period, 92 patients (28%) experienced a major cardiac event. Mean free triiodothyronine levels were lower and median brain natriuretic peptide levels were higher in patients with major cardiac events than in those without. A significant negative correlation was found between free triiodothyronine and brain natriuretic peptide levels. Receiver operating characteristic curve analysis showed that the predictive cutoff values were <2.12 pg/mL for free triiodothyronine and >686 pg/mL for brain natriuretic peptide. Cumulative survival was significantly lower among patients with free triiodothyronine <2.12 pg/mL and among patients with brain natriuretic peptide >686 pg/mL. In multivariate analysis, the significant independent predictors of major cardiac events were age, free triiodothyronine, and brain natriuretic peptide.
In the present study, free triiodothyronine and brain natriuretic peptide had similar prognostic values for predicting long-term prognosis in chronic heart failure patients. These results also suggested that combining these biomarkers may provide an important risk indicator for patients with heart failure.
Key words: Biological markers/blood; cardiomyopathy, dilated/blood; euthyroid sick syndromes; heart failure/complications/chronic/pathology; hypothalamo-hypophyseal system/metabolism; multivariate analysis; natriuretic peptide, brain/blood; prognosis; triiodothyronine/blood/deficiency; survival rate
Alterations of thyroid hormone metabolism are described in association with all stages of chronic heart failure.1–6 This well-known abnormality has been called the “euthyroid sick syndrome,” which is a derangement of thyroid hormone metabolism despite normal thyroid function.7 The aberration is characterized by decreased circulating levels of the biologically active form of triiodothyronine (T3) and by increased levels of reverse T3 in serum as a result of impaired conversion of serum thyroxine (T4) to T3 in peripheral tissues, despite the presence of normal thyroid-stimulating hormone (TSH) and T4 levels.8–10 This syndrome occurs in approximately 30% of patients with advanced chronic heart failure. The presence of low T3 levels has been found to be an independent predictor of poor prognosis; indeed it can be used as a predictor of death in chronic heart failure patients.1,4,9
Brain natriuretic peptide (BNP) is a cardiac neurohormone secreted mainly from both ventricles as a response to volume expansion, pressure overload, and elevated end-diastolic pressure.11 In patients with heart failure, BNP is elevated in correlation with the degree of cardiac insufficiency.12–14 It has potent diuretic, natriuretic, and vascular smooth muscle relaxing effects and also important central and peripheral sympathoinhibitory effects. Brain natriuretic peptide inhibits the renin-angiotensin-aldosterone axis.15–18 For patients with left ventricular (LV) dysfunction, increased BNP levels have both diagnostic and prognostic properties. Increased levels have predicted the worst prognosis—cardiac death—in heart failure patients.19–25
The aims of this study were 1) to determine free-T3 (FT3) levels in patients who have worsening heart failure due to ischemic and nonischemic dilated cardiomyopathy, 2) to determine the relationship between FT3 and BNP levels, and 3) to compare the prognostic value of FT3 with that of BNP in long-term follow-up.
Patients and Methods
Study Population
For this prospective study, we screened 592 consecutive patients who were admitted to our clinic from April 2003 through June 2007 with the diagnosis of worsening ischemic or nonischemic dilated cardiomyopathy. The diagnosis of dilated cardiomyopathy was made on the basis of transthoracic echocardiographic findings (LV end-diastolic diameter, >56 mm; and ejection fraction, <0.45). All patients underwent diagnostic coronary angiography for determining the cause of the heart failure.
Exclusion criteria were as follows: concomitant presence of any severe systemic illness; overt or subclinical hyper- or hypothyroidism; therapy with thyroid hormone or derivatives, steroids, nonsteroidal anti-inflammatory drugs, lithium, phenobarbital, amiodarone, dopamine, dobutamine, iodine-containing compounds, clofibrate, carbamazepine, antithyroid agents, or heparin; or receipt of radiographic contrast medium within 2 weeks before measurement of thyroid hormone levels. Patients with symptoms of acute heart failure due to conditions or diseases other than dilated cardiomyopathy (for example, acute mitral regurgitation, acute aortic regurgitation, or pericardial tamponade) were also excluded.
A total of 258 patients were excluded, and the remaining 334 patients (218 men and 116 women; mean age, 62 ± 13 yr) underwent statistical analysis. The study was conducted in accordance with the Declaration of Helsinki and was approved by our local institutional ethics committee. All patients gave informed consent before entering the study.
Echocardiographic Assessment
All participants underwent transthoracic echocardiography by means of an echocardiograph equipped with a broadband transducer (Vivid 7®, GE VingMed Ultrasound AS; Horten, Norway). Measurements of the left atrium, LV, and right ventricle (RV) were obtained from the parasternal long-axis and apical 4-chamber views, in accordance with standard criteria. Left ventricular ejection fraction (LVEF) was calculated using the modified Simpson rule in the apical 2- and 4-chamber views. Mitral flow was measured from the apical 4-chamber view with pulsed-wave Doppler, by placing the sample volume at the tips of the mitral leaflets. Four types of diastolic filling were defined: normal filling (E/A [ratio of early/late peak diastolic velocities], 1–2; DT [deceleration time], 160–240 ms, and IVRT [isovolumic relaxation time], 70–90 ms); relaxation abnormality in filling (E/A, <1; DT, >240 ms; and IVRT, >90 ms); pseudonormal pattern in filling (E/A, <1–1.5; DT, 160–200 ms; and IVRT, <90 ms); and restrictive filling (E/A, >1.5; DT, <160 ms; and IVRT, <70 ms).26
Blood Samples
During the first 1 to 3 days of hospitalization, each patient had fasting blood samples drawn from a large antecubital vein for the determination of biochemical and hemostatic values. The samples were centrifuged for 10 min and serum FT3, FT4 (free-T4), and TSH levels were measured by means of an Immulite® 2000 advanced immunoassay system (Siemens Medical Solutions USA, Inc.; Malvern, Pa). The reference intervals of our laboratory were as follows: TSH, 0.4 to 4 μIU/mL; FT3, 1.57 to 4.71 pg/mL; and FT4, 0.8 to 1.9 ng/dL. The BNP levels were analyzed by means of the Triage® BNP test (Biosite Incorporated; San Diego, Calif) within 24 hr after hospitalization. The normal value for the Triage® BNP test was <100 pg/mL. Sedimentation, albumin, hemoglobin, and lipid levels were measured by standard methods.
Patient Follow-Up
Follow-up was started upon the taking of BNP and thyroid hormone measurements. Clinical follow-up was done by telephone contact and by periodic examination of patients in the outpatient clinic. All patients were monitored for a mean duration of 17 ± 13 months (range, 1–49 mo). The primary endpoint of the study was a major cardiac event (MCE), which we defined as any of the following: sudden death, cardiac transplantation, death attributable to advanced heart failure, and, for patients with an implantable cardioverter-defibrillator, the receipt of a shock due to ventricular fibrillation. The physicians adjudicating these endpoints were blinded with respect to patients' T3 and BNP levels.
Statistical Methods
The SPSS 13.0 (SPSS Inc., an IBM company; Chicago, Ill) and MedCalc® 8.1.0.0 statistical software (MedCalc Software; Mariakerke, Belgium) packages were used for statistical analyses. Results are presented as mean ± SD, as median and interquartile ranges, or as percentages and numbers for categorical data. Normality tests were used for all variables. In comparing patients with and without MCEs, continuous variables that were normally distributed were analyzed with the 2-tailed t test, and unequally distributed variables were analyzed with the Mann-Whitney U test. Categorical data and proportions were analyzed using the χ2 or Fisher exact test where appropriate. Correlations between thyroid hormones and echocardiographic or biochemical values were determined by Spearman correlation analysis. To compare values between and within the various types of diastolic filling, a 1-way analysis of variance was used. Homogeneity of variances was tested for all variables with Levene's test. If equal variances were assumed, Tukey's HSD post hoc test was applied; if not, the Tamhane T2 test was used to compare the parameters within groups. The Bonferroni correction was used to determine statistically significant values among patient groups with various types of diastolic filling.
During the follow-up period, clinical and laboratory values were compared between patients with and without MCE. For values that were significantly different in patients with MCE, the predictive cutoff values for determining event development were detected with receiver operating characteristic (ROC) curve analysis by using the MedCalc 8.1.0.0 statistical software package. Natural log transformation of BNP achieved a normal distribution. Thus the log (BNP) values were used in the multivariate analyses. The Kaplan-Meier method was used to analyze the timing of events during follow-up. Statistical assessment was performed with the log-rank test, with values of P <0.05 considered significant. Cox proportional hazard analysis was used to evaluate independent predictors of survival. Variables evaluated in the model were age, sex, diabetes mellitus, hypertension, albumin, FT3, log (BNP) levels, prior use of diuretics, spironolactone, β-blockers, or angiotensin-converting enzyme (ACE) inhibitors or angiotensin-II receptor blockers (ARBs), and the patient's LVEF, New York Heart Association (NYHA) functional class, and RV diameter.
Results
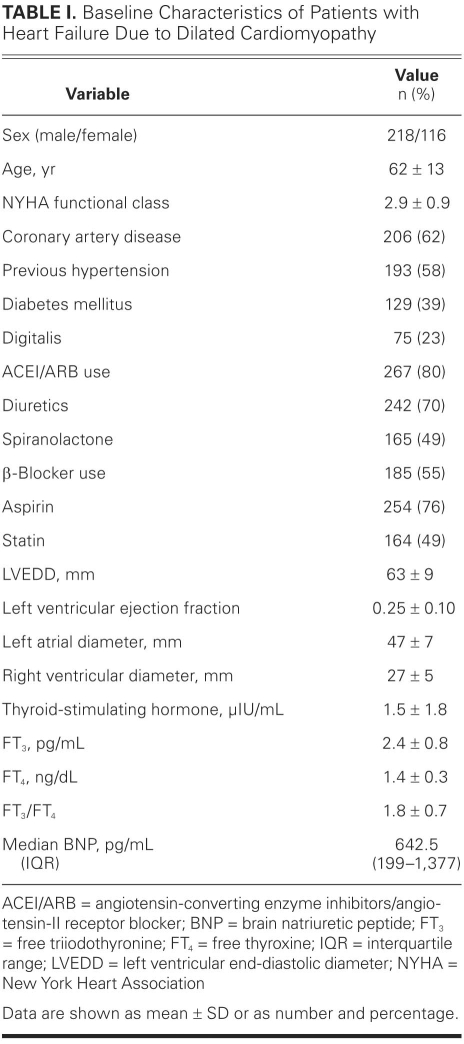
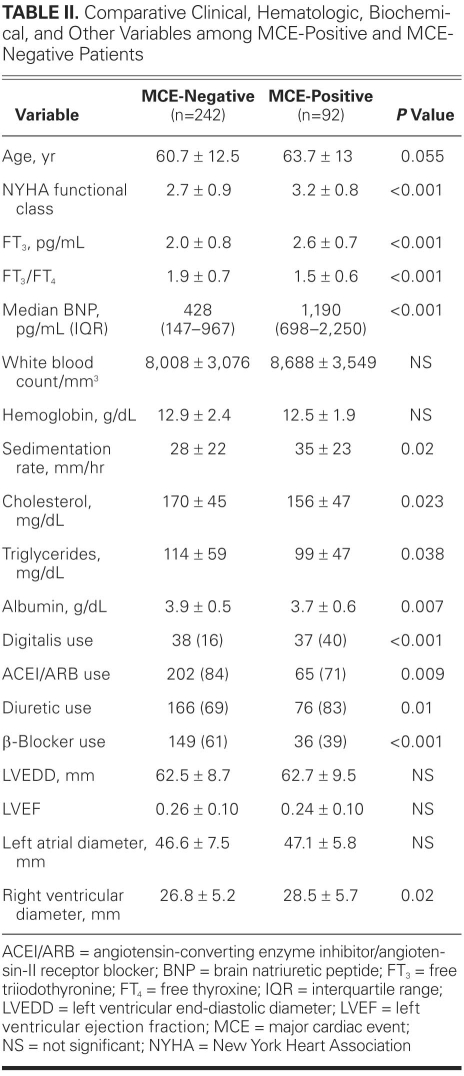
Baseline characteristics of the study group are shown in Table I. During the follow-up period, 92 MCEs (28%) occurred. Table II compares the demographic, clinical, hematologic, biochemical, and echocardiographic values of MCE-positive patients with those of MCE-negative patients. Patients who experienced MCEs were older and had worse functional capacity. No significant difference between the 2 groups was found in regard to sex or the presence of coronary artery disease. The presence of diabetes mellitus was higher in patients who experienced MCEs than in those who did not (38% vs 21%; P = 0.001). Median levels of BNP were significantly higher in patients with MCEs than in those without MCEs, whereas mean values of cholesterol, triglycerides, and albumin, and FT3/FT4 ratios, were lower. Prior use of ACE inhibitors or ARBs and β-blockers seemed to have a beneficial effect on survival, but prior use of digitalis and diuretic agents was significantly higher among patients with MCEs than among those without (Table II). Comparison of echocardiographic results between MCE-positive and MCE-negative patients showed that the only significantly different value between the 2 groups was diameter of the RV. Ejection fraction, LV end-diastolic dimension, LV end-systolic dimension, and left atrial size were similar between the 2 groups. Among 239 patients with sinus rhythm, 70 patients (29%) had a restrictive filling pattern and 86 patients (36%) had a relaxation abnormality. Fifteen patients (17%) with a relaxation abnormality and 25 patients (36%) with a restrictive filling pattern experienced MCEs during the follow-up period (P = 0.007).
TABLE I. Baseline Characteristics of Patients with Heart Failure Due to Dilated Cardiomyopathy
TABLE II. Comparative Clinical, Hematologic, Biochemical, and Other Variables among MCE-Positive and MCE-Negative Patients
Although the relationship between rehospitalization and FT3 or BNP levels was not a specified endpoint, we also investigated that. Median levels of BNP were significantly higher in rehospitalized patients than in those who did not require rehospitalization: 718 pg/mL (range, 243.3–1,537.5 pg/mL) vs 360 pg/mL (range, 144.5–950.7 pg/mL); P <0.001. Nevertheless, FT3 levels of rehospitalized patients were similar to those of patients who were not rehospitalized.
Factors Correlating with FT3 and BNP Levels
Levels of BNP correlated negatively with levels of FT3 (r = −0.22, P <0.001), albumin (r = −0.23, P <0.001), hemoglobin (r = −0.17, P <0.001), and total cholesterol (r = −0.22, P <0.001), and with LVEF (r = −0.18, P <0.001). A positive correlation was found between BNP levels and NYHA functional class, and between BNP and physical examination findings of heart failure. The BNP levels also correlated positively with RV diameter (r=0.32, P <0.001). Levels of FT3 correlated negatively with NYHA functional class and with physical examination findings of heart failure, but FT3 levels correlated positively with albumin (r=0.34, P <0.001), hemoglobin (r=0.23, P <0.001), and total cholesterol (r=0.17, P <0.001). There was a positive correlation between FT3 levels and LVEF (r=0.14, P = 0.002), whereas a negative correlation was found between FT3 levels and RV diameter (r = −0.22, P <0.001).
Cutoff Values of Free T3 and Brain Natriuretic Peptide for Predicting Major Cardiac Events
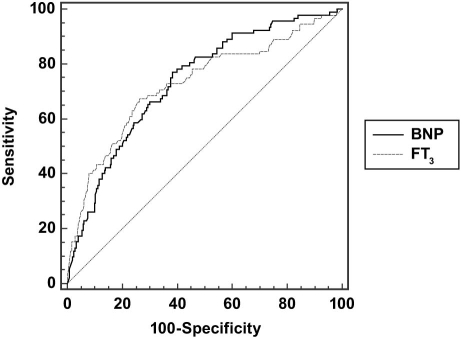
To define the low T3 level in the study population, we used ROC curve analysis to detect the predictive cutoff values of FT3 for the occurrence of MCEs. The same analysis was done for BNP. The cutoff values for predicting MCEs were >686 pg/mL for BNP and <2.12 pg/mL for FT3. The ROC curves and values of area under the curve (AUC) for each are shown in Figure 1. There were 126 patients with FT3 <2.12 pg/mL; therefore, the incidence of low T3 level was 37.7%.
Fig. 1 Receiver operating characteristic (ROC) curve analysis and comparison of the ROC curves in assessing the predictive cutoff values of brain natriuretic peptide (BNP) and free tri-iodothyronine (FT3) levels for major cardiac events. The area under the curve for BNP was 0.735 (95% CI, 0.68–0.78; P <0.001), and the area under the curve for FT3 was 0.728 (95% CI, 0.67–0.77; P <0.001). In comparing the ROC curves, we found that the difference between areas was 0.00725 (SE, 0.0414; 95% CI, –0.0738 to 0.0883, Z score, 0.175; P = 0.861).
BNP = brain natriuretic peptide; CI = confidence interval; FT3 = free triiodothyronine
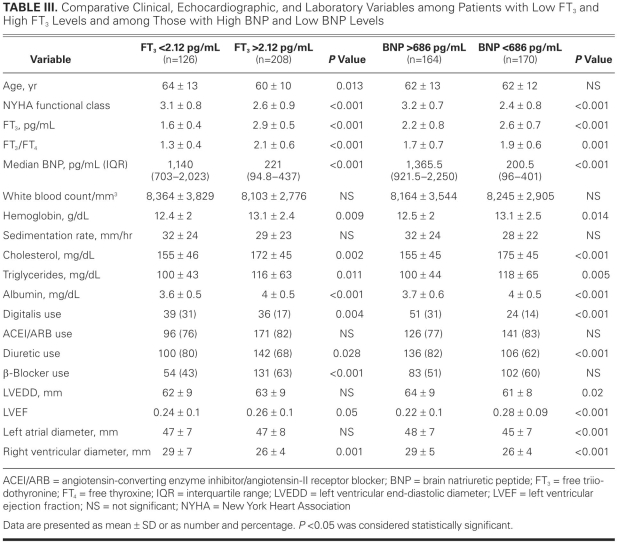
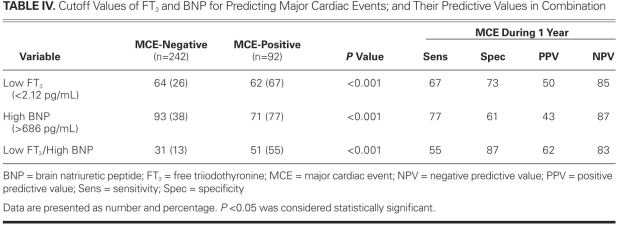
Comparative clinical and laboratory variables among patients with low and high FT3 and among those with low and high BNP levels are shown in Table III. The relationship between low FT3, high BNP, and MCE during the follow-up period and the prognostic values of those 2 variables for MCEs appear in Table IV. Although the 2 variables had similar AUC values for predicting MCE, the positive predictive value of FT3 seemed to be greater than the positive predictive value of BNP. When we combined the 2 measures as low FT3/high BNP, sensitivity decreased, whereas both specificity and positive predictive value increased (Table IV).
TABLE III. Comparative Clinical, Echocardiographic, and Laboratory Variables among Patients with Low FT3 and High FT3 Levels and among Those with High BNP and Low BNP Levels
TABLE IV. Cutoff Values of FT3 and BNP for Predicting Major Cardiac Events; and Their Predictive Values in Combination
Low Free-T3 Level and Prognosis
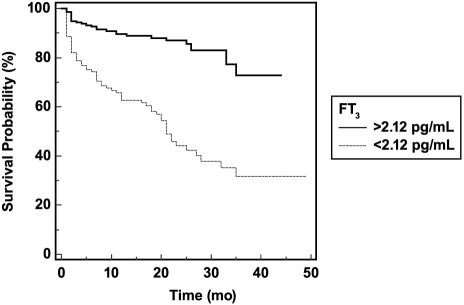
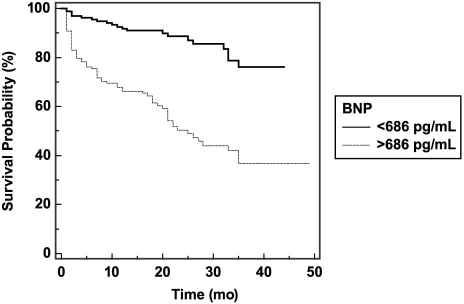
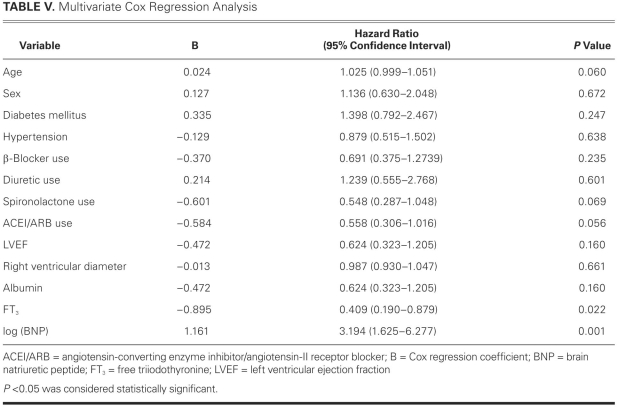
We used Kaplan-Meier analyses according to the ROC-curve-derived cutoff values of FT3 and BNP levels. Cumulative survival was significantly lower according to log-rank test among patients with an FT3 <2.12 pg/mL and among those with a BNP >686 pg/mL (Figs. 2 and 3). In the Cox regression analysis adjusted for age; sex; diabetes mellitus; hypertension; albumin; FT3; log (BNP) levels; prior usage of diuretic agents; spironolactone; β-blockers; and ACE inhibitors or ARBs; LVEF; NYHA functional class; and RV diameter, we found that age, FT3, and log (BNP) were significant independent predictors of MCEs. Hazard ratios and 95% confidence intervals of each variable evaluated in the model appear in Table V.
Fig. 2 Cumulative probability of event-free survival in accordance with free triiodothyronine (FT3) levels.
Fig. 3 Cumulative probability of event-free survival according to brain natriuretic peptide (BNP) levels.
TABLE V. Multivariate Cox Regression Analysis
Discussion
In the present study, we enrolled patients with ischemic and nonischemic dilated cardiomyopathy who had been admitted at least once to our in-patient clinic due to worsening heart failure. The main aim of the present study was to investigate the prognostic efficacy of BNP and FT3 levels. Although we found a weak negative correlation between BNP and FT3 levels, our multivariate analyses showed that FT3 levels might add prognostic strength to the information gained from BNP levels. Both high BNP and low FT3 levels were significantly associated with the severity of illness, poor LV function, and adverse outcomes. Use of the 2 biomarkers individually or in combination strongly predicted adverse outcomes and death. Among patients with ischemic and nonischemic dilated cardiomyopathy, BNP levels may be used to predict rehospitalization. But FT3 levels yielded no benefits in predicting rehospitalization in those patients.
Low FT3 level is a frequent finding in chronic heart failure patients.3,5 Nevertheless, the clinical importance of a low FT3 level in heart failure has not been adequately evaluated.5,9,27,28 It has been emphasized that a decreased FT3 level is a beneficial adaptive mechanism to preserve energy.29 Low thyroid hormone activity might be a response to derangement of the neuroendocrine and proinflammatory systems and an important adaptive mechanism during advanced heart failure.22,30–34 As previously described,35–37 the interleukin system and interactions between cytokine pathways have a negative impact on the peripheral conversion of T4 into T3. Thyroid hormones affect the expression of several enzymes and of functional and structural proteins in the heart and cardiovascular system.38–40 These effects can improve myocardial contractile function and diastolic properties, can increase stroke volume and cardiac output, and can decrease systemic and coronary vascular resistance.41
Thyroid hormone levels begin to decline at a very early stage of heart failure, and this decrease is seen even in asymptomatic or mildly symptomatic patients with idiopathic LV dysfunction.6 These observations suggest that there is a decrease in the conversion of FT4 into FT3, despite the finding of a normal range of FT3 levels in the very early stages of heart failure. Patients who have decreased FT3 levels may also have increased plasma renin activity and increased aldosterone, noradrenalin, BNP, and atrial natriuretic peptide levels.6 Because low FT3 levels can indicate higher activity of the neuroendocrine and proinflammatory systems, they contribute to a poor prognosis. Indeed, low T3 syndrome has been found to be a strong and independent predictor of death in heart-failure patients.2,6,9,28
Increased BNP levels have also been predictive of adverse outcomes, including recurrent chronic heart failure and subsequent death due to chronic heart failure or other causes, whereas very low BNP levels have shown a very high negative predictive value for future cardiac events among heart-failure patients.42–44 Brain natriuretic peptide is a well-known cardiovascular hormone that might have both inflammatory and anti-inflammatory properties in heart-failure patients. It was shown recently45–47 that BNP regulates the production of major inflammatory molecules, such as reactive oxygen species, leukotriene B4, and prostaglandin E2. This regulation of inflammatory-molecule production modulates, in turn, the cytokines—such as tumor necrosis factor (TNF)-a, and interleukin (IL-12 and IL-10)—and it affects cell motility. These results furnish new evidence of BNP's ability to modulate the production of inflammatory mediators in macrophages; and this role of BNP has broad implications in inflammatory states wherein increased BNP levels have been observed.47 The inflammatory process appears to regulate BNP in a singular manner.48 There have been significant correlations between natriuretic peptide levels in chronic heart failure patients and markers of inflammation and myocardial dysfunction.49 Moreover, TNF can causally contribute to cardiac dysfunction, thereby stimulating BNP secretion.49 Relationships have been found between higher IL-6 and IL-10 levels and lower T3 and T4 levels in nonthyroidal illness.50 Therefore, inflammation may be a key mechanism in the readjustment of thyroid hormone levels in patients with chronic heart failure.
In the present study, multivariate analysis showed that both low FT3 and high BNP levels were strong predictors of MCE. These results suggest that during the course of heart failure, FT3 begins to decrease and BNP begins to increase. The 2 measures may have similar prognostic values in chronic heart failure, because activation of both the inflammatory and neurohormonal pathways affects FT3 and BNP levels. In a recent study, Passino and colleagues51 showed that monitoring the combination of low FT3 and high BNP levels is a useful approach for determining long-term prognosis in patients with heart failure. In the present study, both specificity and positive predictive values increased when we combined the 2 biomarkers, rather than use one alone. In accordance with the findings of Passino and colleagues,51 our results suggest that this combination biomarker might be an important risk indicator among patients with heart failure. Although BNP levels in our study group were able to predict rehospitalization, we found no such relationship between rehospitalization and FT3.
Conclusion
We infer that high BNP levels and low FT3 levels may be predictive markers for worse outcomes, including rehospitalization and death, among patients with ischemic and nonischemic dilated cardiomyopathy. Their combination may provide a new management approach to the risk stratification of these patients. Further studies and large clinical trials are needed to establish the interaction between thyroid hormone metabolism and BNP levels, as well as the role of combining these biomarkers in making long-term prognoses.
Footnotes
Address for reprints: Gokhan Ertas, MD, Department of Cardiology, Kocaeli University Medical Faculty, Umuttepe Central Campus, Eski Istanbul Yolu 10 km, 41380 Izmit, Kocaeli, Turkey
E-mail: drgokhanertas@yahoo.com.tr
References
- 1.Hamilton MA, Stevenson LW, Luu M, Walden JA. Altered thyroid hormone metabolism in advanced heart failure. J Am Coll Cardiol 1990;16(1):91–5. [DOI] [PubMed]
- 2.Manowitz NR, Mayor GH, Klepper MJ, DeGroot LJ. Subclinical hypothyroidism and euthyroid sick syndrome in patients with moderate-to-severe congestive heart failure. Am J Ther 1996;3(12):797–801. [DOI] [PubMed]
- 3.Shanoudy H, Soliman A, Moe S, Hadian D, Veldhuis JD, Iranmanesh A, Russell DC. Early manifestations of “sick euthyroid” syndrome in patients with compensated chronic heart failure. J Card Fail 2001;7(2):146–52. [DOI] [PubMed]
- 4.Pingitore A, Landi P, Taddei MC, Ripoli A, L'Abbate A, Iervasi G. Triiodothyronine levels for risk stratification of patients with chronic heart failure. Am J Med 2005;118(2):132–6. [DOI] [PubMed]
- 5.Kozdag G, Ural D, Vural A, Agacdiken A, Kahraman G, Sahin T, et al. Relation between free triiodothyronine/free thyroxine ratio, echocardiographic parameters and mortality in dilated cardiomyopathy. Eur J Heart Fail 2005;7(1):113–8. [DOI] [PubMed]
- 6.Pingitore A, Iervasi G, Barison A, Prontera C, Pratali L, Emdin M, et al. Early activation of an altered thyroid hormone profile in asymptomatic or mildly symptomatic idiopathic left ventricular dysfunction. J Card Fail 2006;12(7):520–6. [DOI] [PubMed]
- 7.Kaptein EM, Grieb DA, Spencer CA, Wheeler WS, Nicoloff JT. Thyroxine metabolism in the low thyroxine state of critical nonthyroidal illnesses. J Clin Endocrinol Metab 1981;53 (4):764–71. [DOI] [PubMed]
- 8.Ascheim DD, Hryniewicz K. Thyroid hormone metabolism in patients with congestive heart failure: the low triiodothyronine state. Thyroid 2002;12(6):511–5. [DOI] [PubMed]
- 9.Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M, et al. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation 2003;107(5): 708–13. [DOI] [PubMed]
- 10.Docter R, Krenning EP, de Jong M, Hennemann G. The sick euthyroid syndrome: changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf) 1993;39(5):499–518. [DOI] [PubMed]
- 11.Baughman KL. B-type natriuretic peptide – a window to the heart. N Engl J Med 2002;347(3):158–9. [DOI] [PubMed]
- 12.Hunt PJ, Richards AM, Nicholls MG, Yandle TG, Doughty RN, Espiner EA. Immunoreactive amino-terminal pro-brain natriuretic peptide (NT-PROBNP): a new marker of cardiac impairment. Clin Endocrinol (Oxf) 1997;47(3):287–96. [DOI] [PubMed]
- 13.Hammerer-Lercher A, Neubauer E, Muller S, Pachinger O, Puschendorf B, Mair J. Head-to-head comparison of N-terminal pro-brain natriuretic peptide, brain natriuretic peptide and N-terminal pro-atrial natriuretic peptide in diagnosing left ventricular dysfunction. Clin Chim Acta 2001;310(2):193–7. [DOI] [PubMed]
- 14.Richards AM, Doughty R, Nicholls MG, MacMahon S, Sharpe N, Murphy J, et al. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: prognostic utility and prediction of benefit from carvedilol in chronic ischemic left ventricular dysfunction. Australia-New Zealand Heart Failure Group. J Am Coll Cardiol 2001;37(7):1781–7. [DOI] [PubMed]
- 15.Clerico A, Iervasi G, Mariani G. Pathophysiologic relevance of measuring the plasma levels of cardiac natriuretic peptide hormones in humans. Horm Metab Res 1999;31(9):487–98. [DOI] [PubMed]
- 16.Mair J, Hammerer-Lercher A, Puschendorf B. The impact of cardiac natriuretic peptide determination on the diagnosis and management of heart failure. Clin Chem Lab Med 2001; 39(7):571–88. [DOI] [PubMed]
- 17.Maisel A. B-type natriuretic peptide levels: a potential novel “white count” for congestive heart failure. J Card Fail 2001;7 (2):183–93. [DOI] [PubMed]
- 18.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet 2003;362(9380): 316–22. [DOI] [PubMed]
- 19.Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation 1994;90(1):195–203. [DOI] [PubMed]
- 20.Wei CM, Heublein DM, Perrella MA, Lerman A, Rodeheffer RJ, McGregor CG, et al. Natriuretic peptide system in human heart failure. Circulation 1993;88(3):1004–9. [DOI] [PubMed]
- 21.Tsutamoto T, Wada A, Maeda K, Hisanaga T, Maeda Y, Fukai D, et al. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 1997;96(2):509–16. [DOI] [PubMed]
- 22.Koglin J, Pehlivanli S, Schwaiblmair M, Vogeser M, Cremer P, vonScheidt W. Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol 2001;38(7):1934–41. [DOI] [PubMed]
- 23.Gegenhuber A, Mueller T, Dieplinger B, Poelz W, Pacher R, Haltmayer M. B-type natriuretic peptide and amino terminal proBNP predict one-year mortality in short of breath patients independently of the baseline diagnosis of acute destabilized heart failure. Clin Chim Acta 2006;370(1–2):174–9. [DOI] [PubMed]
- 24.Jourdain P, Jondeau G, Funck F, Gueffet P, Le Helloco A, Donal E, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol 2007;49(16):1733–9. [DOI] [PubMed]
- 25.Cortes R, Rivera M, Salvador A, Garcia de Burgos F, Bertomeu V, Rosello-Lleti E, et al. Urinary B-type natriuretic peptide levels in the diagnosis and prognosis of heart failure. J Card Fail 2007;13(7):549–55. [DOI] [PubMed]
- 26.Oh JK, Seward JB, Tajik AJ. The echo manual. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 45–8.
- 27.Alevizaki M, Synetou M, Xynos K, Pappa T, Vemmos KN. Low triiodothyronine: a strong predictor of outcome in acute stroke patients. Eur J Clin Invest 2007;37(8):651–7. [DOI] [PubMed]
- 28.Carrero JJ, Qureshi AR, Axelsson J, Yilmaz MI, Rehnmark S, Witt MR, et al. Clinical and biochemical implications of low thyroid hormone levels (total and free forms) in euthyroid patients with chronic kidney disease. J Intern Med 2007;262(6): 690–701. [DOI] [PubMed]
- 29.Utiger RD. Altered thyroid function in nonthyroidal illness and surgery. To treat or not to treat? N Engl J Med 1995;333 (23):1562–3. [DOI] [PubMed]
- 30.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 1984;311(13):819–23. [DOI] [PubMed]
- 31.Mann DL. Mechanisms and models in heart failure: a combinatorial approach. Circulation 1999;100(9):999–1008. [DOI] [PubMed]
- 32.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation 1990;82(5): 1724–9. [DOI] [PubMed]
- 33.Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation 1988;77(4):721–30. [DOI] [PubMed]
- 34.Opie LH. Cellular basis for therapeutic choices in heart failure. Circulation 2004;110(17):2559–61. [DOI] [PubMed]
- 35.Boelen A, Platvoet-Ter Schiphorst MC, Wiersinga WM. Association between serum interleukin-6 and serum 3,5,3′-triiodothyronine in nonthyroidal illness. J Clin Endocrinol Metab 1993;77(6):1695–9. [DOI] [PubMed]
- 36.Boelen A, Maas MA, Lowik CW, Platvoet MC, Wiersinga WM. Induced illness in interleukin-6 (IL-6) knock-out mice: a causal role of IL-6 in the development of the low 3,5,3′-triiodothyronine syndrome. Endocrinology 1996;137(12):5250–4. [DOI] [PubMed]
- 37.Boelen A, Kwakkel J, Platvoet-Ter Schiphorst M, Baur A, Kohrle J, Wiersinga WM. Contribution of interleukin-12 to the pathogenesis of non-thyroidal illness. Horm Metab Res 2004;36(2):101–6. [DOI] [PubMed]
- 38.Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev 2005;26(5):704–28. [DOI] [PubMed]
- 39.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001;344(7):501–9. [DOI] [PubMed]
- 40.Sabatino L, Colantuoni A, Iervasi G. Is the vascular system a main target for thyroid hormones? From molecular and biochemical findings to clinical perspectives. Curr Vasc Pharmacol 2005;3(2):133–45. [DOI] [PubMed]
- 41.Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation 1993;87(5):1435–41. [DOI] [PubMed]
- 42.Harrison A, Morrison LK, Krishnaswamy P, Kazanegra R, Clopton P, Dao Q, et al. B-type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea. Ann Emerg Med 2002;39(2):131–8. [DOI] [PubMed]
- 43.Rosolova H, Cech J, Simon J, Spinar J, Jandova R, Widimsky sen J, et al. Short to long term mortality of patients hospitalised with heart failure in the Czech Republic–a report from the EuroHeart Failure Survey. Eur J Heart Fail 2005;7(5):780–3. [DOI] [PubMed]
- 44.Dokainish H, Zoghbi WA, Lakkis NM, Ambriz E, Patel R, Quinones MA, Nagueh SF. Incremental predictive power of B-type natriuretic peptide and tissue Doppler echocardiography in the prognosis of patients with congestive heart failure. J Am Coll Cardiol 2005;45(8):1223–6. [DOI] [PubMed]
- 45.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med 1994;180(6):2365–70. [DOI] [PMC free article] [PubMed]
- 46.Oh-ishi S, Utsunomiya I, Yamamoto T, Komuro Y, Hara Y. Effects of prostaglandins and cyclic AMP on cytokine production in rat leukocytes. Eur J Pharmacol 1996;300(3):255–9. [DOI] [PubMed]
- 47.Chiurchiu V, Izzi V, D'Aquilio F, Carotenuto F, Di Nardo P, Baldini PM. Brain natriuretic peptide (BNP) regulates the production of inflammatory mediators in human THP-1 macrophages. Regul Pept 2008;148(1–3):26–32. [DOI] [PubMed]
- 48.de Bold AJ. Cardiac natriuretic peptides gene expression and secretion in inflammation. J Investig Med 2009;57(1):29–32. [DOI] [PubMed]
- 49.Vaz Perez A, Doehner W, von Haehling S, Schmidt H, Zimmermann AV, Volk HD, et al. The relationship between tumor necrosis factor-alpha, brain natriuretic peptide and atrial natriuretic peptide in patients with chronic heart failure. Int J Cardiol 141(1):39–43. [DOI] [PubMed]
- 50.Abo-Zenah HA, Shoeb SA, Sabry AA, Ismail HA. Relating circulating thyroid hormone concentrations to serum interleukins-6 and -10 in association with non-thyroidal illnesses including chronic renal insufficiency. BMC Endocr Disord 2008;8:1. [DOI] [PMC free article] [PubMed]
- 51.Passino C, Pingitore A, Landi P, Fontana M, Zyw L, Clerico A, et al. Prognostic value of combined measurement of brain natriuretic peptide and triiodothyronine in heart failure. J Card Fail 2009;15(1):35–40. [DOI] [PubMed]