Abstract
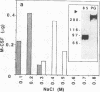
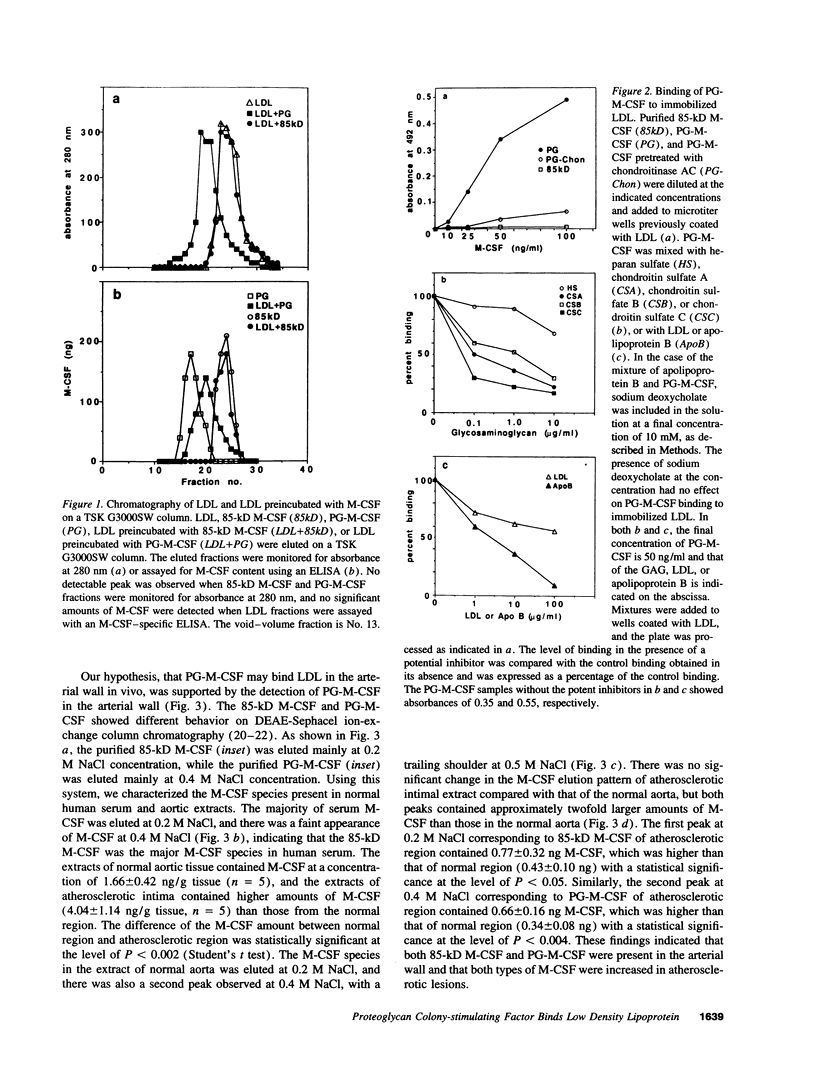
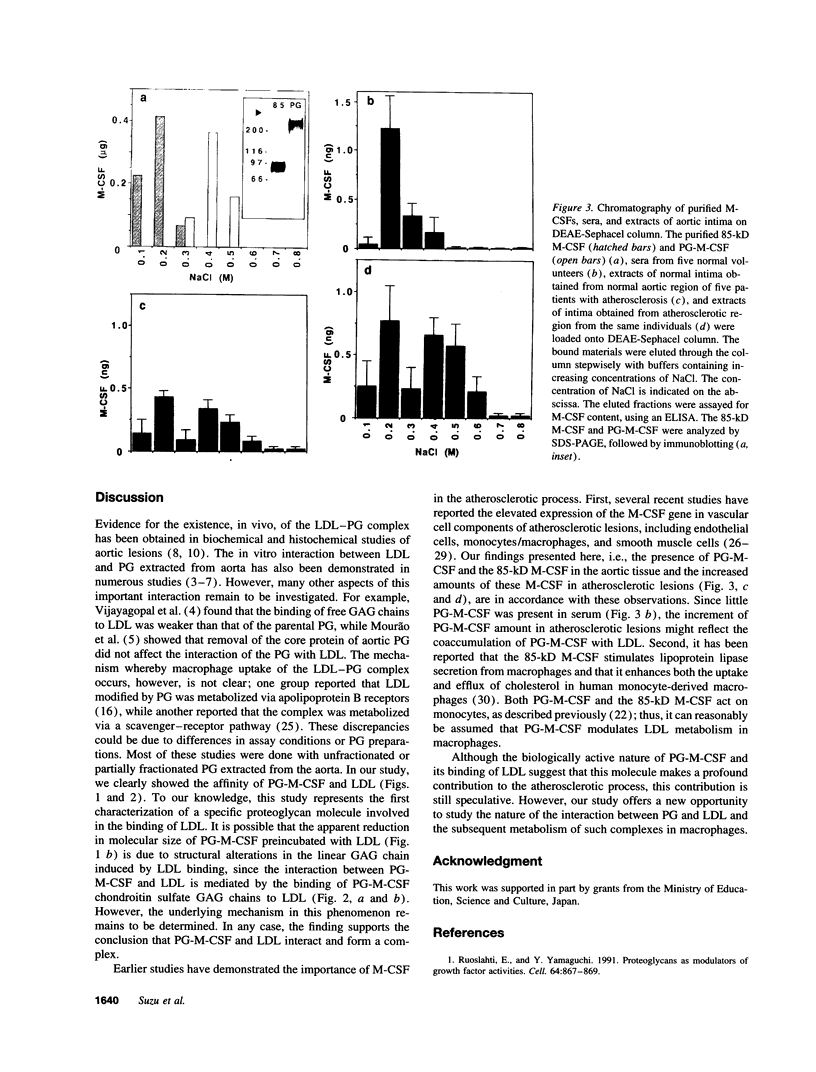
We recently isolated a proteoglycan form of macrophage colony-stimulating factor (PG-M-CSF) that carries a chondroitin sulfate glycosaminoglycan chain. Here, we examined the interaction of PG-M-CSF with low density lipoprotein (LDL). When LDL preincubated with PG-M-CSF was fractionated by molecular size sieving chromatography, it was eluted earlier than untreated LDL. When LDL was preincubated with chondroitin sulfate-free 85-kD M-CSF instead of PG-M-CSF, the elution profile of LDL remained unchanged, indicating specific interaction between PG-M-CSF and LDL. The level of PG-M-CSF binding in the wells of a plastic microtitration plate precoated with LDL was significant, this binding being completely abolished by pretreatment of PG-M-CSF with chondroitinase AC, which degrades chondroitin sulfate. The addition of exogenous chondroitin sulfate or apolipoprotein B inhibited the binding of PG-M-CSF to LDL in a dose-dependent manner, indicating that the interaction between PG-M-CSF and LDL was mediated by the binding of the chondroitin sulfate chain of PG-M-CSF to LDL apolipoprotein B. PG-M-CSF was also demonstrated in the arterial wall, and there were increased amounts of PG-M-CSF in atherosclerotic lesions. The in vitro interaction between PG-M-CSF and LDL thus appears to have physiological significance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alavi M., Moore S. Glycosaminoglycan composition and biosynthesis in the endothelium-covered neointima of de-endothelialized rabbit aorta. Exp Mol Pathol. 1985 Jun;42(3):389–400. doi: 10.1016/0014-4800(85)90088-7. [DOI] [PubMed] [Google Scholar]
- Camejo G., Acquatella H., Lalaguna F. The interaction of low density lipoproteins with arterial proteoglycans. An additional risk factor? Atherosclerosis. 1980 May;36(1):55–65. doi: 10.1016/0021-9150(80)90198-7. [DOI] [PubMed] [Google Scholar]
- Christner J. E., Baker J. R. A competitive assay of lipoprotein: proteoglycan interaction using a 96-well microtitration plate. Anal Biochem. 1990 Feb 1;184(2):388–394. doi: 10.1016/0003-2697(90)90698-9. [DOI] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Clinton S. K., Underwood R., Hayes L., Sherman M. L., Kufe D. W., Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992 Feb;140(2):301–316. [PMC free article] [PubMed] [Google Scholar]
- Hara I., Okazaki M. High-performance liquid chromatography of serum lipoproteins. Methods Enzymol. 1986;129:57–78. doi: 10.1016/0076-6879(86)29062-x. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., Wagner W. D. Plasma low density lipoprotein accumulation in aortas of hypercholesterolemic swine correlates with modifications in aortic glycosaminoglycan composition. Atherosclerosis. 1986 Sep;61(3):231–236. doi: 10.1016/0021-9150(86)90143-7. [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Inaba T., Shimano H., Harada K., Inoue I., Mokuno H., Mori N., Gotoda T., Takaku F., Yamada N. Monocyte colony-stimulating factor enhances uptake and degradation of acetylated low density lipoproteins and cholesterol esterification in human monocyte-derived macrophages. J Biol Chem. 1990 Aug 25;265(24):14109–14117. [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Motoyoshi K., Takaku F., Mizoguchi H., Miura Y. Purification and some properties of colony-stimulating factor from normal human urine. Blood. 1978 Nov;52(5):1012–1020. [PubMed] [Google Scholar]
- Mourão P. A., Bracamonte C. A. The binding of human aortic glycosaminoglycans and proteoglycans to plasma low density lipoproteins. Atherosclerosis. 1984 Feb;50(2):133–146. doi: 10.1016/0021-9150(84)90017-0. [DOI] [PubMed] [Google Scholar]
- Owens R. T., Wagner W. D. Proteoglycans produced by cholesterol-enriched macrophages bind plasma low density lipoprotein. Atherosclerosis. 1991 Dec;91(3):229–240. doi: 10.1016/0021-9150(91)90170-8. [DOI] [PubMed] [Google Scholar]
- Price L. K., Choi H. U., Rosenberg L., Stanley E. R. The predominant form of secreted colony stimulating factor-1 is a proteoglycan. J Biol Chem. 1992 Feb 5;267(4):2190–2199. [PubMed] [Google Scholar]
- Rajavashisth T. B., Andalibi A., Territo M. C., Berliner J. A., Navab M., Fogelman A. M., Lusis A. J. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990 Mar 15;344(6263):254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Ylä-Herttuala S., Lipton B. A., Ord V. A., Witztum J. L., Steinberg D. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992 Feb;140(2):291–300. [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Salisbury B. G., Falcone D. J., Minick C. R. Insoluble low-density lipoprotein-proteoglycan complexes enhance cholesteryl ester accumulation in macrophages. Am J Pathol. 1985 Jul;120(1):6–11. [PMC free article] [PubMed] [Google Scholar]
- Salisbury B. G., Hajjar D. P., Minick C. R. Altered glycosaminoglycan metabolism in injured arterial wall. Exp Mol Pathol. 1985 Jun;42(3):306–319. doi: 10.1016/0014-4800(85)90081-4. [DOI] [PubMed] [Google Scholar]
- Shimada M., Inaba T., Shimano H., Gotoda T., Watanabe Y., Yamamoto K., Motoyoshi K., Yazaki Y., Yamada N. Platelet-derived growth factor BB-dimer suppresses the expression of macrophage colony-stimulating factor in human vascular smooth muscle cells. J Biol Chem. 1992 Aug 5;267(22):15455–15458. [PubMed] [Google Scholar]
- Srinivasan S. R., Radhakrishnamurthy B., Dalferes E. R., Jr, Berenson G. S. Collagenase-solubilized lipoprotein--glycosaminoglycan complexes of human aortic fibrous plaque lesions. Atherosclerosis. 1979 Oct;34(2):105–118. doi: 10.1016/0021-9150(79)90134-5. [DOI] [PubMed] [Google Scholar]
- Steele R. H., Wagner W. D., Rowe H. A., Edwards I. J. Artery wall derived proteoglycan-plasma lipoprotein interaction: lipoprotein binding properties of extracted proteoglycans. Atherosclerosis. 1987 May;65(1-2):51–62. doi: 10.1016/0021-9150(87)90007-4. [DOI] [PubMed] [Google Scholar]
- Suzu S., Ohtsuki T., Makishima M., Yanai N., Kawashima T., Nagata N., Motoyoshi K. Biological activity of a proteoglycan form of macrophage colony-stimulating factor and its binding to type V collagen. J Biol Chem. 1992 Aug 25;267(24):16812–16815. [PubMed] [Google Scholar]
- Suzu S., Ohtsuki T., Yanai N., Takatsu Z., Kawashima T., Takaku F., Nagata N., Motoyoshi K. Identification of a high molecular weight macrophage colony-stimulating factor as a glycosaminoglycan-containing species. J Biol Chem. 1992 Mar 5;267(7):4345–4348. [PubMed] [Google Scholar]
- Suzu S., Yanai N., Sato-Somoto Y., Yamada M., Kawashima T., Hanamura T., Nagata N., Takaku F., Motoyoshi K. Characterization of macrophage colony-stimulating factor in body fluids by immunoblot analysis. Blood. 1991 May 15;77(10):2160–2165. [PubMed] [Google Scholar]
- Vijayagopal P., Srinivasan S. R., Radhakrishnamurthy B., Berenson G. S. Human monocyte-derived macrophages bind low-density-lipoprotein-proteoglycan complexes by a receptor different from the low-density-lipoprotein receptor. Biochem J. 1993 Feb 1;289(Pt 3):837–844. doi: 10.1042/bj2890837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayagopal P., Srinivasan S. R., Radhakrishnamurthy B., Berenson G. S. Interaction of serum lipoproteins and a proteoglycan from bovine aorta. J Biol Chem. 1981 Aug 10;256(15):8234–8241. [PubMed] [Google Scholar]
- Wegrowski J., Moczar M., Robert L. Proteoglycans from pig aorta. Comparative study of their interactions with lipoproteins. Biochem J. 1986 May 1;235(3):823–831. doi: 10.1042/bj2350823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. G., Temple P. A., Leary A. C., Witek-Giannotti J. S., Yang Y. C., Ciarletta A. B., Chung M., Murtha P., Kriz R., Kaufman R. J. Human CSF-1: molecular cloning and expression of 4-kb cDNA encoding the human urinary protein. Science. 1987 Mar 20;235(4795):1504–1508. doi: 10.1126/science.3493529. [DOI] [PubMed] [Google Scholar]