Abstract
The technique of microdialysis enables sampling and collecting of small-molecular-weight substances from the interstitial space. It is a widely used method in neuroscience and is one of the few techniques available that permits quantification of neurotransmitters, peptides, and hormones in the behaving animal. More recently, it has been used in tissue preparations for quantification of neurotransmitter release. This unit provides a brief review of the history of microdialysis and its general application in the neurosciences. The authors review the theoretical principles underlying the microdialysis process, methods available for estimating extracellular concentration from dialysis samples (i.e., relative recovery), the various factors that affect the estimate of in vivo relative recovery, and the importance of determining in vivo relative recovery to data interpretation. Several areas of special note, including impact of tissue trauma on the interpretation of microdialysis results, are discussed. Step-by-step instructions for the planning and execution of conventional and quantitative microdialysis experiments are provided.
Keywords: microdialysis, quantitative microdialysis, neurochemistry, no net flux method, analytical chemistry, extraction fraction, neurotransmitter quantification
INTRODUCTION
The technique of microdialysis enables the monitoring of neurotransmitters and other molecules in interstitial tissue fluid. This method is widely used for sampling and quantifying neurotransmitters, neuropeptides, and hormones in the brain and periphery. Depending on the availability of an appropriate analytical assay, virtually any soluble molecule in the interstitial space fluid can be measured by microdialysis. This unit describes the principles of conventional and quantitative microdialysis, as well as strategies for designing a dialysis experiment. It establishes the groundwork for the basic techniques of preparation, conduct, and analysis of dialysis experiments in rodents and subhuman primates (UNITS 7.2–7.4). Although the methods described are those used for monitoring CNS function, they can easily be adapted to other organ systems.
HISTORICAL PERSPECTIVE
Initial studies measuring transmitter release in anesthetized and freely moving behaving animals employed the push-pull technique developed by Gaddum (1961) and Myers (1972). The concept of using a dialysis bag to collect samples from small interstitial tissue, such as the brain, was first introduced by Bito et al. (1966). Delgado refined the technique, developing the first “dialytrode” in which a solution was slowly perfused through a dialysis bag and carried to a site immediately accessible to the experimenter (Delgado et al., 1972). By enlarging the surface area of the dialysis membrane and increasing the efficiency of the microdialysis probe in collecting analyte, Ungerstedt and colleagues substantially improved the design of the microdialysis probe (Ungerstedt and Pycock, 1974; Ungerstedt et al., 1982). The successful use of microdialysis to quantify monoamine levels in neural tissue by Ungerstedt and colleagues during the late 1970s and early 1980s contributed significantly to the worldwide use of the microdialysis method.
Since 1960, over 11,000 published studies have used microdialysis techniques to sample substances from tissue. The majority of these studies have conducted dialysis in the extracellular milieu of neural tissue in the laboratory rat, in particular, to collect samples for the analysis of monoamines and their metabolites. It is perhaps no accident that this technique has been applied so often to the study of monoamines. Monoamines are widely distributed throughout the peripheral and central nervous system. They have been implicated in a variety of brain functions and their dys-regulation contributes to a range of neuropsychiatric disorders including Parkinson’s disease, depression, pain, and drug addiction. The extracellular concentration of monoamines is typically high, of neuronal origin, and concentrated in large brain structures such as striatum and accumbens. The physicochemical characteristics of monoamines are well understood (i.e., hydrophilicity, size, structure), and sensitive analytical instruments are available for their quantification in dialysis samples [e.g., electrochemical (EC) detection, capillary electrophoresis (CE)].
Many other analytes have been successfully sampled by microdialysis. The range of microdialysis applications is illustrated in Tables 7.1.1, 7.1.2, and 7.1.3. Since the late 1990s, the use of microdialysis in the mouse and nonhuman primate has increased, as has its use with in vivo imaging techniques and for clinical diagnostics in human subjects (Cavus et al., 2005; Rambeck et al., 2006; Montgomery et al., 2007).
Table 7.1.1.
Compounds Analyzed by Microdialysis
Abbreviations: GABA, γ -aminobutyric acid; DA, dopamine; LHRH, luteinizing hormone releasing hormone; MHPG, 4-hydroxy-3-methylphenylglycol.
Table 7.1.2.
Tissues Analyzed by Microdialysis
| Organ system | Reference |
|---|---|
| Adipose tissue | Hallstrom et al., 1989 |
| Adrenal glands | Kuzmin et al., 1990 |
| Blood | Sjoberg et al., 1992 |
| Bone | Thorsen et al., 1996 |
| Brain | Hallstrom et al., 1989 |
| Heart | Kuzmin et al., 1992 |
| Ileum | Fukui et al., 1993 |
| Kidney | Siragy, 1992 |
| Liver | Okuda et al., 1992b |
| Lung | Larsson, 1991 |
| Luteal tissue | Sauerwein et al., 1992 |
| Muscle | Hallstrom et al., 1989 |
| Pancreas | Jonsson et al., 1992 |
| Retina | Louzada-Junior et al., 1992 |
| Skin | Petersen et al., 1992b |
| Spinal cord | Linderoth et al., 1992 |
| Spleen | Shimizu et al., 1994 |
| Subcutaneous tissue | Deleu et al., 1993 |
| Uterus | Nordenvall et al., 1989 |
Table 7.1.3.
Species Analyzed by Microdialysis
| Species | Reference |
|---|---|
| Carnivoran | |
| Cat | Linderoth et al., 1992 |
| Dog | Kuzmin et al., 1992 |
| Primate | |
| Baboon | Khan-Dawood et al., 1994 |
| Common marmoset | Nomoto et al., 1994 |
| Cynomolgus monkey | Ljungdahl-Stahle et al., 1992 |
| Human | Petersen et al., 1992a,b |
| Rhesus monkey | Saunders et al., 1993 |
| Rodentian | |
| Gerbil | Yue et al., 1992 |
| Guinea pig | Callaghhan et al., 1994 |
| Mouse | Rollema et al., 1989 |
| Rat | Wright et al., 1992 |
| Siberian hamster | Glass et al., 1992 |
| Artiodactylan | |
| Cow | Einspanier et al., 1990 |
| Goat | Hashizume et al., 1995 |
| Pig | Jonsson et al., 1992 |
| Sheep | Kendrick et al., 1989 |
| Other | |
| Horse | Ingvast-Larsson et al., 1991 |
| Pigeon | Hasegawa and Ebihara, 1992 |
| Rabbit | Wang and Sawchuck, 1995 |
| Rainbow trout | Hylland et al., 1995 |
| Crayfish | Cebada et al., 2006 |
The advantages and limitations of microdialysis techniques have been reviewed in detail (Westerink et al., 1987; Benveniste, 1989; Westerink and Justice, 1991; Khan and Shuaib, 2001; Plock and Kloft, 2005) and are only briefly summarized here. The primary advantage of microdialysis over other in vivo perfusion techniques lies in the construction of the probe. First, the microdialysis probe is smaller than devices used for push-pull or cortical cup perfusion and, thus, displaces a smaller area of tissue. Second, the microdialysis membrane provides a physical barrier between the perfusate and tissue that protects the tissue not only from the turbulent flow of the perfusate but also from high-molecular-weight substances, such as bacteria, that cannot cross the membrane. The perfusate flow rate is lower than that used in other perfusion techniques, and thereby reducing analyte depletion. Another advantage of microdialysis is that it can be used to infuse drugs or known compounds through the probe, a technique referred to as retrodialysis.
Finally, a more precise characterization of the quantity and distribution of an analyte can be achieved using microdialysis because the membrane constrains the flow of perfusate, limiting the underlying mechanisms for analyte transport into the probe, and, as a result of the membrane geometry, generates an evenly distributed area of tissue impacted by the probe. Thus, the processes of microdialysis can be well defined mathematically to yield predictions about the characteristic distribution and activity of an analyte of interest (Morrison et al., 1991). These advantages do not hold when comparing microdialysis to in vivo voltammetry. However, microdialysis does have one significant advantage over voltammetry in that it is not limited to the analysis of electroactive substances and multiple analytes can be sampled simultaneously. This is because microdialysis is an analyte collection procedure and once the sample is obtained, any analytical technique can be used to quantify the analyte(s) of interest. Furthermore, for electroactive substances, lower concentrations are detectable using microdialysis than in vivo voltammetry. This distinction is important for studies in which quantification of basal neurotransmitter dynamics is desired.
Microdialysis has several disadvantages relative to other techniques. It has limited time resolution (≥1 min; more typically, 10-min collection periods are employed) in comparison to voltammetry (seconds and milliseconds). Although the use of highly sensitive analytical techniques (e.g., capillary electrophoresis) can reduce sampling times to 1 min or less, microdialysis samples still do not provide real time information regarding changes in the neurochemical environment, which can be done with fast-scan cyclic voltammetry. Second, microdialysis is an invasive technique and therefore causes some damage to the tissue under study. In this respect, the use of a guide cannula in which a removable probe is inserted when the animal is sufficiently recovered from the surgery is more preferable than implantation of the microdialysis probe itself during surgery. Third, the dialysis process creates an area around the probe in which all solutes capable of crossing the probe membrane are depleted. Changes in the neurochemical milieu may affect basal levels and/or the pharmacological responsiveness of the substance under study. Some investigators, however, have suggested that perfusion of a neurotransmitter-free artificial cerebrospinal fluid (aCSF) during conventional microdialysis results in the formation of an artificial neurotransmitter concentration gradient, which enables detection of neurotransmitter changes in the implanted area (Di Chiara et al., 1996). On the other hand, Sam and Justice (1996) showed that basal levels of dopamine (DA), in the striatum, are unaffected by variations in the amount of a non-DA solute depleted around the probe. Fourth, low-molecular-weight solutes contained in the perfusate may diffuse into the interstitial space disturbing the homeostatic balance of the extracellular environment and artificially changing the level or activity of the analyte. For example, failure to provide appropriate concentrations of Ca2+ or Na+ ions in the perfusate can dramatically alter basal levels of neurotransmitters (Westerink et al., 1988; Moghaddam and Bunney, 1989; Osborne et al., 1991). Use of a high concentration of calcium (e.g., 2.4 as compared to 1.2 mM) can result in quantification of stimulated rather than basal neurotransmitter overflow. Fifth, the microdialysis probe occupies space of a particular dimension and length. Thus, use of the microdialysis technique is limited to areas large enough and long enough to surround the microdialysis probe. The smallest probe is still relatively large compared to many brain or spinal cord regions, and damage to local tissue could potentially confound experimental results. Finally, the quantity of analyte collected by microdialysis most often represents a fraction of the actual extracellular concentration because at most flow rates (i.e., >0.1 μl/min), the rate of analyte removal from the inside of the probe is higher than the rate of analyte replacement to the probe membrane surface.
This last limitation requires additional comment, as the search for the proper mathematical expression of these differences has led to some unanticipated conclusions regarding interpretation of microdialysis data. At usual perfusate flow rates (e.g., 1 μl/min) the ratio between the actual extracellular concentration of an analyte and its dialysate concentration is typically <40%. This ratio is termed the extraction fraction, relative recovery, or probe efficiency. Because flow rate is inversely related to the extraction fraction (Wages et al., 1986; Zetterstrom et al., 1988), it is possible to reduce the flow to a rate suitable to achieve near equilibrium. However, the sample collection volume and collection time at these very low perfusate flow rates are either insufficient or inappropriate to test most hypotheses.
Several groups have attempted to characterize and quantify the extraction fraction. An understanding of the source of diffusivity during dialysis has greatly aided this aim. When extraction fraction is low, as is typically the case, the greatest source of resistance to diffusion occurs in the tissue. Tissue resistance differs for each analyte and, in turn, it is influenced by neurochemical processes that affect analyte supply and degradation. As a result, experimental manipulations that modify these neurochemical processes may also modify the extraction fraction and confound interpretation of changes in dialysate concentration. Consequently, changes in dialysate concentration do not mirror changes in extracellular concentration, but instead reflect the net change in extraction fraction and extracellular concentration. Thus, the study of the diffusional and/or convective processes underlying analyte sampling by microdialysis (or solute delivery by the microdialysis probe) has been developed (i.e., quantitative microdialysis) to determine in vivo extraction fraction and the proportional relationship between dialysate and extracellular concentrations of an analyte under study. The theoretical and empirical findings to date are discussed in detail in the following sections.
DESCRIPTION OF THE MICRODIALYSIS PROCESS
A principal element of microdialysis is the probe, which is used to capture molecules from the extracellular space. All microdialysis probes are composed of a length of tubular dialysis membrane through which a solution, usually devoid of the analyte of interest, is constantly perfused. The dialysis membrane is semipermeable and permits free transport of some but not all solutes. Permeability is typically limited to compounds with molecular masses <20,000 Da. A variety of membranes are available which differ in pore size and the material used. Once the probe is inserted into an external medium (i.e., tissue) and perfusion begins, substances on the outside surface of the dialysis membrane diffuse through the membrane into the probe along their concentration gradient. The perfusate is infused slowly and continuously through the length of the dialysis probe into the outflow tubing where it can be collected for subsequent analyte quantification. Perfusion rates of 0.3 to 3 μl/min are typically used depending upon the volume, sample collection time, and analytical sensitivity needed with typical sample collection times ranging from 1 to 20 min. The presence of the microdialysis probe impacts the external medium surrounding it. As low-molecular-weight solutes are removed from the external medium by the probe, a zone around the probe becomes depleted of solute. Depletion is not complete because the external medium is continuous and large, so that the solutes can be partially replaced by diffusion from areas of higher concentration that are undisturbed by the probe. Moreover, the perfusion itself may induce changes in the surrounding brain tissue, which may influence the release and metabolism of the sampled transmitter. Therefore, a description of the microdialysis process should include an account of the changes in concentration of the analyte in the tissue around the probe. Of particular interest will be the difference in concentration between the undisturbed tissue and the dialysate and the size of the area of analyte depletion (penetration distance). This information will aid in the interpretation of the results from a microdialysis experiment by providing a measure of probe efficiency that makes it possible to calibrate the concentration of the analyte in the dialysate with respect to its extracellular concentration, and to identify the anatomical site from which the microdialysis sample is collected.
The underlying process driving analyte transport during microdialysis is generally accepted to be diffusion, although ultrafiltration may occur in some circumstance (e.g., increased pressure associated with manual changes in the syringe infusing the perfusate). However, because ultrafiltration should be prevented during experiments, it has not been included in the mathematical consideration of the microdialysis process. The occurrence of ultrafiltration during microdialysis can be identified by “sweating of the probe” and, if noted, perfusate flow rate should be decreased or greater care should be taken when manipulating the infusion process. Diffusion of a given analyte involves transport of that analyte down its concentration gradient from the “undisturbed” tissue into the perfusate. An analysis of the slope of the concentration gradient, the radial distance over which the concentration gradient occurs, and the net change in analyte concentration (extraction fraction) over the course of the concentration gradient are used to describe the transport of analyte during microdialysis. It is important to note that the concentration gradient is influenced not only by the physical attributes of the analyte (i.e., molecular weight, hydrophobicity, and tertiary structure), but also by the physical composition of the medium in which transport occurs. Therefore, a complete characterization of the transport of an analyte during microdialysis involves a description of the concentration gradient in the external medium (e.g., tissue), the concentration gradient across the membrane, and the concentration gradient across the annular width of the microdialysis probe.
PRINCIPLES UNDERLYING MICRODIALYSIS
During microdialysis, substances move from an area of higher concentration to an area of lower concentration. The direction of movement is set by the experimenter, who biases the procedure so that the concentration of the analyte under study is higher on one side of the probe membrane. In this way, the technique is used either to sample a substance from an external solution or to deliver a substance to the external solution (e.g., retrodialysis) or both.
The extraction fraction (i.e., probe efficiency or relative recovery) defines the proportional difference in concentration between the highest concentration of substance and the lowest concentration of the substance (i.e., “undisturbed” extracellular concentration versus dialysate concentration as illustrated in Fig. 7.1.1). Knowing the extraction fraction is essential for determining the external concentration of a substance relative to its concentration in the dialysate. In addition, for practical reasons, knowledge of the extraction fraction facilitates the choice of flow rate and collection period that will supply dialysate samples with sufficient concentration of the substance for detection.
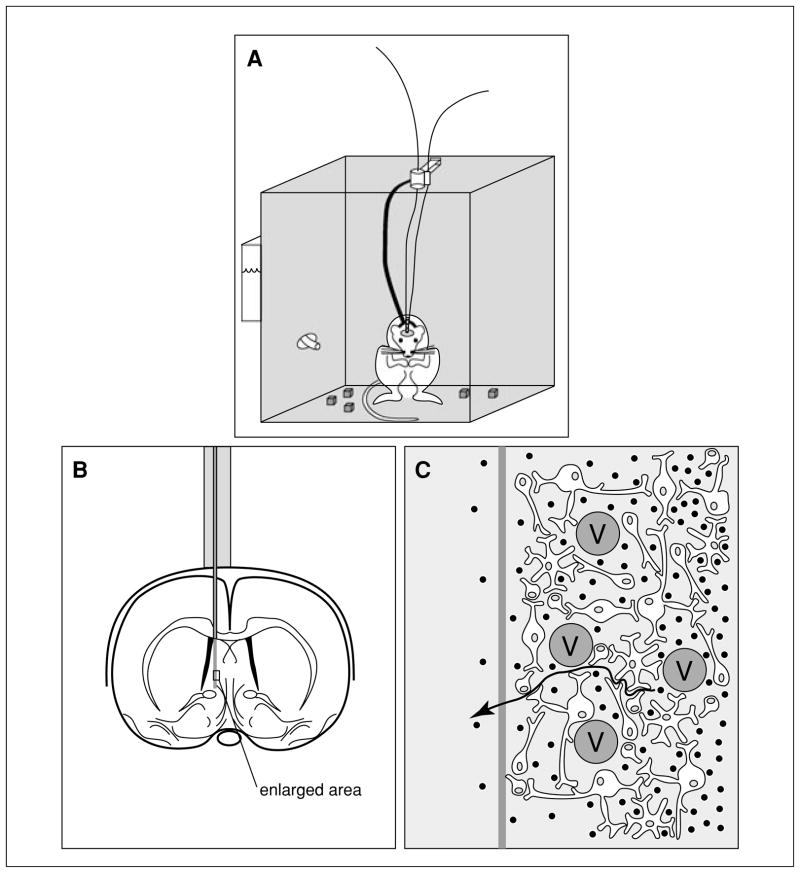
Figure 7.1.1.
Representation of the “inside view” of a microdialysis probe. The microdialysis probe, which consists of an inflow and outflow tubing (A) separated by tubing made of dialysis membrane, is implanted surgically into a specific area within the brain (B). The enlarged view (C) illustrates the complex composition of the fluid through which analytes (black dots) must diffuse to get to the microdialysis probe. The presence of impermeable cells such as blood vessels (V) reduces the fluid volume surrounding the probe and increases the diffusional path (arrow) of analytes moving toward the probe. The net effect is a decreased diffusivity in this phase.
Relative recovery can be determined empirically if the external concentration of the substance is known or directly measurable. Such is the case for in vitro preparations, in which direct measurement of the substance in the external solution is easily accomplished (see UNIT 7.2 for microdialysis in vitro). Although in vitro relative recovery estimates have been used to correct dialysate values, this method has proved imprecise (Glick et al., 1994) because diffusion in tissue differs significantly from diffusion in a solution (Nicholson and Rice, 1986). An empirical determination of in vivo recovery can also be obtained by using one of several quantitative methods described in the next section.
A number of mathematical models have been developed to describe the processes governing analyte transport during in vitro and in vivo microdialysis (Amberg and Lindefors, 1989; Benveniste, 1989; Lindefors et al., 1989a; Bungay et al., 1990). These models provide testable hypotheses regarding how and why recovery in tissue deviates from recovery in solution, and provide the basis with which to understand how analyte dialysate levels relate to extracellular concentration. A brief theoretical treatment of the microdialysis process is given below.
The physical laws governing diffusion and convective processes describe the movement of substances across a medium in which a concentration gradient exists (for discussion of this subject, see Friedman, 1986). The description of analyte transport during microdialysis has been limited to the consideration of diffusive processes alone, as the impact of convective processes during microdialysis, such as osmotic and/or hydrostatic pressure differences across the membranes, are thought to be negligible (Benveniste, 1989; Bungay et al., 1990). The rate of analyte transport by diffusion can be described mathematically by Fick’s law of diffusion, which states that:
where J is the flux (i.e., moles of solute transported over the area of diffusion), D is the diffusion coefficient, Δc is the change in analyte concentration over the area of diffusion, and Δr is the small length over which diffusion occurs. The assumptions underlying this expression are that the transport of analyte occurs through an ideal solution that is homogeneous in composition and that the concentration gradient is at steady state. Since these assumptions are not met during microdialysis, the expression of flux must be altered to reflect analyte diffusion in a microdialysis preparation.
Consider first the composition of the solution or medium through which diffusion occurs. During microdialysis, substances move across three media, each of distinct composition: perfusate, membrane, and external medium (i.e., tissue). The factors that guide diffusion will vary in each medium. Thus, the concentration profile will differ. Therefore, the diffusional characteristics of a substance in each medium must be considered separately. An expression of flux that allows for the consideration of analyte transport across several media is given by the following equation:
Here transport is expressed as resistance R instead of diffusivity D because resistances, in series, can be added (Friedman, 1986). In this equation, the subscripts 1, 2, and 3 refer to the three media over which diffusion occurs (external medium, membrane, and perfusate), ΔC1–3 is the overall concentration difference between the phases, and Rx is the resistance to diffusion, or inverse permeability, of the solute in medium x of Rx. The form of the expression can be modified to account for the way in which the medium of each phase deviates from the ideal solution.
Bungay et al. (1990, 2003, 2007), who have provided the most comprehensive discussion of analyte transport during microdialysis under steady-state conditions, use the expression of mass transport resistance to describe the proportional difference in analyte concentration between the undisturbed external medium and the microdialysis probe perfusate or extraction fraction (Ed). Solving the mass transport resistance equation, above, for the appropriate geometry and boundary conditions, Ed under steady-state conditions is given by:
where Qd is the perfusate flow rate and Rd, Rm, and Re are the resistances for the dialysate, membrane, and external medium, respectively. Cout is the concentration of solute in the dialysate sample, Cin is the concentration of solute in the perfusate (typically zero for conventional microdialysis experiments), and Cext is the concentration of solute in tissue undisturbed by the action of the microdialysis probe.
Ed provides a measure of probe efficiency. It is the correction factor by which to transform analyte concentration in dialysates to extracellular concentration. Changes in Ed will occur when there are changes in diffusional resistance or changes in flow rate. For small hydrophilic substances, the greatest source of resistance during microdialysis has been found to be tissue (Re > Rm > Rd; Bungay et al., 1990). Changes in the processes underlying differential resistance in tissue will change Ed.
Changes in Ed can simply appear as increases or decreases in dialysate concentration of the analyte. Therefore, changes in dialysate concentration do not necessarily mean that extracellular concentration has changed. Decreased Ed may mean reduced relative recovery or increased radial distance between undisturbed tissue and the probe. An increase in Ed may mean increased relative recovery or reduced radial distance between the undisturbed tissue and the probe. In most conventional microdialysis reports, an underlying assumption is that Ed remains unchanged over the course of dialysate collection, so any change in dialysate concentration is interpreted as a change in extracellular concentration. Increasing evidence suggests that this assumption is not always valid (Justice, 1993; Parsons and Justice, 1994; Thompson et al., 2000). A theoretical consideration of the factors affecting tissue resistance, and therefore Ed, is presented below.
The general expression proposed by Bungay et al. (1990) to describe resistance in a microdialysis preparation is:
where r is the length over which diffusion occurs, Deff is the (effective) diffusion coefficient corrected for the complex geometry of the extracellular space, φ is the volume fraction, and S is the probe surface area. Resistance to analyte transport in each medium can be determined by finding the distance over which the concentration gradient occurs (e.g., from the boundary of the “undisturbed” area in the external solution to the outer boundary of the membrane), and dividing that distance by the product of the volume fraction (e.g., 1.0 for water), surface area (e.g., of the membrane), and effective diffusion coefficient. The specific mathematical description of resistance in each medium will also contain elements that specify the way in which diffusion in that medium deviates from diffusion in an ideal solution.
Specific expressions describing diffusional resistance in the membrane, perfusate, and tissue are detailed in Bungay et al. (1990). The parameters (i.e., Δr, Deff) needed to solve the resistance equation for the membrane and the perfusate can be determined empirically. It is generally expected that diffusional resistance in the membrane and perfusate is not concentration dependent and is unaffected by experimental manipulation. Therefore, Rm and Rd should remain constant between and within experimental groups and can be determined independently in a solution. The calculation of tissue resistance is complicated by the fact that both Δr and Deff are unknown and not easily measured. The distance over which the concentration gradient develops, propagating from the probe into the tissue, is inversely related to diffusivity and directly related to the diffusional resistance of the analyte in tissue: as resistance of an analyte increases, the area over which the concentration gradient develops increases.
Resistance in tissue is significantly increased by the presence of impermeable cell membranes because of the reduced fluid volume available for diffusion (the extracellular space is estimated to be 20% of the total volume in the brain) and increased complexity in the diffusional path (Nicholson and Rice, 1986). Both factors slow diffusion and increase the Re. In addition, for endogenous substances, generation and removal of the substances are distributed throughout the tissue, regulated by multiple physiological mechanisms, and subject to change as a result of experimental manipulation. To determine the concentration profile of the analyte radiating from the probe in tissue, the mass balance equation, which accounts for all factors that affect diffusion in tissue, including the generation and degradation of the analyte, must be solved.
Consider the general differential mass balance equation proposed by Bungay et al. (1990) to describe the extracellular concentration of an analyte in a differential element at time t:
where Cp, Ce, and Ci are the concentrations of analyte in plasma and in extracellular and intracellular compartments, respectively. In this equation, km is a rate constant for irreversible metabolism, kpe and kep are the rate constants for analyte transport between the extracellular fluid and plasma, and kr and ku are the rate constants for transport between the extracellular and the intracellular compartments by release (kr) and uptake (ku). The components of the differential mass balance equation are intended to describe all factors that affect the concentration profile of the analyte with the following assumptions: metabolism is linear; tissue binding is rapid and linear; tissue diffusion occurs mainly through the extracellular space; the sampled substance may enter or leave tissue by transport into microvessels that are uniformly distributed in tissue; no sources or sinks for the substance exist in the dialysis membrane; and axial diffusion in the perfusate is minor (Bungay et al., 1990; Morrison et al., 1991).
An important assumption in the above mass balance equation is that terms for analyte degradation are dependent on the extracellular concentration (terms containing ku, km, and kep), whereas the terms for analyte generation are dependent on intracellular concentration (i.e., terms containing kpe and kr). This means that the analyte depletion caused by the probe will affect analyte degradation but not analyte generation in tissue falling within the depletion zone (the area defined by r in previous equations). The concentration profile described by the solution of the mass balance equation will provide a measure of probe efficiency, providing information on the relative loss of analyte concentration between the undisturbed tissue and the probe, as well as the area over which the concentration profile will change the efficiency of the probe (relative recovery and penetration distance).
Since the mass balance equation assumes that terms for analyte degradation are dependent on the extracellular concentration, whereas terms for analyte generation are dependent on intracellular concentration, analyte depletion caused by the probe affects degradation, but it does not affect the generation of the analyte in tissue located within the depletion zone. Thus, any external manipulation that changes the rate of degradation will change the steady state concentration profile (slope and distance) and, in turn, the efficiency of the probe (Ed). Increases in analyte clearance increase probe efficiency while decreases in analyte clearance decrease probe efficiency.
The spatial resolution of the probe, or penetration distance (Γ), also changes when the rate of analyte clearance changes. This relationship can be expressed as (Bungay et al., 1990):
where kep is the first-order rate constant representing efflux to the microvasculature, km is the first-order rate constant representing irreversible extracellular metabolism, and ku is the first-order rate constant representing the effect of uptake. Γ is used here to define Δr. This shows that the penetration depth of the concentration gradient induced by the probe is reduced as the rate constants increase. Thus, as the rate of analyte clearance increases, the penetration depth, Γ, decreases and resistance, Re, increases.
Two important predictions arise from the theoretical considerations of tissue resistance. Firstly, experimental manipulation that changes the analyte clearance rate will change Ed. If analyte clearance is altered, dialysate concentration of the analyte will reflect not only changes in extracellular concentration but also a change in analyte clearance. (Note that these changes may be in opposite directions. An increase in transmitter uptake would increase Ed but decrease extracellular concentration.) Secondly, it suggests that the penetration depth of the concentration gradient in tissue will be analyte dependent and potentially variable under conditions when the experimental manipulation modifies analyte clearance. This may be a limiting factor in instances where small areas of tissue are being sampled (e.g., brain nuclei) in that the “undisturbed tissue” may be outside the area of interest. Note that for substances such as neurotransmitters, which are tightly regulated, clearance rates tend to be high, and therefore penetration depths shallow (see Bungay et al., 1990; Dykstra et al., 1992 for discussion).
Theoretical consideration of the processes underlying diffusion during microdialysis has advanced greatly and provided new insight into the complexities of interpreting microdialysis data. Additional discussion is available in Morrison et al. (1991) and in reviews by Justice (1993) and Parsons and Justice (1994). Morrison et al. (1991) extended the mathematical model derived by Bungay et al. (1990) to the transient condition. Empirical evaluation of these theoretical constructs has shown that pharmacological manipulations, which specifically affect the rate of analyte clearance of monoamines and acetylcholine, change Ed in the predicted direction (Parsons and Justice, 1992; Cosford et al., 1994; Smith et al., 1992; Bruhn et al., 1995; Vinson and Justice, 1997). In general, it is advisable to use methods of microdialysis that provide in vivo estimates of relative recovery, particularly when group differences in the rate of analyte clearance are suspected (e.g., following manipulations that affect neurotransmitter uptake) and/or recovery is low. In this way, it will be possible to compare dialysate levels and in vivo recoveries to test hypotheses regarding extracellular concentration. Finally, an important outcome from the theoretical treatment of the underlying processes guiding microdialysis is that it is possible to use in vivo recovery, Ed, as an index of the rate of analyte clearance. Studies using electrochemical or radioligand uptake techniques have shown that changes in Ed provide a sensitive index of changes in dopamine uptake (Chefer et al., 2000, 2005; Thompson et al., 2000).
EMPIRICAL METHODS FOR DETERMINING IN VIVO RELATIVE RECOVERY
Estimates of in vivo probe efficacy (extraction fraction, Ed) are important for two purposes. First, knowledge of in vivo Ed provides a way of accurately converting dialysate levels to extracellular concentration and, thus, serves as a calibration tool. Second, as discussed above (see Principles Underlying Microdialysis), when Ed is low, changes in the in vivo Ed parallel changes in the resistance of tissue to analyte diffusion. Changes in tissue resistance, in turn, may reflect underlying differences in the metabolism and clearance of the analyte (Justice, 1993; Cosford et al., 1996; Vinson and Justice, 1997).
In early studies, estimates of in vivo Ed were obtained from measurements of in vitro Ed. However, it was soon recognized that the main source of resistance to diffusion during microdialysis arises from the external medium (Jacobson et al., 1985; Benveniste, 1989) and not the dialysis membrane. Therefore, estimates of Ed by in vitro calibration were inaccurate. Several groups have developed empirical methods to directly determine in vivo Ed (Jacobson et al., 1985; Lonnroth et al., 1987, 1989; Larsson, 1991). These methods are described below. In addition, the use of in vitro assays to estimate in vivo Ed is reconsidered.
No-net-flux or zero-net-flux microdialysis
Typically, all brain microdialysis experiments can be divided into two basic types: conventional microdialysis and no-net-flux microdialysis. In conventional microdialysis, neurotransmitter-free aCSF is perfused through the probe and the concentration of neurotransmitter that diffuses into the probe is measured. In the no-net-flux or zero-net-flux method of quantitative microdialysis, aCSF containing several different concentrations of neurotransmitter that bracket the anticipated concentration in the extracellular space is perfused through the probe and the amount of analyte gained to or lost from the probe is determined (Lonnroth et al., 1987; Justice, 1993).
Although conventional microdialysis provides valuable information regarding relative changes in neurotransmitter efflux in discrete regions of the brain as a function of time and/or pharmacological manipulation, it does not take into account alterations of the Ed of the microdialysis probe in vivo, which can lead to an over- or under-estimation of actual extracellular levels. Theoretical considerations described above (also see Bungay et al., 2003 for review) and empirical observations (Smith and Justice, 1994) have shown that changes in monoamine re-uptake, but not release or metabolism, modify Ed. Increased dialysate concentrations, therefore, may reflect increased uptake and/or increased extracellular monoamine levels and manipulations that increase uptake and decrease release may result in no apparent change in dialysate levels although extracellular concentrations are reduced. This issue is of considerable importance when using microdialysis to examine the effects of manipulations that affect neurotransmitter transporters.
In contrast to conventional microdialysis, the no-net-flux method of quantitative microdialysis (UNIT 7.2) provides an estimate of extracellular neurotransmitter concentration that is not confounded by changes in analyte clearance. Using this method, an in-vivo Ed value is also obtained and it provides a measure of analyte clearance (Fig. 7.1.2). The Ed is the slope of the linear regression that describes the dialysate concentration of the analyte under study as a function of experimenter-controlled variations in perfusate analyte concentration. The net difference between the perfusate concentration and the dialysate concentration of the analyte is assumed to result solely from the gain or loss of analyte by diffusion across the microdialysis probe membrane. The direction of diffusion (into or out of the probe) is dictated by the undisturbed tissue concentration and it could be either a gain of analyte (if the perfusate concentration is lower than the tissue concentration) or a loss of analyte (if the perfusate concentration is greater than the tissue concentration). In this approach, also referred to as the variation in concentration method (Justice, 1993; Parsons and Justice, 1994), analysis is accomplished by perfusing several concentrations of the analyte of interest through the dialysis probe (Cin) in random order, and determining the net change in perfusate concentration after dialysis (Cin – Cout), where Cout is the dialysate concentration for each Cin. The slope, defined by the linear regression equation describing Cin – Cout (net change) as a function of Cin (starting perfusate concentration), provides the proportional difference between dialysate concentration and the undisturbed tissue concentration of analyte (Cext, the “undisturbed” tissue concentration). This proportion describes the transport of analyte to and from the probe and is defined mathematically by:
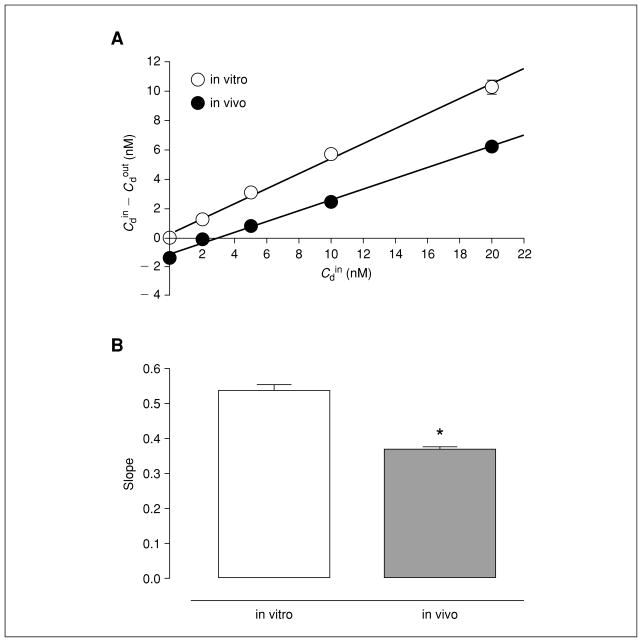
Figure 7.1.2.
No-net-flux microdialysis of DA under well-stirred conditions in vitro at 37°C and in vivo in mouse nucleus accumbens (vehicle-treated controls). (A) Plot of the average gain or loss of DA ( ) to or from perfusate and the average linear regression fit of the data: in vitro (open circles), in vivo (filled circles). The slope of the regression line represents the extraction fraction ( in vitro, Ed in vivo). (B) Bar graph of extraction fraction values expressed as the mean ± S.E.M. (*) Denotes significant difference in between in vitro and in vivo measurements. Reproduced with permission from Chefer et al., 2006.
See UNIT 7.2 for more details on the application of this technique under steady-state and transient conditions (see Principles Underlying Microdialysis above, or Bungay et al., 1990, for a more detailed mathematical description of Ed).
Group comparison of Ed values can easily be made on data collected under steady-state and transient conditions. Typically, under steady-state conditions, all data necessary for calculation of the linear regression equation are collected in each subject. Therefore, an Ed value is obtained for each subject and can be treated as any other dependent variable. Under transient conditions, the data necessary for calculation of the linear regression equation are collected from a group of subjects. In this case, Ed is obtained for each group and can be compared using standard regression statistics that test for parallelism of slopes. Group differences in Ed would suggest that probe efficacy varies as a function of group, and differences in analyte levels of dialysates cannot solely be attributed to an alteration of extracellular concentration (Justice, 1993).
The difference technique is easy to apply and analysis of in vivo Ed data is straightforward. Moreover, when group differences in Ed are observed, it is possible to use the Ed as a calibration tool to correct dialysate data to obtain extracellular concentrations, and to test hypotheses concerning group differences in extracellular concentration.
Flow Rate Method
The flow rate method was one of the first empirical methods designed to determine extracellular concentration by determining the relationship between perfusate flow rate, the active area of the membrane, and the mass transfer coefficient and then extrapolating to the case of zero flow rate (Jacobson et al., 1985). The dialysate concentration at zero flow rate is expected to be equal to the extracellular concentration of undisturbed tissue. The equation:
is written to describe Ed, where Cout is the concentration in the dialysis probe, Cext is the concentration of analyte in undisturbed tissue, K0 is the mass transfer coefficient, A is the active area of the microdialysis probe membrane, and F is the perfusion flow rate. This equation can be related to the mathematical model of Ed described above (see Principles Underlying Microdialysis above). Here K0A is equivalent to 1/(Rd + Rm + Re), and the entire expression is equivalent to Ed (Bungay et al., 1990).
The above equation describes a nonlinear regression to a set of dialysate data collected at varying flow rates. The nonlinearity is not surprising given that the source of greatest resistance will vary by flow rate. The source of maximum resistance to transport of the analyte when Ed is low will lie in the external solution, while the source of maximum resistance to transport of the analyte when Ed is high will lie in the membrane. This is a limiting factor for the use of this technique since it does not yield a single best fit as in linear regression (Parsons and Justice, 1994). Furthermore, most of the data should be obtained at very low flow rates (<1 μl/min) to increase the accuracy of the regression analysis in its most dynamic range. Unfortunately, low flow rates require long collection periods to obtain appropriate sample volumes. Some attempts to overcome these limitations have been addressed; however, this technique has had limited application (Stahle et al., 1991; Menacherry et al., 1992).
Internal Standard Method
The internal standard method measures Ed by measuring the loss of an internal standard from the perfusate (Larsson, 1991; Scheller and Kolb, 1991). Ed is given as the relative loss of the standard (RL) as shown by:
where Cin is the starting concentration of the internal standard and Cout is the dialysate concentration of the internal standard. The internal standard is usually a radiolabeled version of the analyte of interest. The assumption is that perfusion of the internal standard across the dialysis membrane is not limited regardless of the amount of unlabeled analyte present. This assumption has received support from in vitro studies in which increasing concentrations of the analyte of interest in the test solution did not change RL (Scheller and Kolb, 1991). With this method it is necessary to show that the labeled standard is diffusionally similar to the unlabeled analyte (e.g., has a similar diffusion coefficient through the membrane and similar clearance mechanisms in tissue). In addition, one must avoid high concentrations that would tend to make the membrane the limiting site of diffusion in the overall diffusional path (Le Quellec et al., 1995; Lonnroth and Strindberg, 1995).
In Vitro Recovery Method
When the primary resistance of the membrane is greater than the resistance of the external medium, then no difference between in vitro recovery and in vivo recovery is expected. Circumstances in which this might occur are found in microdialysis protocols that use very slow flow rates and/or long dialysis fibers, or diffusionally resistant membranes (e.g., when high-molecular-weight substances are sampled or membranes with very small pore sizes are used). Unfortunately, use of a highly resistant membrane is typically not possible, because Ed becomes so low that the detection of the analyte is compromised (Bungay et al., 1990). Slow perfusate flow rates yield nearly 100% recovery but require a long collection period, or very small sample volume, which may compromise other aspects of the experiment (Menacherry et al., 1992). In vitro recovery methods may become more advantageous as more sensitive detection sampling and storage methods become available.
FACTORS AFFECTING IN VIVO RECOVERY
During microdialysis, analytes pass through a semipermeable membrane from the extracellular fluid (ECF) into a perfusate that is collected over a predetermined time and volume. Because the membrane is semipermeable, only some solutes, namely low-molecular-weight solutes, will be recovered. The concentration of analyte collected by this method will represent only a fraction of the ECF concentration.
The factors determining the amount of analyte recovered during microdialysis are described below. Consideration of these factors during the design phase of a microdialysis experiment will greatly enhance the success of that experiment (see Strategy for Determining Experimental Design) by assuring detectable levels of analyte in each dialysate sample. Furthermore, since a major assumption underlying conventional microdialysis is that analyte recovery is at a steady state throughout the sampling period, understanding the factors that contribute to changes in analyte recovery can aid experimental design and data interpretation.
Flow Rate
Relative recovery of an analyte (concentration of analyte per sample) is inversely proportional to the perfusate flow rate (Johnson and Justice, 1983; Tossman et al., 1986; Wages et al., 1986; Alexander et al., 1988; Benveniste, 1989). Furthermore, at lower flow rates the net depletion of solutes around the probe decreases. For analytes with low extracellular concentration or low diffusivity, reducing the flow rate will increase the relative recovery and, therefore, increase the probability of obtaining a detectable concentration of analyte in each sample.
At extremely low flow rates (<0.1 μl/min) it is possible to reach near 100% recovery of an analyte, in which case the dialysate concentration of that analyte would equal the ECF analyte concentration (Van Wylen et al., 1986; Menacherry et al., 1992; Smith et al., 1992). The flow rate necessary to achieve 100% relative recovery depends on both the diffusional characteristics of the analyte under study, as well as membrane type and length. Smith et al. (1992), using a 4-mm regenerated cellulose membrane in rat striatum, perfused aCSF at a rate of 0.057 μl/min and obtained dialysate concentrations of dopamine and its metabolite, 3,4-dihdroxyphenylacetic acid (DOPAC), similar to the in vivo extracellular concentration of these analytes (as estimated by the difference method). Van Wylen et al. (1986) using a rate of 0.1 μl/min obtained almost 100% recovery of adenosine in rat striatum. These flow rates do not typically yield sufficient time or volume resolution to be practical in most microdialysis experiments. Furthermore, for many analytes, low flow rates are not necessary to achieve detectable concentrations in dialysates. However, for situations in which long times and/or low volume sample handling are possible, slow flow rates offer the advantage of providing direct measurements of extracellular concentration.
The absolute recovery of an analyte (amount of analyte per sample) is proportional to the perfusate flow rate, up to flow rates of 2 μl/min (Wages et al., 1986; Benveniste, 1989). Thus, for analytical assays in which the total amount of analyte, rather than concentration, is measured (e.g., radioimmunoassay) it may be advantageous to use higher, rather than lower, flow rates (up to 2 μl/min) to achieve a sufficient quantity of sample for detection. However, at these higher flow rates, probe efficiency is reduced (probe efficiency is equivalent to relative recovery), and the probe may be more sensitive to changes in the diffusional characteristics of the analyte in tissue.
Microdialysis Probe Membrane Properties (Weight Cut-Off and Membrane Surface Area)
Recovery is proportional to the membrane surface area of the probe (Hamberger et al., 1983; Johnson and Justice, 1983; Sandberg and Lindstrom, 1983; Ungerstedt, 1984; Tossman et al., 1986; Kendrick, 1989, 1990), assuming no change in the homogeneous nature of the tissue surrounding the probe. More often than not, the length of the microdialysis probe is limited by the size of the structure under study.
Probe membrane material may also affect analyte recovery (Ungerstedt, 1984; Kendrick 1989, 1990; Hsiao et al., 1990; Mason and Romano, 1995). The membrane materials currently used in microdialysis probes include regenerated cellulose (Cuprophan from Gambro AB), polyacrylonitrile (PAN), and polycarbonate-ether (proprietary to CMA/Microdialysis). Kendrick (1989, 1990) determined the in vitro recovery for a large number of analytes using probes representative of each type of material and found a great degree of variation in recovery among probe types. These results could be accounted for by differences in the diffusional properties of the analyte in the membrane (see also Mason and Romano, 1995). In vivo, however, relative recovery may be more a function of the diffusional characteristics of the analyte in tissue than in the membrane, and so similar differences in recovery among membrane types may not occur in vivo.
Hsiao et al. (1990) compared the in vitro and in vivo recovery of acetaminophen, DOPAC, hydroxyvalproic acid (HVA), and 5-hydroxyindoleacetic acid (5-HIAA) among the three membrane types and found large, significant differences in the in vitro relative recovery. In contrast, no consistent differences in recovery were found in vivo. The latter results suggest that membrane material may have little impact on in vivo recovery under conditions in which the maximum diffusional resistance resides in the tissue, as is typically true for low-molecular-weight solutes. However, as molecular weight increases or if hindrance to analyte diffusion in the membrane increases (e.g., a “sticky” compound; Kendrick, 1989, 1990), significant differences in probe efficiency (relative recovery) as a function of membrane material would be predicted, since the greatest source of diffusional resistance to the analyte would be found in the membrane (Bungay et al., 1990).
Analyte Properties
A large number of analytes have been collected by microdialysis (see Table 7.1.1). Reports of relative recovery for different analytes vary considerably. This is due, in part, to differences among laboratories in the microdialysis procedure used (i.e., flow rate, membrane length). However, substantial differences in recovery as a function of molecular weight and hydrophobicity can also be shown. By far, the most complete analysis of recovery by analyte type was performed by Kendrick (1989), who determined the in vitro relative recovery of >40 analytes under similar conditions (2 μl/min flow rate using the same probe immersed in a solution maintained at 37°C). By analyte class, relative recovery was highest for amino acids (33% to 40%), followed by monoamines (22% to 30%), and then neuropeptides (1.5% to 24%).
Kendrick (1989) observed a strong negative linear relationship between molecular weight and the log percent recovery, suggesting that one explanation for the differences in relative recovery among analytes may be molecular weight. This is not surprising given the inverse relationship between molecular weight and the diffusion coefficient: as molecular weight increases the diffusion coefficient decreases. The lowest relative recoveries were observed for neuropeptides that had greater hydrophobic properties (“sticky”), suggesting that, for these analytes, hindrance may be a significant impediment to their collection by microdialysis. Dialysate levels obtained from sticky analytes also respond slowly to changes in the external concentration of the analyte (particularly decreases) and generally require considerably longer periods of equilibration to achieve a steady level of recovery (Kendrick, 1989; Thompson et al., 1995). (Note that recovery may be concentration dependent for sticky analytes, confounding the interpretation of experimental manipulations on dialysis data.)
Impact of Temperature on the Velocity of the Diffusion Process
The relative recovery for different molecules can differ according to their diffusion coefficient, which is directly proportional to temperature. On average, a 1% to 2% increase in the diffusion coefficient is observed for every degree Celsius increase in temperature (Bard and Faulkner, 1980). Significant differences in relative recovery between solutions maintained at 23° and 37°C have been observed during in vitro assays for a number of analytes (Wages et al., 1986; Alexander et al., 1988; Benveniste et al., 1989; Lindefors et al., 1989b; Parry et al., 1990). Analyte recovery evaluated at room temperature (23° to 25°C) underestimates in vivo analyte recovery. Since the goal of an in vitro assay is to correspond as closely as possible to the in vivo condition, recovery should be assessed at the in vivo temperature (typically 37°C) and microdialysis experiments should be carried out at a constant, body temperature.
Tissue Factors
Diffusion in tissue is typically slower than in an aqueous solution. This is due, in great part, to the reduced fluid volume and increased diffusional path (or tortuosity) characteristic of tissue (Nicholson and Rice, 1986; Amberg and Lindefors, 1989; Lindefors et al., 1989a; Benveniste and Huttemeier, 1990). Moreover, diffusion in tissue may be slowed further by analyte binding to cell surface proteins along the diffusional path (Rice et al., 1985). This means in vivo recovery of an analyte should be less than in vitro recovery of that analyte in a stirred solution held at 37°C. However, these factors alone do not adequately describe the difference between in vitro and in vivo estimates of relative recovery, perhaps because, in tissue, diffusion of an analyte is also influenced by the rate of analyte clearance (Bungay et al., 1990; Morrison et al., 1991; Dykstra et al., 1992). As the rate of analyte clearance increases, resistance to diffusion decreases. Diffusivity in tissue, then, is somewhat greater for substances, like neurotransmitters, that are rapidly cleared from the extracellular space.
The extent to which relative recovery, or probe efficiency, during in vivo microdialysis is affected by tissue factors depends on which medium (tissue, membrane, or perfusate) has the greatest resistance to diffusion. When recovery is low, most low-molecular-weight hydrophilic substances will meet the greatest diffusional resistance in tissue. In these cases, changes in the physiological characteristics of tissue (i.e., fluid volume, tortuosity, hindrance, and rate of analyte clearance) that affect diffusional resistance will modify probe efficiency in a given microdialysis experiment. This leads to two problems. Firstly, decreases in recovery in vivo because of reduced fluid volume, increased tortuosity, and/or increased hindrance in tissue may reduce the dialysate levels of the analyte below the sensitivity of analytical detection. Second, experimental treatments (e.g., lesions, drug treatments) or procedures (e.g., implantation of the microdialysis probe) that affect these physiological characteristics of the tissue may lead to changes in probe efficiency.
IN VITRO AND IN VIVO RECOVERY AND THEIR SIGNIFICANCE FOR DATA INTERPRETATION
One of the advantages of the no-net-flux procedure relative to conventional microdialysis is that Ed is obtained in situ. Importantly, the Ed value is sensitive to changes in analyte clearance from the extracellular space. The relationship between Ed and processes that control neurotransmitter clearance was convincingly demonstrated by Smith and Justice (1994) in studies of the nucleus accumbens (NAc). These investigators showed that, in contrast to the change in extracellular DA concentrations that occurs in response to pharmacological manipulations that inhibit synthesis, release, or metabolism of DA, treatments that reduce DA uptake decrease Ed. The finding that 6-hydroxydopamine lesions of the NAc produce a ~50% reduction in the Ed for dopamine provided further evidence of the sensitivity of Ed to changes in uptake (Parsons et al., 1991b). Subsequent studies have shown that the relationship between Ed and changes in analyte clearance extend to other neurotransmitters. The Ed for norepinephrine was reduced by administration of the norepinephrine uptake inhibitor, desipramine, and the Ed for serotonin diminished following treatment with the serotonin uptake inhibitor, paroxetine (Cosford et al., 1996). Administration of neostigmine to inhibit acetylcholinesterase, the enzyme responsible for clearance of acetylcholine, lowered the Ed for this neurotransmitter in rat striatum (Vinson and Justice, 1997). These experimental observations are in accord with mathematical models of microdialysis, which predict that the magnitude of Ed for any applicable analyte is dependent upon the efficiency of processes that remove the analyte from the extracellular space. The study of Smith and Justice (1994) supports the equally important corollary prediction that Ed is independent of processes that supply the analyte to the extracellular space, if the rate of supply is independent of the extracellular analyte concentration (zero-order assumption). The mathematical models predict, as well, that enhancing clearance processes should increase Ed (see Bungay et al., 2003, 2007). The experiments mentioned above all relate to diminishing the rate of analyte removal from the extracellular space. However, the predicted effect on Ed of enhanced clearance was borne out by a study showing an increase in the Ed for dopamine in the rat NAc following 10 days of cocaine administration (Parsons et al., 1991a). Increases in Ed were also observed in rat medial prefrontal cortex following repeated, intermittent administration of cocaine (Chefer et al., 2000), and in mouse NAc following acute administration of the D3-preferring agonist (+)-PD128907 (Zapata and Shippenberg, 2002). More recently, a significant augmentation of Ed was observed in mouse NAc in response to pharmacological inactivation or genetic deletion of kappa-opioid receptors (Chefer et al., 2005). In each of theses cases, microdialysis theory was used to interpret the increase in Ed as representing a treatment-induced augmentation in DA uptake in vivo. The increase in dopamine uptake was confirmed in vitro using radioligand uptake or electrochemical techniques.
The utility of Ed measurements for the detection of enhanced dopamine uptake was recently questioned by Tang et al. (2003). These investigators determined the Ed for dopamine in vivo in the NAc of freely moving rats and obtained Ed values in vitro for the same probes immersed in a well-stirred medium maintained at 37°C. There was no statistically significant difference between the mean in vivo and in vitro Ed values, suggesting that the limiting factor under both conditions was the mass transport properties of the probe. Since tissue processes were apparently not contributing to Ed in vivo, the authors concluded that Ed is insensitive to increases in the rate of DA uptake in the vicinity of the microdialysis probe. This finding appears to conflict with the observations cited in the preceding paragraph, as an increase in Ed can only be detected if the Ed values in vivo for both control and treatment conditions are less than the corresponding well-stirred in vitro values. However, in vitro values were not measured in the prior studies showing increases in Ed in vivo. A recent study by Chefer et al. (2006) addressed this controversy by comparing in vitro Ed values obtained under well-stirred conditions to in situ values in the mouse NAc, and determining if increases in Ed can be observed in one test case [treatment with the kappa-opioid receptor antagonist, norbinaltorphimine (nor-BNI)]. Another objective of this study was to determine whether the magnitude of increase in dopamine uptake produced by nor-BNI treatment could be quantified based on a mathematical model of dopamine microdialysis in which the difference between in vivo and in vitro Ed values is used to isolate the specific tissue contribution to Ed. The results clearly showed that in vitro Ed values obtained at 37°C under well-stirred conditions were significantly higher than in vivo Ed values for the same microdialysis probes in mouse NAc, and that increases in Ed could be detected in vivo. These observations suggest that in vitro probe characterization is especially important for assessing changes in dopamine uptake, because the in vitro Ed value obtained under well-stirred conditions delineates the upper limit for Ed in vivo.
Few microdialysis studies have actually directly compared the in vitro and in vivo characteristics of the same microdialysis probes, and the results of these were inconsistent. Sam and Justice (1996) reported that Ed values were less in vitro than in vivo in the striatum of the anesthetized rat, but the same probes were not used in both experiments. Tang et al. (2003) found no statistically significant difference between values in vitro and in vivo in the NAc of the awake rat, whereas Bungay et al. (2003) described a relatively small difference in Ed measurements in vitro and in vivo in anesthetized rat striatum. In all three studies, in vitro measurements were made at 37°C in stirred or flowing media. A number of factors could contribute to the apparent lack of agreement among the studies. First are the species difference (rat versus mouse) and the difference in the type of microdialysis probes employed: CMA 7 in the more recent experiments (Chefer et al., 2006) versus handmade probes in the prior studies (Sam and Justice, 1996; Bungay et al., 2003; Tang et al., 2003). Differences in perfusion flow rate (0.6 μl/min versus 2.0 μl/min), the method of microdialysis probe insertion (gentle restraint of awake animals versus halothane anesthesia), and the concentrations of neurotransmitter used may also be factors which contributed to the different results obtained.
Overall, the results of Chefer et al. (2006) show that no-net-flux microdialysis can be an effective technique for monitoring increases, as well as decreases in dopamine uptake that occur in discrete regions of the brain. The finding that both the post-treatment and control Ed in vivo values were less than the well-stirred in vitro value demonstrates that an assessment of both increase and decrease in uptake is feasible with this method. Further, they show that determination of the ECS clearance rate constant (kt) may provide more sensitive information than Ed regarding the magnitude of uptake changes. However, additional studies are needed to evaluate the use of this parameter for more precise quantification of changes in neurotransmitter dynamics that occur in response to particular pharmacological or genetic manipulations.
Finally, it should be noted that in addition to monoamines, no- net-flux microdialysis has been used to quantify alterations in basal dynamics of excitatory amino acids (Melendez et al., 2005; Szumlinski et al., 2005) and GABA (Xi et al., 2003).
THE TRAUMA LAYER SURROUNDING THE MICRODIALYSIS PROBE
Michael and co-workers (Lu et al., 1998; Peters and Michael, 1998; Yang et al., 1998, 2000; Borland et al., 2005) have used small voltammetric microsensors placed 1 mm from, immediately adjacent to, and at the outlet of microdialysis probes acutely implanted in the striatum to examine the influence of trauma on microdialysate measures. They showed that dopamine release sites adjacent to the microdialysis probe were damaged compared to the corresponding uptake sites in the same region. The authors proposed that a trauma layer surrounding the microdialysis probe suppresses dopamine release and that uptake removes much of the dopamine diffusing toward the probe from adjacent tissue. As a result, the no-net-flux intercept for dopamine underestimates normal tissue extracellular concentration. They provided evidence that disruption associated with probe implantation extends at least 220 to 250 μm from the probes and possibly as far as 1 mm from the probe. This work has called into question the use of Ed in determining extracellular concentrations because it suggests that Ed may not equal relative recovery. Consequently, other laboratories (Bungay et al., 2003; Chen, 2005a,b) have begun to examine the effects of tissue trauma on the sampling composition and accuracy of microdialysis methods. Bungay et al. (2003) used mathematical modeling to quantify the impact of trauma on estimates of tissue extracellular concentrations of dopamine. Modeling predicted that the trauma layer would cause the dopamine Ed obtained from in vivo calibration techniques, such as no-net-flux, to differ from relative recovery and lead to an underestimation of extracellular concentration in the surrounding normal tissue. However, Chen (2005a,b; 2006) showed that while destruction of the neurotransmitter release process always reduces the relative recovery, it still may rise or fall with uptake inhibition in the tissue depending on the ratio of the remaining neurotransmitter release to that of uptake in the tissue trauma layer. Subsequently, extracellular neurotransmitter concentration may be over- or underestimated, respectively. Thus, it would appear that, although no-net-flux microdialysis may not predict actual neurotransmitter levels, it does permit analysis of relative changes in extracellular neurotransmitter levels, as well as changes in neurotransmitter reuptake.
PERFUSATE COMPOSITION
Perfusate composition is important because its components diffuse into the tissue in the same way that the analyte of interest diffuses into the microdialysis probe. Furthermore, any solute in the extracellular fluid that is small enough to pass through the dialysis membrane, and is in a lower concentration than that in the perfusate, will diffuse into the perfusate and be depleted from the extracellular fluid surrounding the probe. This, in turn, may disturb the homeostatic balance of the extracellular environment.
Ideally, the perfusate should contain the exact concentration of all solutes that diffuse through the probe membrane and that are found in the extracellular fluid, except of course the solute meant to be sampled. In practice, however, this is neither possible nor pragmatic. Instead, microdialysis perfusates are most often made with the ionic composition and pH of plasma (Ringer’s solutions) or mock aCSF. Maintaining proper ionic composition is critical in the evaluation of neurochemical events. The ionic milieu of neuronal extracellular fluid differs from plasma in both concentration and lability (Bradbury, 1979; Wood and Wood, 1980). Numerous reports have shown that small differences in perfusate Ca2+ concentration produce marked effects on basal neurotransmitter levels (Westerink et al., 1988; Moghaddam and Bunney, 1989; Osborne et al., 1991; Westerink, 2000). Moreover, changing the concentration of K+ in the perfusate is a standard technique used to induce neuronal release of many neurotransmitters (Spanagel et al., 1990; Parsons and Justice, 1992; Thompson et al., 1995). Interestingly, Obrenovitch et al. (1995) showed that the concentration of ions in aCSF might act to “buffer” experimentally induced changes in the ionic composition of neural tissue (i.e., spreading depression). In this case, increasing the K+ concentration in the perfusate was necessary to permit the development of spreading depression in neural tissue. These effects are not surprising given the importance of ion concentration and distribution in the regulation of neuronal activity and emphasize the importance of maintaining appropriate ionic concentrations.
Information regarding the issue of solute depletion around the probe is limited. The magnitude of solute depletion is directly related to the flow rate. Sam and Justice (1996) used this relationship to directly test the effect of increasing the solute depletion (by increasing the flow rate) on basal extracellular dopamine concentration in the rat striatum. They found that changes in the amount of total solute depletion around the probe did not, in fact, affect basal extracellular dopamine concentrations. More research is necessary to determine how well this finding generalizes to other neurotransmitters. It is known that the extracellular concentrations of many analytes are regulated by other neurochemicals in the interstitial space. Thus, during microdialysis, there could be depletion of a substance or substances that in turn regulate the analyte under study.
Finally, it should be noted that the perfusate can be used to deliver agents into tissue, as well as to provide preservative agents (e.g., antioxidants) or other substances that aid in the recovery of the analyte once it has diffused into the perfusate (e.g., bovine serum albumin or antibodies). Microdialysis probes can be used to locally administer either a continuous or a pulse infusion of drugs. One advantage of this delivery method over microinjections is that the drug is delivered by measurable parameters of diffusion. As a result, precise calculations of the “dose” and area affected can be determined (Bungay et al., 1990).
Preservatives are usually not diffusible through the membrane, thus eliminating the possibility of adding an undesirable compound to the tissue. Bovine serum albumin, which is too large to pass through most microdialysis membranes, has been used to reduce the adsorption of sticky analytes to the probe tubing and collection vials (Kendrick, 1989). Antibodies have also been used for similar purposes (Lambert et al., 1994).
Ascorbic acid is frequently included in perfusate when monoamines are being collected to reduce analyte degradation after collection. Ascorbic acid readily diffuses through most microdialysis membranes and it is known to impact on the neurochemical milieu, but it is also found endogenously. Its perfusate concentration should be limited to no more than that found in tissue (~0.2 mM in interstitial fluid). Ascorbate is also electroactive and typically has a short retention time. Samples containing exogenous ascorbate will typically have a long solvent front in HPLC chromatograms. Therefore, standards containing known concentrations of an analyte with ascorbate, or any protective agent, should be tested before adopting such an agent into the experimental protocol.
NEURONAL ORIGIN OF THE SAMPLED NEUROTRANSMITTERS
Because of the dimensions of microdialysis probes, microdialysis does not sample neurotransmitter directly from the synaptic cleft, but detects compounds relatively far away from the site of release. Therefore, a critical question is whether dialysate levels reflect true synaptic release and/or uptake of the measured analyte or overflow from nonsynaptic sources. A classical feature of neuronal activity is membrane depolarization that is initiated by the opening of fast Na+ channels to allow influx of Na+ ions and release of neurotransmitter from vesicles by exocytosis, a process that is Ca2+ dependent. Both the sodium-channel and calcium dependency can be evaluated during microdialysis by retrograde dialysis of the sodium-channel blocker tetrodotoxin (TTX), or by omitting calcium from the perfusion fluid. The TTX- and Ca2+ dependency of biogenic amines sampled by microdialysis has been demonstrated in numerous studies (see Westerink et al., 1988; Westerink, 2000; for review). It can be concluded with certainty that the major part of dopamine, norepinephrine, serotonin, and acetylcholine present in brain dialysates is derived from action-potential controlled release.
Basal dialysate levels of glutamate and GABA show a very low sensitivity or complete insensitivity to TTX and low dependence or independence with respect to calcium (Moghaddam 1993; Morari et al., 1993; Timmerman et al., 1999; Del Arco et al., 2003). However, many microdialysis studies show changes in extracellular concentrations of glutamate and GABA under specific pharmacological and behavioral conditions that could be interpreted as a consequence of neuronal activation (Nash and Yamamoto, 1993; Keefe et al., 1993; Venero and Borrell, 1999; Timmerman et al., 1999; Hotsenpiller et al., 2001). Growing evidence suggests that synaptic glutamate and GABA are only involved in very short distance signaling and therefore may not reach the microdialysis probe, and alterations in dialysate amino acids could reflect changes in the activation of neuron-astrocytic networks (see Del Arco et al., 2003 for review).
STRATEGY FOR DETERMINING EXPERIMENTAL DESIGN
The challenge in designing and implementing a microdialysis experiment is to collect a sample with both sufficient volume and concentration of analyte to permit separation and detection by the analytical technique employed, while still satisfying the experimental design specified by the research question. Therefore, the first step in designing a dialysis experiment is to obtain information regarding the analytical sensitivity and minimum volume of dialysate necessary to isolate and quantify the analyte(s) of interest. Familiarity with the physicochemical properties of the analyte of interest (e.g., molecular weight, lipophilicity) and its distribution, concentration, and clearance from the tissue of interest will greatly facilitate subsequent experimental design.
The volume of the collected dialysis sample is determined by the flow rate (μl/min) of the fluid that is perfused through the probe (perfusate) and the collection period (min). The concentration of analyte in the collected sample is dependent on the relative recovery of the analyte by the microdialysis probe (also referred to as probe efficiency) and the analyte concentration in tissue. An estimate of tissue concentration can generally be obtained from the literature. Relative recovery is dependent on a number of factors that affect diffusion of the analyte from the tissue to the perfusate, including perfusate composition, membrane composition, active area (or length) of the membrane, flow rate, temperature, and, in many cases, diffusional characteristics of the analyte(s) in tissue. Small changes in these factors may produce significant changes in relative recovery of the analyte(s). In addition, other factors such as the material and surface area of the outflow tubing employed, tubing length, and method of sample storage may diminish the actual concentration of an analyte in the sample. For example, some analytes are particularly prone to adsorption to materials such as the surface of the outflow tubing or the inner surface of the collection vial. Others may have low thermal stability (e.g., monoamines that are oxidized) and degrade quickly following collection. Fortunately, many of these problems have been identified in the literature or can be resolved using in vitro assays.
The following strategy is recommended for investigators first setting up microdialysis or for those establishing procedures for the collection of substances not already described in the literature.
Determine the sensitivity of the analytical equipment and the minimum sample volume required for the handling of physiologically relevant levels of analyte.
Determine if any loss of the analyte might occur after its diffusion into the microdialysis probe. This can be assessed by perfusing a known concentration of the analyte of interest through the inflow/outflow tubing into a collection vial. The concentration of analyte in the collection vial should be the same as the starting concentration. A physiologically relevant concentration(s) of the analyte of interest should be used, and any additional equipment, such as a liquid switch, or swivel that the analyte might contact should also be tested in this way. If significant loss of analyte is determined, then the source of the loss can be identified by systematically testing each component of the dialysis setup. Loss of analyte is often the result of surface adsorption to the tubing, collection vial, or any other piece of equipment through which the perfusate flows. However, degradation of the sample by enzymes (from a bacterial source) or physicochemical interactions (e.g., oxidation) with metal or tubing may also contribute to sample loss.
Evaluate the sample storage method. Two issues should be considered. First, some analytes will degrade rapidly at room temperature (e.g., monoamines). Therefore, optimal collection and storage conditions must be determined. Frequently, reducing the temperature of the sample once it is collected or including a protective agent in the collection vial or perfusate will increase analyte stability. For example, the addition of ascorbic acid (typical concentration used is 0.25 mM) to the perfusate or perchloric acid (typical concentration used is 0.1 mM) to the collection vial will protect catecholamines from oxidative degradation. Second, because microdialysis samples tend to be very small (in the microliter range), evaporation of the sample may occur over long collection periods. In this case, it would be advisable to seal the collection vials during sample collection.
Consider the length and diameter of the inflow/outflow tubing. The combination of long, narrow tubing and higher flow rates may produce considerable back pressure in the probe, resulting in ultrafiltration (“sweating”) of the perfusate. The net impact on sample collection will be a reduction in relative recovery. In addition, it is important to know the “dead volume” for each dialysis setup, as this will affect the calculated time course of a given neurochemical response. For example, if the capacity of the outflow tubing is 10 μl and the perfusion rate is 2.0 μl/min, there will be a 5-min lag in measuring the response to a given manipulation.
Choosing a probe membrane. The molecular weight cutoff should be considerably larger than the analyte of interest yet small enough to maintain the semi-permeable nature of the membrane. Several types of membranes are used commercially, and at least one report has shown that some membrane materials may be better suited for the collection of analytes that prove to be sticky, e.g., hydrophobic neuropeptides; see Kendrick (1990) and UNIT 7.2.
Determine the maximum length of active membrane accessible to the tissue under study. The goal here is to increase the surface area through which dialysis occurs. The surface area of a microdialysis probe is increased by increasing the length, and not the diameter, of the probe. In tissue, the maximum length of the probe is generally dictated by the size of the region in which the probe will be implanted. When probe length is not limited by tissue size, the length should be great enough to produce maximum or near-maximum recovery. The effect of probe length on analyte recovery can be easily determined in vitro.
Choose a perfusate that is compatible with the organ system and analyte being measured. As a rule, perfusion fluids should be isoosmotic. Remember that diffusion during microdialysis is bidirectional, so that low-molecular-weight solutes in the perfusate will diffuse out of the probe and into tissue.
Determine the longest collection period that will still permit hypothesis testing. Typically, the longer the collection period the slower the flow rate necessary to yield a sufficient volume for analytical detection.
Choose a flow rate that will yield sufficient volume for the desired collection period. Slower flow rates will increase the relative recovery of the analyte of interest.
Use an in vitro assay to determine if the flow rate and collection period selected yield a sample with a detectable concentration of analyte (use an estimate of actual extracellular tissue concentration).
Consider an in vivo pilot procedure to assure adequate sample recovery before investing in a larger experiment.
LITERATURE CITED
- Abercrombie ED, Zigmond MJ. Partial injury to central noradrenergic neurons: Reduction of tissue norepinephrine content is greater than reduction of extracellular norepinephrine measured by microdialysis. J Neurosci. 1989;9:4062–4067. doi: 10.1523/JNEUROSCI.09-11-04062.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Grothusen JR, Schwartzman RJ. Flow dependent changes in the effective surface area of microdialysis probes. Life Sci. 1988;42:595–601. doi: 10.1016/0024-3205(88)90063-x. [DOI] [PubMed] [Google Scholar]
- Amberg G, Lindefors N. Intracerebral microdialysis: II. Mathematical studies of diffusion kinetics. J Pharmacol Meth. 1989;22:157–183. doi: 10.1016/0160-5402(89)90012-0. [DOI] [PubMed] [Google Scholar]
- Bard AG, Faulkner LR. Electrochemical Methods: Fundamentals and applications. John Wiley & Sons; New York: 1980. [Google Scholar]
- Benveniste H. Brain microdialysis. J Neurochem. 1989;52:1667–1679. doi: 10.1111/j.1471-4159.1989.tb07243.x. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Huttemeier PC. Microdialysis: Theory and application. Prog Neurobiol. 1990;35:195–215. doi: 10.1016/0301-0082(90)90027-e. [DOI] [PubMed] [Google Scholar]
- Benveniste H, Hansen AJ, Ottosen NS. Determination of brain interstitial concentrations by microdialysis. J Neurochem. 1989;52:1741–1750. doi: 10.1111/j.1471-4159.1989.tb07252.x. [DOI] [PubMed] [Google Scholar]
- Bito L, Davson H, Levin E, Murray M, Snider N. The concentration of free amino acids and other electrolytes in cerebrospinal fluid: in vivo dialysis of brain and blood plasma of the dog. J Neurochem. 1966;13:1057–1067. doi: 10.1111/j.1471-4159.1966.tb04265.x. [DOI] [PubMed] [Google Scholar]
- Blomquist L, Dizdar N, Karlsson M, Kagedal B, Ossowicki H, Pettersson A, Smeds S. Microdialysis of 5-S-cysteinyldopa from interstitial fluid in cutaneous human melanoma transplanted to athymic mice. Melanoma Res. 1991;1:23–32. doi: 10.1097/00008390-199104000-00004. [DOI] [PubMed] [Google Scholar]
- Borland LM, Shi G, Yang H, Michael AC. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J Neurosci Meth. 2005;146:149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Bourdelais AJ, Kalivas PW. Modulation of extracellular gamma-aminobutyric acid in the ventral pallidum using in vivo microdialysis. J Neurochem. 1992;58:2311–2320. doi: 10.1111/j.1471-4159.1992.tb10979.x. [DOI] [PubMed] [Google Scholar]
- Bradbury MWB. The Concept of a Blood-Brain Barrier. John Wiley & Sons; New York: 1979. [Google Scholar]
- Bruhn T, Christensen T, Diemer NH. Microdialysis as a tool for in vivo investigation of glutamate transport capacity in rat brain. J Neurosci Meth. 1995;59:169–174. doi: 10.1016/0165-0270(94)00179-k. [DOI] [PubMed] [Google Scholar]
- Brust P, Diemer NH. Blood-brain transfer of L-phenylalanine declines after peripheral but not central nervous administration of vasopressin. J Neurochem. 1990;55:2098–2104. doi: 10.1111/j.1471-4159.1990.tb05801.x. [DOI] [PubMed] [Google Scholar]
- Bungay PM, Morrison PF, Dedrick RL. Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro. Life Sci. 1990;46:105–119. doi: 10.1016/0024-3205(90)90043-q. [DOI] [PubMed] [Google Scholar]
- Bungay PM, Newton-Vinson P, Isele W, Garris PA, Justice JB. Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. Microdialysis of dopamine interpreted with quantitative model incorporating probe implantation trauma. J Neurochem. 2003;86:932–946. doi: 10.1046/j.1471-4159.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungay PM, Morrison PF, Dedrick RL, Chefer VI, Zapata A. Principles of quantitative microdialysis. In: Westerink BHC, Cremers TIFH, editors. Handbook of Microdialysis: Methods, Applications and Perspectives Vol. 16: Handbook of Behavioral and Neuroscience. Academic Press/Elsevier B.V.; Amsterdam: 2007. pp. 131–167. [Google Scholar]
- Callaghhan DH, Yergey JA, Rousseau P, Masson P. Respiratory tract eicosanoid measurement using microdialysis sampling and GC/MS detection. Pulm Pharmacol. 1994;7:35–41. doi: 10.1006/pulp.1994.1003. [DOI] [PubMed] [Google Scholar]
- Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, Abi-Saab WM. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol. 2005;57:226–235. doi: 10.1002/ana.20380. [DOI] [PubMed] [Google Scholar]
- Cebada J, Alvarado-Alvarez R, Becerra E, Neri-Bazán L, Rocha L, García U. An improved method for long-term measuring of hemolymph fluctuations of non-essential amino acids, GABA and histamine from freely moving crayfish. J Neurosci Meth. 2006;153:1–7. doi: 10.1016/j.jneumeth.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Moron JA, Hope B, Rea W, Shippenberg TS. Kappa-opioid receptor activation prevents alterations in mesocortical dopamine neurotransmission that occur during abstinence from cocaine. Neuroscience. 2000;101:619–627. doi: 10.1016/s0306-4522(00)00417-6. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci. 2005;25:5029–5037. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Zapata A, Shippenberg TS, Bungay PM. Quantitative no-net-flux microdialysis permits detection of increases and decreases in dopamine uptake in mouse nucleus accumbens. J Neurosci Meth. 2006;155:187–193. doi: 10.1016/j.jneumeth.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Chen Y, Graham DI, Stone TW. Release of endogenous adenosine and its metabolites by the activation of NMDA receptors in the rat hippocampus in vivo. Br J Pharmacol. 1992;106:632–638. doi: 10.1111/j.1476-5381.1992.tb14387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC. Preferentially impaired neurotransmitter release sites not their discreteness compromise the validity of microdialysis zero-net-flux method. J Neurochem. 2005a;92:29–45. doi: 10.1111/j.1471-4159.2004.02847.x. [DOI] [PubMed] [Google Scholar]
- Chen KC. Evidence on extracellular dopamine level in rat striatum: Implications for the validity of quantitative microdialysis. J Neurochem. 2005b;92:46–58. doi: 10.1111/j.1471-4159.2004.02848.x. [DOI] [PubMed] [Google Scholar]
- Chen KC. Effects of tissue trauma on the characteristics of microdialysis zero-net-flux method sampling neurotransmitters. J Theor Biol. 2006;238:863–881. doi: 10.1016/j.jtbi.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Krishna G, Tulsi P, Obata T, Lang K, Huang SJ, Murphy DL. Intracranial microdialysis of salicylic acid to detect hydroxyl radical generation through dopamine auto-oxidation in the caudate nucleus: Effects of MPP+ Free Radic Biol Med. 1992;13:581–583. doi: 10.1016/0891-5849(92)90151-6. [DOI] [PubMed] [Google Scholar]
- Consolo S, Wu CF, Fusi R. D-1 receptor linked mechanism modulates cholinergic neurotransmission in rat striatum. J Pharmacol Exp Ther. 1987;242:300–305. [PubMed] [Google Scholar]
- Cook CJ. Measuring of extracellular cortisol and corticotropin-releasing hormone in the amygdala using immunosensor coupled microdialysis. J Neurosci Methods. 2001;110:95–101. doi: 10.1016/s0165-0270(01)00421-6. [DOI] [PubMed] [Google Scholar]
- Cosford RJ, Parsons LH, Justice JB., Jr Effect of tetrodotoxin and potassium infusion on microdialysis extraction fraction and extracellular dopamine in the nucleus accumbens. Neurosci Lett. 1994;178:175–178. doi: 10.1016/0304-3940(94)90753-6. [DOI] [PubMed] [Google Scholar]
- Cosford RJ, Vinson AP, Kukoyi S, Justice JB., Jr Quantitative microdialysis of serotonin and norepinephrine: Pharmacological influences on in vivo extraction fraction. J Neurosci Methods. 1996;68:39–47. doi: 10.1016/0165-0270(96)00057-x. [DOI] [PubMed] [Google Scholar]
- Damsma G, Westerink BHC, Imperato A, Rollema H, de Vries JB, Horn AS. Automated brain dialysis of acetylcholine in freely moving rats: Detection of basal acetylcholine. Life Sci. 1987;41:873–876. doi: 10.1016/0024-3205(87)90184-6. [DOI] [PubMed] [Google Scholar]
- de Boer J, Plijter-Groendijk H, Korf J. Continuous monitoring of glucose with a transcutaneous microdialysis probe [letter] Lancet. 1992;340:547–548. doi: 10.1016/0140-6736(92)91743-r. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Segovia G, Fuxe K, Mora F. Changes in dialysate concentrations of glutamate and GABA in the brain: An index of volume transmission mediated actions? J Neurochem. 2003;85:23–33. doi: 10.1046/j.1471-4159.2003.01692.x. [DOI] [PubMed] [Google Scholar]
- Deleu D, Sarre S, Ebinger G, Michotte Y. Simultaneous monitoring of levodopa, dopamine, and their metabolites in skeletal muscle and subcutaneous tissue in different pharmacological conditions using microdialysis. J Pharm Biomed Anal. 1993;11:557–585. doi: 10.1016/0731-7085(93)80008-o. [DOI] [PubMed] [Google Scholar]
- Delgado JMR, DeFeudis FV, Roth RH, Ryugo DK, Mitruka BK. Dialytrode for long term intracerebral perfusion in awake monkeys. Arch Int Pharmacodyn. 1972;198:9–21. [PubMed] [Google Scholar]
- Dev BR, Mason PA, Freed CR. Drug-induced changes in blood pressure lead to changes in extracellular concentrations of epinephrine, dihydroxyphenylacetic acid, and 5-hydroxyindoleacetic acid in the rostral ventrolateral medulla of the rat. J Neurochem. 1992;58:1386–1394. doi: 10.1111/j.1471-4159.1992.tb11354.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Tanda G, Carboni E. Estimation of in-vivo neurotransmitter release by brain microdialysis: The issue of validity. Behav Pharmacol. 1996;7:640–657. [PubMed] [Google Scholar]
- Dietze S, Kushinsky K. Determination of extracellular glutamate after local K+ stimulation in the striatum of non-anaesthetized rats after treatment with dopaminergic drugs: Studies using microdialysis. J Neural Transm Gen Sect. 1992;90:1–11. doi: 10.1007/BF01250513. [DOI] [PubMed] [Google Scholar]
- Dizdar N, Kagedal B, Smeds S, Arstrand K. A high sensitivity fluorometric high-performance liquid chromatographic method for determination of glutathione and other thiols in cultured melanoma cells, microdialysis samples from melanoma tissue, and blood plasma. Melanoma Res. 1991;1:33–42. doi: 10.1097/00008390-199104000-00005. [DOI] [PubMed] [Google Scholar]
- During MJ, Bean AJ, Roth RH. Effects of CNS stimulants on the in vivo release of the colocalized transmitters, dopamine and neurotensin, from rat prefrontal cortex. Neurosci Lett. 1992;140:129–133. doi: 10.1016/0304-3940(92)90698-7. [DOI] [PubMed] [Google Scholar]
- Dykstra KH, Hsiao JK, Morrison PF, Bungay PM, Mefford IN, Scully MM, Dedrick RL. Quantitative examination of tissue concentration profiles associated with microdialysis. J Neurochem. 1992;58:931–940. doi: 10.1111/j.1471-4159.1992.tb09346.x. [DOI] [PubMed] [Google Scholar]
- Einspanier R, Miyamoto A, Schams D, Muller M, Brem G. Tissue concentration, mRNA expression and stimulation of IGF-I in luteal tissue during the oestrous cycle and pregnancy of cows. J Reprod Fertil. 1990;90:439–445. doi: 10.1530/jrf.0.0900439. [DOI] [PubMed] [Google Scholar]
- Ferraro TN, Weyers P, Carrozza DP, Vogel WH. Continuous monitoring of brain ethanol levels by intracerebral microdialysis. Alcohol. 1990;7:129–132. doi: 10.1016/0741-8329(90)90073-l. [DOI] [PubMed] [Google Scholar]
- Friedman MH. Principles and Models of Biological Transport. Springer-Verlag; New York and Heidelberg: 1986. Free diffusion; pp. 22–41. [Google Scholar]
- Fukui H, Yamamoto M, Ando T, Sasaki S, Sato S. Increase in serotonin levels in the dog ileum and blood by cisplatin as measured by microdialysis. Neuropharmacology. 1993;32:959–968. doi: 10.1016/0028-3908(93)90060-g. [DOI] [PubMed] [Google Scholar]
- Gaddum J. Push-pull cannulae. J Physiol. 1961;155:1–2. [Google Scholar]
- Glass JD, Randolph WW, Ferriera SA, Rea MA, Hauser UE, Blank JL, De Vries MJ. Diurnal variation in 5-hydroxyindoleacetic acid output in the suprachiasmatic region of the Siberian hamster assessed by in vivo microdialysis: Evidence for nocturnal activation of serotonin release. Neuroendocrinology. 1992;56:582–590. doi: 10.1159/000126277. [DOI] [PubMed] [Google Scholar]
- Glick SD, Dong N, Keller RW, Jr, Carlson JN. Estimating extracellular concentration of dopamine and 3,4-dihydroxyphenylacetic acid in nucleus accumbens and striatum using microdialysis: Relationship between in vitro and in vivo recoveries. J Neurochem. 1994;62:2017–2021. doi: 10.1046/j.1471-4159.1994.62052017.x. [DOI] [PubMed] [Google Scholar]
- Golden PL, Brouwer KR, Pollack GM. Assessment of valproic acid serum-cerebrospinal fluid transport by microdialysis. Pharmacol Res. 1993;10:1765–1771. doi: 10.1023/a:1018982300285. [DOI] [PubMed] [Google Scholar]
- Guadalupe T, Gonzalez-Mora JL, Fumero B, Mas M. Voltammetric monitoring of brain extracellular levels of serotonin, 5-hydroxyindoleacetic acid and uric acid as assessed by simultaneous microdialysis. J Neurosci Methods. 1992;45:159–164. doi: 10.1016/0165-0270(92)90072-l. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Andersson P, Lacarewicz J, Jacobson I, Butcher S, Sandberg M. Extracellular adenosine, inosine, hypoxanthine, and xanthine in relation to tissue nucleotides and purines in rat striatum during transient ischemia. J Neurochem. 1987;49:227–231. doi: 10.1111/j.1471-4159.1987.tb03419.x. [DOI] [PubMed] [Google Scholar]
- Hallstrom A, Carlsson A, Hillered L, Ungerstedt U. Simultaneous determination of lactate, pyruvate, and ascorbate in microdialysis samples from the rat brain, blood, fat, and muscle using high-performance liquid chromatography. J Pharmacol Methods. 1989;22:113–124. doi: 10.1016/0160-5402(89)90040-5. [DOI] [PubMed] [Google Scholar]
- Hamberger A, Berthold C-H, Karlsson B, Lehmann A, Nystrom B. Extracellular GABA, glutamate, and glutamine in vivo perfusion dialysis of the rabbit hippocampus. In: Herz L, Krammer E, McGee EG, Schousboe A, editors. Neurology and Neurobiology, Vol. 7: Glutamine, Glutamate and GABA in the Central Nervous System. Wiley-Liss; New York: 1983. pp. 473–492. [Google Scholar]
- Hasegawa M, Ebihara S. Circadian rhythms of pineal melatonin release in the pigeon measured by in vivo microdialysis. Neurosci Lett. 1992;148:89–92. doi: 10.1016/0304-3940(92)90811-k. [DOI] [PubMed] [Google Scholar]
- Hashizume T, Nitta Y, Kasuya K, Kanematsu S. Microdialysis of intracerebral somatostatin in the goat. Domest Anim Endocr. 1995;12:363–370. doi: 10.1016/0739-7240(95)00049-k. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behavior and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Hsiao JK, Ball BA, Morrison PF, Mefford IN, Bungay P. Effects of different semipermeable membranes on in vitro and in vivo performance of microdialysis probes. J Neurochem. 1990;54:1449–1452. doi: 10.1111/j.1471-4159.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zielke CL, Tidon JT, Zielke HR. Monitoring in vivo oxidation of 14C-labeled substrates to 14CO2 by brain microdialysis. Dev Neurosci. 1993;15:233–239. doi: 10.1159/000111339. [DOI] [PubMed] [Google Scholar]
- Hylland P, Nilsson GE, Johansson D. Anoxic brain failure in an ectothermic vertebrate: Release of amino acids and K+ in rainbow trout thalamus. Am J Physiol. 1995;269:R1077–R1084. doi: 10.1152/ajpregu.1995.269.5.R1077. [DOI] [PubMed] [Google Scholar]
- Imperato A, Di Chiara G. Trans-striatal dialysis coupled to reverse-phase high performance liquid chromatography with electrochemical detection: A new method for study of the in vivo release of endogenous dopamine and metabolites. J Pharmacol Exp Ther. 1984;4:966–977. doi: 10.1523/JNEUROSCI.04-04-00966.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingvast-Larsson C, Appelgren LE, Nyman G. Microdialysis of theophylline in the rat and horse. Acta Vet Scand Suppl. 1991;87:330–332. [Google Scholar]
- Itoh Y, Oishi R, Nishibori M, Saeki K. in vivo measurement of noradrenaline and 3,4-dihydroxyphenylethyleneglycol in the rat hypothalamus by microdialysis: Effects of various drugs affecting noradrenaline metabolism. J Pharmacol Exp Ther. 1990;255:1090–1097. [PubMed] [Google Scholar]
- Jacobson I, Sandberg M, Hamberger A. Mass transfer in brain dialysis devices: A new method for the estimation of extracellular amino acids concentration. J Neurosci Methods. 1985;15:263–268. doi: 10.1016/0165-0270(85)90107-4. [DOI] [PubMed] [Google Scholar]
- Johnson RD, Justice JB. Model studies for brain dialysis. Brain Res Bull. 1983;10:567–571. doi: 10.1016/0361-9230(83)90156-9. [DOI] [PubMed] [Google Scholar]
- Jonsson P, Borgstrom A, Ohlsson K. Measurements of exocrine proteins in the pig pancreas using microdialysis. Gastroenterol Jpn. 1992;27:529–535. doi: 10.1007/BF02777790. [DOI] [PubMed] [Google Scholar]
- Justice JB., Jr Quantitative microdialysis of neurotransmitters. J Neurosci Methods. 1993;48:263–276. doi: 10.1016/0165-0270(93)90097-b. [DOI] [PubMed] [Google Scholar]
- Keefe KA, Sved AF, Zigmond MJ, Abercrombie ED. Stress-induced dopamine release in the neostriatum: Evaluation of the role of action potentials in nigrostriatal dopamine neurons or local initiation by endogenous excitatory amino acids. J Neurochem. 1993;61:1943–1952. doi: 10.1111/j.1471-4159.1993.tb09837.x. [DOI] [PubMed] [Google Scholar]
- Kendrick KM. Use of microdialysis in neuroendocrinology. Methods Enzymol. 1989;168:182–205. doi: 10.1016/0076-6879(89)68013-5. [DOI] [PubMed] [Google Scholar]
- Kendrick KM. Microdialysis measurement of in vivo neuropeptide release. J Neurosci Methods. 1990;34:35–46. doi: 10.1016/0165-0270(90)90040-m. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Chapman C, Baldwin BA. Microdialysis measurement of oxytocin, aspartate, gamma-aminobutyric acid and glutamate release from olfactory bulb of the sheep during vaginocervical stimulation. Brain Res. 1988;442:171–174. doi: 10.1016/0006-8993(88)91447-3. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, De la Riva C, Hinton M, Baldwin BA. Microdialysis measurements of monoamine and amino acid release from the medial preoptic region of the sheep in response to heat exposure. Brain Res Bull. 1989;22:541–544. doi: 10.1016/0361-9230(89)90108-1. [DOI] [PubMed] [Google Scholar]
- Khan SH, Shuaib A. The technique of intracerebral microdialysis. Methods. 2001;23:3–9. doi: 10.1006/meth.2000.1101. [DOI] [PubMed] [Google Scholar]
- Khan-Dawood FS, Gargiulo AR, Dawood MY. Baboon corpus lutem: Autonomous pulsatile progesterone secretion and evidence for an intraluteal oscillator demonstrated by in vitro microretrodialysis. J Clin Endocrinol Metab. 1994;79:1790–1796. doi: 10.1210/jcem.79.6.7989486. [DOI] [PubMed] [Google Scholar]
- Kubota M, Atobe M, Hirano M, Nakagawara M, Kariya T. Hypothalamic MHPG measured by microdialysis in rats with PNMT inhibitors. Jpn J Psychiat Neurol. 1993;47:428. doi: 10.1111/j.1440-1819.1993.tb02135.x. [DOI] [PubMed] [Google Scholar]
- Kuzmin AI, Selivanov VN, Sysoev AB, Medvedev OS. Study of catecholamine secretion by the rat adrenal glands using microdialysis in vivo. Fiziol Zh. 1990;36:14–20. [PubMed] [Google Scholar]
- Kuzmin AI, Tskitishvili OV, Serebryakova LI, Saprygina TV, Kapelko VI, Medvedev OS. Cardiac microdialysis measurement of extracellular adenine nucleotide breakdown products during regional ischemia and reprofusion in canine heart: Protective effect of propranolol against reprofusion injury. J Cardiovasc Pharmacol. 1992;20:961–968. doi: 10.1097/00005344-199212000-00017. [DOI] [PubMed] [Google Scholar]
- Lahtinen H, Brankack J, Koivisto E, Riekkinen PJ. Somatostatin release in rat neocortex during gamma-hydroxybutyrate-provoked seizures: Microdialysis combined with EEG recording. Brain Res Bull. 1992;29:837–841. doi: 10.1016/0361-9230(92)90152-n. [DOI] [PubMed] [Google Scholar]
- Lambert PD, Wilding JP, Turton MD, Ghatel MA, Bloom SR. Effects of food deprivation and streptozotocin-induced diabetes on hypothalamic neuropeptide Y release as measured by a radioimmunoassay-linked microdialysis procedure. Brain Res. 1994;656:135–140. doi: 10.1016/0006-8993(94)91374-9. [DOI] [PubMed] [Google Scholar]
- Larsson CI. The use of an “internal standard” for control of the recovery in microdialysis. Life Sci. 1991;49:PL73–PL78. doi: 10.1016/0024-3205(91)90082-m. [DOI] [PubMed] [Google Scholar]
- Le Quellec A, Dupin S, Genissel P, Saivin S, Marchand B, Houin G. Microdialysis probes calibration: Gradient and tissue dependent changes in no net flux and reverse dialysis methods. J Pharmacol Exp Ther. 1995;33:11–16. doi: 10.1016/1056-8719(94)00049-a. [DOI] [PubMed] [Google Scholar]
- Lincoln GA. Administration of melatonin into the mediobasal hypothalamus as a continuous or intermittent signal affects the secretion of follicle stimulating hormone and prolactin in ram. J Pineal Res. 1992;12:135–144. doi: 10.1111/j.1600-079x.1992.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Brodin E, Ungerstedt U. Amphetamine facilitates the in vivo release of neurokinin A in the nucleus accumbens of the rat. Eur J Pharmacol. 1989a;160:417–420. doi: 10.1016/0014-2999(89)90100-3. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Amberg G, Ungerstedt U. Intracerebral microdialysis: I. Experimental studies of diffusion kinetics. J Pharmacol Methods. 1989b;22:141–156. doi: 10.1016/0160-5402(89)90011-9. [DOI] [PubMed] [Google Scholar]
- Linderoth B, Gazel-Gazaliuss B, Franck J, Brodin E. Dorsal column stimulation induces release of serotonin and substance P in the cat dorsal horn. Neurosurgery. 1992;31:289–296. doi: 10.1227/00006123-199208000-00014. [DOI] [PubMed] [Google Scholar]
- Ljungdahl-Stahle E, Guzeda E, Bottiger D, Wahren B, Oberg B, Stahle L. Penetration of zidovudine and 3′-fluoro-3′-deoxythymidine into the brain, muscle tissue and veins in cynomolgus monkeys: Relation to antiviral action. Antimicrob Agents Chemother. 1992;36:2418–2422. doi: 10.1128/aac.36.11.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonnroth P, Strindberg L. Validation of the “internal reference technique” for calibrating microdialysis catheters in situ. Acta Physiol Scand. 1995;153:375–380. doi: 10.1111/j.1748-1716.1995.tb09875.x. [DOI] [PubMed] [Google Scholar]
- Lonnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987;253:E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Lonnroth P, Jansson PA, Fredholm BB, Smith U. Microdialysis of intercellular adenosine concentration in subcutaneous tissue in humans. Am J Physiol. 1989;256:E250–E255. doi: 10.1152/ajpendo.1989.256.2.E250. [DOI] [PubMed] [Google Scholar]
- Lonnroth P, Carlsten J, Johnson L, Smith U. Measurements by microdialysis of free tissue concentrations of propranolol. J Chromatogr. 1991;568:419–425. doi: 10.1016/0378-4347(91)80179-g. [DOI] [PubMed] [Google Scholar]
- Louzada-Junior P, Dias JJ, Santos WF, Lachat JJ, Bradford HF, Coutinho-Netto J. Glutamate release in experimental ischaemia of the retina: An approach using microdialysis. J Neurochem. 1992;59:358–363. doi: 10.1111/j.1471-4159.1992.tb08912.x. [DOI] [PubMed] [Google Scholar]
- Lu Y, Peters JL, Michael AC. Direct comparison of the response of voltammetry and microdialysis to electrically evoked release of striatal dopamine. J Neurochem. 1998;70:584–593. doi: 10.1046/j.1471-4159.1998.70020584.x. [DOI] [PubMed] [Google Scholar]
- Maidment NT, Brumbaugh DR, Rudolph VD, Erdelyi E, Evans CJ. Microdialysis of extracellular endogenous opioid peptides from rat brain in vivo. Neuroscience. 1989;33:549–557. doi: 10.1016/0306-4522(89)90407-7. [DOI] [PubMed] [Google Scholar]
- Mason PA, Romano WF. Recovery characteristics of a rigid, nonmetallic microdialysis probe for use in an electromagnetic field. Bioelectromagnetics. 1995;16:113–118. doi: 10.1002/bem.2250160207. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Nakashima M, Nakaboh Y, Ichikawa M, Yano T, Satoh S. Application of in vivo microdialysis to transdermal absorption of methotrexate in rats. Pharmacol Res. 1994;11:684–686. doi: 10.1023/a:1018972112077. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–33. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Menacherry S, Hubert W, Justice JB., Jr in vivo calibration of microdialysis probes of exogenous compounds. Anal Chem. 1992;64:577–583. doi: 10.1021/ac00030a003. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Schams D. Oxytocin stimulates progesterone release from microdialyzed bovine corpus luteum in vitro. Biol Reprod. 1991;44:1163–1170. doi: 10.1095/biolreprod44.6.1163. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: Comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS. Ionic composition of microdialysis perfusing solution alters the pharmacological responsiveness and basal outflow of striatal dopamine. J Neurochem. 1989;53:652–654. doi: 10.1111/j.1471-4159.1989.tb07383.x. [DOI] [PubMed] [Google Scholar]
- Montgomery AJ, Lingford-Hughes AR, Egerton A, Nutt DJ, Grasby PM. The effect of nicotine on striatal dopamine release in man: A [(11)C]raclopride PET study. Synapse. 2007;61:637–645. doi: 10.1002/syn.20419. [DOI] [PubMed] [Google Scholar]
- Morari M, O’Connor WT, Ungerstedt U, Fuxe K. N-methyl-D-aspartic acid differentially regulates extracellular dopamine, GABA, and glutamate levels in the dorsolateral neostriatum of the halothane-anesthetized rat: An in vivo microdialysis study. J Neurochem. 1993;60:1884–1893. doi: 10.1111/j.1471-4159.1993.tb13416.x. [DOI] [PubMed] [Google Scholar]
- Morrison PF, Bungay PM, Hsiao JK, Mefford IN, Dykstra KH, Dedrick RL. Quantitative microdialysis. In: Robinson TE, Justice JB Jr, editors. Microdialysis in the Neurosciences. Elsevier Science Publishing; New York: 1991. pp. 47–80. [Google Scholar]
- Myers R. Methods for perfusing different brain structures. In: Myers R, editor. Methods in Psychobiology. Vol. 2. Academic Press; London: 1972. pp. 169–211. [Google Scholar]
- Nash JF, Yamamoto BK. Effect of d-amphetamine on the extracellular concentrations of glutamate and dopamine in iprindole-treated rats. Brain Res. 1993;627:1–8. doi: 10.1016/0006-8993(93)90741-5. [DOI] [PubMed] [Google Scholar]
- Nicholson C, Rice ME. The migration of substances in the neuronal microenvironment. Ann NY Acad Sci. 1986;481:55–71. doi: 10.1111/j.1749-6632.1986.tb27139.x. [DOI] [PubMed] [Google Scholar]
- Nielsen EO, Aarslew-Jensen M, Diemer NH, Krogsgaard-Larsen P, Schousboe A. Baclofen-induced, calcium-dependent stimulation of in vivo release of D-[3H]aspartate from rat hippocampus monitored by intracerebral microdialysis. Neurochem Res. 1989;14:321–326. doi: 10.1007/BF01000034. [DOI] [PubMed] [Google Scholar]
- Nomoto M, Irifune M, Fukuzaki K, Fukuda T. Effects of bifemelane on parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the common marmoset. Neurosci Lett. 1994;178:95–98. doi: 10.1016/0304-3940(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Nordenvall M, Ulmsten U, Ungerstedt U. Influence of progesterone on the sodium and potassium concentrations of the rat uterine fluid investigated by microdialysis. Gynecol Obstet Invest. 1989;28:73–77. doi: 10.1159/000293518. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP, Zikha E, Urenjak J. Intracerebral microdialysis: Electrophysiological evidence of a critical pitfall. J Neurochem. 1995;64:1884–1887. doi: 10.1046/j.1471-4159.1995.64041884.x. [DOI] [PubMed] [Google Scholar]
- Okuda C, Sawa T, Harada M, Murakami T, Matsuda T, Tanaka Y. Lactate in rat skeletal muscle after hemorrhage measured by microdialysis probe calibrated in situ. Am J Physiol. 1992a;263:E1035–E1039. doi: 10.1152/ajpendo.2006.263.6.E1035. [DOI] [PubMed] [Google Scholar]
- Okuda C, Sawa T, Harada M, Murakami T, Tanaka Y. Continuous measurement of lactate concentration in skeletal muscle and liver interstitium using a microdialysis method after acute hemorrhage in anesthetized rats. Circ Shock. 1992b;37:230–235. [PubMed] [Google Scholar]
- Osborne PG, O’Connor WT, Ungerstedt U. Effect of varying the ionic concentration of a microdialysis perfusate on basal striatal dopamine levels in awake rats. J Neurochem. 1991;56:452–456. doi: 10.1111/j.1471-4159.1991.tb08171.x. [DOI] [PubMed] [Google Scholar]
- Ota M, Crofton JT, Festavan G, Share L. Central carbachol stimulates vasopressin release into interstitial fluid adjacent to the paraventricular nucleus. Brain Res. 1992;592:249–254. doi: 10.1016/0006-8993(92)91682-5. [DOI] [PubMed] [Google Scholar]
- Parry TJ, Carter TL, McElligott JG. Physical and chemical considerations in the in vitro calibration of microdialysis probes for biogenic amine neurotransmitters and metabolites. J Neurosci Methods. 1990;32:175–183. doi: 10.1016/0165-0270(90)90138-6. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB., Jr Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991a;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB., Jr The in vivo microdialysis recovery of dopamine is altered independently of basal level by 6-hydroxydopamine lesions to the nucleus accumbens. J Neurosci Meth. 1991b;40:139–147. doi: 10.1016/0165-0270(91)90063-6. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr Extracellular concentration and in vivo recovery of 26 dopamine in the nucleus accumbens using microdialysis. J Neurochem. 1992;58:212–218. doi: 10.1111/j.1471-4159.1992.tb09298.x. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr Quantitative approaches to in vivo brain microdialysis. Crit Rev Neurobiol. 1994;8:189–220. [PubMed] [Google Scholar]
- Peters JL, Michael AC. Modeling voltammetry and microdialysis of striatal extracellular dopamine: The impact of dopamine uptake on extraction and recovery ratios. J Neurochem. 1998;70:594–603. doi: 10.1046/j.1471-4159.1998.70020594.x. [DOI] [PubMed] [Google Scholar]
- Petersen LJ, Skov PS, Bindslev-Jensen C, Sondergaard J. Histamine release in immediate-type hypersensitivity reactions in intact human skin measured by microdialysis: A preliminary study. Allergy. 1992a;47:635–637. doi: 10.1111/j.1398-9995.1992.tb02387.x. [DOI] [PubMed] [Google Scholar]
- Petersen LJ, Kristensen JK, Bulow J. Microdialysis of the interstitial water space in human skin in vivo: Quantitative measurement of cutaneous glucose concentrations. J Invest Dermatol. 1992b;99:357–360. doi: 10.1111/1523-1747.ep12616676. [DOI] [PubMed] [Google Scholar]
- Plock N, Kloft C. Microdialysis—Theoretical background and recent implementation in applied life-sciences. Eur J Pharm Sci. 2005;25:1–24. doi: 10.1016/j.ejps.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Rambeck B, Jurgens UH, May TW, Pannek HW, Behne F, Ebner A, Gorji A, Straub H, Speckmann EJ, Pohlmann-Eden B, Loscher W. Comparison of brain extracellular fluid, brain tissue, cerebrospinal fluid, and serum concentrations of antiepileptic drugs measured intraoperatively in patients with intractable epilepsy. Epilepsia. 2006;47:681–694. doi: 10.1111/j.1528-1167.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- Rice ME, Gerhardt GA, Hierl PM, Nagy G, Adams RN. Diffusion coefficients of neurotransmitters and their metabolites in brain extracellular fluid space. Neuroscience. 1985;15:891–902. doi: 10.1016/0306-4522(85)90087-9. [DOI] [PubMed] [Google Scholar]
- Rollema H, Alexander GM, Grothusen JR, Matos FF, Castagnoli N. Comparison of the effects of intracerebrally administered MPP+ (1-methyl-4-phenylpyridinium) in three species: Microdialysis of dopamine and metabolites in mouse, rat and monkey striatum. Neurosci Lett. 1989;106:275–281. doi: 10.1016/0304-3940(89)90176-6. [DOI] [PubMed] [Google Scholar]
- Russi P, Alesiani M, Lombardi G, Davolio G, Pellicciari R. Nicotinylalanine increases the formation of kynurenic acid in the brain and antagonizes convulsions. J Neurochem. 1992;59:2076–2080. doi: 10.1111/j.1471-4159.1992.tb10097.x. [DOI] [PubMed] [Google Scholar]
- Sam P, Justice JB., Jr Effect of general microdialysis-induced depletion of extracellular dopamine. Anal Chem. 1996;68:724–728. doi: 10.1021/ac950754+. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Lindstrom S. Amino acids in the dorsal lateral geniculate nucleus of the cat: Collection in vivo. J Neurosci Methods. 1983;9:65–74. doi: 10.1016/0165-0270(83)90110-3. [DOI] [PubMed] [Google Scholar]
- Sato Y, Shibanoki S, Sugahara M, Ishikawa K. Measurement and pharmacokinetic analysis of imipramine and its metabolite by brain microdialysis. Br J Pharmacol. 1994;112:625–629. doi: 10.1111/j.1476-5381.1994.tb13120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerwein H, Miyamoto A, Gunther J, Meyer HH, Schams D. Binding and action of insulin-like growth factors and insulin in bovine luteal tissue during the oestrous cycle. J Reprod Fertil. 1992;96:103–115. doi: 10.1530/jrf.0.0960103. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Kolachana BS, Weinberger DR. Local pharmacological manipulation of dopamine in prefrontal cortex and caudate in the rhesus monkey: An in vivo microdialysis study. Exp Brain Res. 1993;98:44–52. doi: 10.1007/BF00229108. [DOI] [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: A practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Methods. 1991;40:31–37. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Scheller D, Kolb J, Tegtmeier F, Lehmenkuhler A. Extracellular changes of inorganic phosphate are different during spreading depression and global cerebral ischemia of rats. Neurosci Lett. 1992;141:269–272. doi: 10.1016/0304-3940(92)90911-p. [DOI] [PubMed] [Google Scholar]
- Scheyer RD, During MJ, Spencer DD, Cramer JA, Mattson RH. Measurements of carbamazepine and carbamazepine epoxide in the human brain using in vivo microdialysis. Neurology. 1994a;44:1469–1472. doi: 10.1212/wnl.44.8.1469. [DOI] [PubMed] [Google Scholar]
- Scheyer RD, During MJ, Hochholzer JM, Spencer DD, Cramer JA, Mattson RH. Phenytoin concentrations in the human brain: An in vivo microdialysis study. Epilepsy Res. 1994b;18:227–232. doi: 10.1016/0920-1211(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Hori T, Nakane H. An interleukin-1 β-induced noradrenaline release in the spleen is mediated by brain corticotropin-releasing factor: An in vivo microdialysis study in conscious rats. Brain Behav Immun. 1994;8:14–23. doi: 10.1006/brbi.1994.1002. [DOI] [PubMed] [Google Scholar]
- Siragy HM. Endothelium-derived relaxing factor modulates renal interstitial cycle GMP. J Cardiovasc Pharmacol. 1992;20:S163–S165. doi: 10.1097/00005344-199204002-00046. [DOI] [PubMed] [Google Scholar]
- Siragy HM, Jaffa AA, Margolius HS. Stimulation of renal interstitial bradykinin by sodium depletion. Am J Hypertens. 1993;6:863–866. doi: 10.1093/ajh/6.10.863. [DOI] [PubMed] [Google Scholar]
- Sjoberg P, Olofsson IM, Lundqvist T. Validation of different microdialysis methods for the determination of unbound steady-state concentrations of theophylline in blood and brain tissue. Pharm Res. 1992;9:1592–1598. doi: 10.1023/a:1015816509140. [DOI] [PubMed] [Google Scholar]
- Smith AD, Justice JB. The effect of inhibition of synthesis, release, metabolism and uptake on the microdialysis extraction fraction of dopamine. J Neurosci Methods. 1994;54:75–82. doi: 10.1016/0165-0270(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Smith AD, Olson RJ, Justice JB., Jr Quantitative microdialysis of dopamine in the striatum: Effect of circadian variation. J Neurosci Methods. 1992;44:33–41. doi: 10.1016/0165-0270(92)90111-p. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: An in vivo microdialysis study. J Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Specialle C, Ungerstedt U, Schwarcz R. Effect of kynurenine loading on quinolinic acid production in the rat: Studies in vitro and in vivo. Life Sci. 1988;43:777–786. doi: 10.1016/0024-3205(88)90178-6. [DOI] [PubMed] [Google Scholar]
- Stahle L, Sebersvard S, Ungerstedt U. A comparison between three methods for estimating the extracellular concentration of endogenous and exogenous compounds by microdialysis. J Pharmacol Methods. 1991;25:42–52. doi: 10.1016/0160-5402(91)90021-v. [DOI] [PubMed] [Google Scholar]
- Steele MK, Stephenson KN, Meredith JM, Levine JE. Effects of angiotensin II on LHRH release as measured by in vivo microdialysis of the anterior pituitary gland of conscious female rats. Neuroendocrinology. 1992;55:276–281. doi: 10.1159/000126126. [DOI] [PubMed] [Google Scholar]
- Stone EA, John SM. Stress induced increases in extracellular levels of cyclic AMP in rat cortex. Brain Res. 1992;597:144–147. doi: 10.1016/0006-8993(92)91516-h. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Kleschen MJ, Oleson EB, Dehoff MH, Schwarz MK, Seeburg PH, Worley PF, Kalivas PW. Behavioral and neurochemical phenotyping of Homer1 mutant mice: Possible relevance to schizophrenia. Genes Brain Behav. 2005;4:273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- Tang A, Bungay PM, Gonzales RA. Characterization of probe and tissue factors that influence interpretation of quantitative microdialysis experiments for dopamine. J Neurosci Methods. 2003;126:1–11. doi: 10.1016/s0165-0270(03)00003-7. [DOI] [PubMed] [Google Scholar]
- Taylor DL, Davies SE, Obrenovitch TP, Urenjak J, Richards DA, Clark JB, Symon L. Extracellular N-acetylaspartate in the rat brain: in vivo determination of basal levels and changes evoked by high K+ J Neurochem. 1994;62:2233–2240. doi: 10.1046/j.1471-4159.1994.62062349.x. [DOI] [PubMed] [Google Scholar]
- Thompson AC, Justice JB, Jr, McDonald JK. Quantitative microdialysis of neuropeptide Y. J Neurosci Methods. 1995;60:189–198. doi: 10.1016/0165-0270(95)00012-j. [DOI] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB, Jr, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20:9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen K, Kristoffersson AO, Lerner UH, Lorentzon RPK. In situ microdialysis in bone tissue: Stimulation of prostaglandin E2 release by weight-bearing mechanical loading. J Clin Invest. 1996;98:2446–2449. doi: 10.1172/JCI119061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman W, Cisci G, Nap A, De Vries JB, Westerink BH. Effects of handling on extracellular levels of glutamate and other amino acids in various areas of the brain measured by microdialysis. Brain Res. 1999;833:150–160. doi: 10.1016/s0006-8993(99)01538-3. [DOI] [PubMed] [Google Scholar]
- Tossman U, Wieloch T, Ungerstedt U. Gamma-aminobutyric acid and taurine release in the striatum of the rat during hypoglycemic coma, studied by microdialysis. Neurosci Lett. 1985;62:231–235. doi: 10.1016/0304-3940(85)90360-x. [DOI] [PubMed] [Google Scholar]
- Tossman U, Jonsson G, Ungerstedt U. Regional distribution and extracellular levels of amino acids in rat central nervous system. Acta Physiol Scand. 1986;127:533–545. doi: 10.1111/j.1748-1716.1986.tb07938.x. [DOI] [PubMed] [Google Scholar]
- Tsai G, Forloni G, Robinson MB, Stauch BL, Coyle JT. Calcium-dependent evoked release of N[3H]acetylaspartylglutamate from the optic pathway. J Neurochem. 1988;51:1956–1959. doi: 10.1111/j.1471-4159.1988.tb01186.x. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. Measurement of neurotransmitter release by intracranial dialysis. In: Marsden CA, editor. Measurement of Neurotransmitter Release In Vivo. John Wiley & Sons; New York: 1984. pp. 81–105. [Google Scholar]
- Ungerstedt U, Pycock C. Functional correlates of dopamine neurotransmission. Bull Schweitz Akad Med Wiss. 1974;1278:1–13. [PubMed] [Google Scholar]
- Ungerstedt U, Herrera-Marchintz M, Jungnelius U, Stahle L, Tossman U, Zetterstrom T. Dopamine synaptic mechanisms reflected in studies combining behavioral recordings and brain dialysis. In: Kotisaka M, editor. Advances in Dopamine Research. Pergamon Press; Elmsford, N.Y.: 1982. pp. 219–231. [Google Scholar]
- Van Wylen DGL, Park TS, Rubio R, Berne RM. Increases in cerebral interstitial fluid adenosine concentration during hypoxia, local potassium infusion, and ischemia. J Cereb Blood Flow Metab. 1986;6:522–528. doi: 10.1038/jcbfm.1986.97. [DOI] [PubMed] [Google Scholar]
- Venero C, Borrell J. Rapid glucocorticoid effects on excitatory amino acid levels in hippocampus: a microdialysis study in freely moving rats. Eur J Neurosci. 1999;11:2465–2473. doi: 10.1046/j.1460-9568.1999.00668.x. [DOI] [PubMed] [Google Scholar]
- Vinson PN, Justice JB., Jr Effect of neostigmine on concentration and extraction fraction of acetylcholine using quantitative microdialysis. J Neurosci Methods. 1997;73:61–67. doi: 10.1016/s0165-0270(96)02213-3. [DOI] [PubMed] [Google Scholar]
- Wages SA, Church WH, Justice JB. Sampling considerations for online microbore liquid chromatography of brain dialysis. Anal Chem. 1986;58:1649–1656. doi: 10.1021/ac00121a012. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sawchuk RJ. Zidovudine transporter in the rabbit brain during intravenous and intracerebroventricular infusion. J Pharm Sci. 1995;84:871–876. doi: 10.1002/jps.2600840717. [DOI] [PubMed] [Google Scholar]
- Westerink BH. Analysis of biogenic amines in microdialysates of the brain. J Chromatogr B Biomed Sci Appl. 2000;747:21–32. doi: 10.1016/s0378-4347(00)00338-8. [DOI] [PubMed] [Google Scholar]
- Westerink BHC, Justice JB., Jr . Microdialysis compared with other in vivo release models. In: Robinson TE, Justice JB Jr, editors. Microdialysis in the Neurosciences. Elsevier Science Publishing; New York: 1991. pp. 23–43. [Google Scholar]
- Westerink BHC, Damsma G, Rollema H, de Vries JB, Horn AS. Scope and limitations of in vivo brain dialysis: A comparison of its application to various neurotransmitter systems. Life Sci. 1987;41:1763–1776. doi: 10.1016/0024-3205(87)90695-3. [DOI] [PubMed] [Google Scholar]
- Westerink BHC, Hofsteede HM, Damsma G, de Vries JB. The significance of extracellular calcium for the release of dopamine, acetylcholine and amino acids in conscious rats, evaluated by brain microdialysis. Naunyn-Schmiedeberg’s Arch Pharmacol. 1988;337:373–378. doi: 10.1007/BF00169526. [DOI] [PubMed] [Google Scholar]
- Wood JH, Wood JH, editors. Neurobiology of cerebrospinal fluid. Vol. 1. Plenum; New York: 1980. [Google Scholar]
- Wright IK, Upton N, Marsden CA. Effects of established and putative anxiolytics on extracellular 5-HT and 5-HIAA in the ventral hippocampus of rats during behavior on the elevated X-maze. Psychopharmacology. 1992;109:338–346. doi: 10.1007/BF02245882. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Shen H, Lake R, Samuvel DJ, Kalivas PW. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J Neurosci. 2003;23:3498–3505. doi: 10.1523/JNEUROSCI.23-08-03498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Peters JL, Michael AC. Coupled effects of mass transfer and uptake kinetics on in vivo microdialysis of dopamine. J Neurochem. 1998;71:684–692. doi: 10.1046/j.1471-4159.1998.71020684.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Peters JL, Allen C, Chern SS, Coalson RD, Michael AC. A theoretical description of microdialysis with mass transport coupled to chemical events. Anal Chem. 2000;72:2042–2049. doi: 10.1021/ac991186r. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Komura S, Kawamura K. Occurrence in vivo of 5-hydroxytryptophol in the brain of rats treated with ethanol. Alcohol. 1992;27:131–136. [PubMed] [Google Scholar]
- Yue TL, Gu JL, Lysko PG, Cheng HY, Barone FC, Feursteun G. Neuroprotective effects of phenyl-t-butylnitrone in gerbil global brain ischemia and in cultured rat cerebellar neurons. Brain Res. 1992;574:193–197. doi: 10.1016/0006-8993(92)90816-r. [DOI] [PubMed] [Google Scholar]
- Zapata A, Shippenberg TS. D(3) receptor ligands modulate extracellular dopamine clearance in the nucleus accumbens. J Neurochem. 2002;81:1035–1042. doi: 10.1046/j.1471-4159.2002.00893.x. [DOI] [PubMed] [Google Scholar]
- Zetterstrom T, Sharp T, Collin AK, Ungerstedt U. in vivo measurement of extracellular dopamine and DOPAC in the rat striatum after various dopamine-releasing drugs: Implications for the origin of extracellular DOPAC. Eur J Pharmacol. 1988;148:327–334. doi: 10.1016/0014-2999(88)90110-0. [DOI] [PubMed] [Google Scholar]