Introduction
Asthma is among the most common serious medical problems in pregnancy1, estimated to affect between 3.7% and 8.4% of pregnant women2. Further, population data suggest that these rates may be rising3, 4. Management of asthma in pregnancy poses a dilemma for both physicians and their pregnant patients: asthma may itself present risks to the fetus, such as low birth weigh and premature delivery,5, 6 but the fetal risks of medications used to treat asthma are largely unknown. To improve management, the National Asthma Education and Prevention Program (NAEPP) has published recommendations for treatment of asthma in pregnancy, initially in 19937 and most recently in January, 20051, which advise that inhaled steroids be used as first-line treatment for persistent asthma. However, the extent to which these guidelines are followed is unclear.
Using data on mothers of non-malformed infants collected as part of an ongoing program of case-control surveillance of risk factors for birth defects, we examined the prevalence of asthma, levels of asthma control, the use of asthma medications among pregnant women, and the relationship between pregnancy and asthma symptoms. By examining trends in the use of specific asthma medications over time, we also evaluated the impact of changes in both asthma treatment guidelines and the introduction of new medications.
Methods
The Slone Epidemiology Center (SEC) at Boston University has been conducting case-control surveillance to identify risk factors for birth defects since 1976; the methods have been described previously8, 9. In 1998, we began including in our study population a random sample of Massachusetts births of non-malformed infants. On a bi-monthly basis, the SEC receives a data file from the Massachusetts Department of Public Health containing all births reported in the previous five months and from this file, a random sample of mothers of infants between 12 and 18 weeks of age are invited to participate in the study. Known neonatal deaths are excluded. The current report is based on this population-based sample of mothers of normal infants born in Massachusetts between 1998 and 2006.
Mothers of eligible subjects are interviewed within six months of the baby's birth by trained nurse-interviewers using a standardized questionnaire. The interview elicits demographic information about the mother and father and detailed information regarding maternal illnesses and details of all medications used from 2 months prior to the last menstrual period (LMP) through the end of pregnancy. This study has been approved by Boston University Medical Center's Institutional Review Board (IRB) and the IRBs of all participating institutions as appropriate, and verbal informed consent is obtained from all participants.
The interview included a series of questions related specifically to asthma diagnosis, symptoms, and management. The questions began by asking about the presence, in the past five years, of provider-diagnosed asthma, bronchitis, or reactive airway disease. Because one objective of these questions was to identify women who may have undiagnosed asthma, women who responded “no” to these questions were asked whether, within the past five years, they experienced episodes of coughing, wheezing, chest tightness, or shortness of breath. Women who responded affirmatively were asked about the timing of these symptoms: whether they occurred seasonally; at night; during or after colds; after exercise; after exposure to animals, cut grass or dust; or only during pregnancy. They were also asked whether their symptoms changed during pregnancy and, if so, the date of the change. Next, a series of questions inquired about level of asthma control: whether asthma required unscheduled medical visits, prednisone or cortisone, hospitalization, or intubation. Finally, women were asked questions relating to the extent to which their symptoms interfered with activities or sleep.
The asthma-related questions were the basis for classifying the likelihood of asthma:
Physician-diagnosed asthma was defined as health-care-provider-diagnosed asthma that was present within the past five years;
Possible asthma was defined as either a) bronchitis, wheezing, chest tightness, or persistent cough within the past five years; or b) shortness of breath in the past five years with at least one positive response (other than “only during pregnancy”) to the questions about timing of symptoms;
Past asthma was defined as provider-diagnosed asthma that was not present within the past five years.
Subjects not meeting these definitions were classified as no asthma.
We also created a scale reflecting degree of symptom control for provider-diagnosed asthma:
Asthma that required hospitalization or involved symptoms that were reported to interfere with activities or sleep “constantly” was considered to be poorly-controlled;
Asthma that required the use of prednisone, caused one or more unscheduled medical visits, or involved symptoms that interfered with activities or sleep “often” was considered to be not well-controlled (this is modeled after the EPR310 definition which could not be directly applied here because of the 9-month pregnancy timeframe versus the 12-month EPR3 risk domain timeframe);
Remaining cases of provider-diagnosed asthma were classified as well-controlled.
Since symptom frequency and pulmonary function were not captured, the NAEPP severity and control classification could not be utilized. However, symptoms interfering with sleep and activity are part of the impairment domain of asthma severity and control, and exacerbations requiring oral corticosteroids are part of the risk domains of asthma severity and control 10 While this study predated the publication of validated asthma control questionnaires11-13, questions regarding asthma symptoms interfering with sleep and activity are included in all of those tools.
Drug treatments were grouped into five major classes: corticosteroids, beta-2 agonists, leukotriene modifiers, combination products, and others (primarily mast cell stabilizers). Corticosteroids and beta-2 agonists were further subdivided according to route of administration. Exposure was considered separately for each of these classes, and, where numbers were sufficient, for specific medications. We also classified asthma medications as either controller or reliever drugs: controllers included inhaled steroids, salmeterol, cromolyn, nedocromil, theophylline, leukotriene modifiers, and formoterol; relievers included all other asthma medications.
We examined rates of asthma among study women, identified factors associated with asthma presence and control, examined the timing and direction of changes in the control of symptoms during pregnancy, and determined rates of use of the various classes of medication in each of the asthma categories. We also investigated changes in the use of medications over time.
Multiple logistic regression was used to evaluate each potential risk factor while controlling for the effects of others. Tests for trend were performed using regression to assess changes over time.
Results
Between 1998 and 2006, mothers of 5,323 non-malformed infants in Massachusetts were located and approached for participation; 3,609 (67.8%) agreed to be interviewed. Overall, 502 women (13.9%) met our definition of provider-diagnosed asthma; an additional 578 (16.0%) were classified as possible asthma and 137 (3.8%) as past asthma (Table 1).
Table 1. Rates of Asthma Among 3609 Mothers of Normal Newborns.
| Asthma Status | Number | % |
|---|---|---|
| Provider-diagnosed | 502 | 13.9 |
| Well-controlled | 153 | 4.2 |
| Not well-controlled | 202 | 5.6 |
| Poorly-controlled | 147 | 4.1 |
| Possible | 578 | 16.0 |
| Past | 137 | 3.8 |
| No Asthma | 2392 | 66.3 |
Physician-diagnosed asthma was most prevalent among the youngest women, and decreased with age (Table 2). Higher prevalences were also observed among women who were white, obese, had less than high school education, smoked during pregnancy, and lived in households with 5 or more members. Similarly, possible asthma was more prevalent among women who were young, less well-educated, and who smoked during pregnancy, but, in contrast to physician-diagnosed asthma, possible asthma was most common among underweight women (BMI less that 18.5), and was not associated with household size. Restricting subjects to those with physician-diagnosed asthma (Table 3), women classified as poorly-controlled tended to be older, less well-educated, smoke during pregnancy, have lower family incomes, and live in larger households than women with well-controlled asthma, although only the relationships with lower income and larger household size were statistically significant. Women in the not well-controlled category were more likely to be over age 24 and in the lowest income group.
Table 2. Prevalence of Asthma According to Selected Factors Among 3609 Mothers of Normal Newborns*.
| Physician-diagnosed | Possible | No Asthma | ||||
|---|---|---|---|---|---|---|
| N (%) | Adj OR | N (%) | Adj OR** | N (%) | ||
| Age | ||||||
| ≤24 | 126 (19.4) | Ref | 110 (16.9) | Ref | 391 (60.2) | |
| 25-29 | 122 (15.4) | 0.7 (0.5,1.0) | 116 (14.7) | 0.9(0.6, 1.3) | 512 (64.7) | |
| 30-34 | 149 (11.3) | 0.5 (0.3,0.7) | 214 (16.2) | 1.0 (07, 1.3) | 917 (69.3) | |
| ≥35 | 103 (12.4) | 0.5 (0.4, 0.8) | 137(16.4) | 1.0 (0.7, 1.4) | 565 (67.8) | |
| Race/Ethnicity | ||||||
| White | 418 (14.8) | Ref | 468 (16.6) | Ref | 1835 (65.1) | |
| Black | 23 (13.6) | 0.8 (0.5, 1.4) | 28 (16.6) | 1.1 (0.7, 1.7) | 106 (62.7) | |
| Hispanic | 48 (11.9) | 0.6 (0.4,0.9) | 63 (15.7) | 0.9 (0.7, 1.3) | 272 (67.7) | |
| Asian | 6 (4.2) | 0.2 (0.1, 0.6) | 13 (9.0) | 0.5 (0.3, 1.0) | 119 (82.6) | |
| Other | 7 (9.2) | 0.4 (0.2,0.9) | 6 (7.9) | 0.4 (0.2, 0.9) | 60 (78.9) | |
| Body Mass Index | ||||||
| Underweight | 20 (13.4) | 1.1 (0.6,1.8) | 34 (22.8) | 1.8 (1.2, 2.8) | 92 (61.7) | |
| Normal weight | 287 (12.9) | Ref | 318 (14.3) | Ref | 1540 (69.2) | |
| Overweight | 104 (13.9) | 1.1(0.8, 1.5) | 140 (18.7) | 1.4 (1.1, 1.7) | 470 (62.8) | |
| Obese | 84 (19.8) | 1.8 (1.3,2.3) | 77 (18.2) | 1.5(1.1,2.0) | 248 (58.5) | |
| Education | ||||||
| Less than high school | 51 (18.1) | 1.4 (0.9, 2.1) | 53 (18.8) | 1.3 (0.9, 1.9) | 167 (59.2) | |
| High school | 227 (14.8) | Ref | 260 (17.0) | Ref | 981 (64.1) | |
| Some college | 224 (12.5) | 1.1 (0.8, 1.4) | 265 (14.8) | 1.0 (0.8, 1.3) | 1241 (69.2) | |
| Smoking | ||||||
| Never | 248 (12.2) | Ref | 264 (13.0) | Ref | 1448 (71.1) | |
| Before pregnancy | 132 (14.6) | 1.3 (1.0, 1.7) | 151 (16.7) | 1.4 (1.1, 1.7) | 592 (65.4) | |
| During pregnancy | 122 (18.3) | 1.6 (1.2, 2.2) | 162 (24.3) | 2.4 (1.9, 3.2) | 352 (52.9) | |
| Caffeine | ||||||
| Never | 172 (14.7) | Ref | 183 (15.7) | Ref | 773 (66.2) | |
| Before pregnancy | 141 (13.9) | 0.9 (0.7, 1.2) | 168 (16.6) | 1.0 (0.8, 1.3) | 664 (65.5) | |
| During pregnancy | 81 (15.0) | 0.9 (0.7, 1.3) | 92 (17.0) | 0.9 (0.7, 1.3) | 350 (64.8) | |
| Alcohol | ||||||
| Never | 219 (14.2) | Ref | 241 (15.6) | Ref | 1016 (65.8) | |
| Before pregnancy | 263 (13.6) | 1.0 (0.8, 1.2) | 307 (15.9) | 0.9 (0.8, 1.3) | 1301 (67.3) | |
| During pregnancy | 20 (15.0) | 1.2 (0.7, 2.1) | 30 (22.6) | 1.6 (1.0, 2.6) | 75 (56.4) | |
| Income | ||||||
| <25,000 | 68 (16.8) | 1.0 (0.7, 1.5) | 68 (16.8) | 0.9 (0.7, 1.4) | 249 (61.6) | |
| 25,000-45,000 | 70 (15.2) | 1.0 (0.7, 1.4) | 79 (17.2) | 1.6 (1.0,2.6) | 284 (61.7) | |
| >45,000 | 305 (13.1) | Ref | 368 (15.8) | Ref | 1582 (67.8) | |
| Household size | ||||||
| 1-2 | 178 (14.2) | Ref | 189 (15.0) | Ref | 830 (66.0) | |
| 3-4 | 230 (12.1) | 0.9 (0.7, 1.1) | 316 (16.7) | 1.1 (0.9, 1.3) | 1284 (67.8) | |
| 5+ | 92 (20.6) | 1.5 (1.1, 2.0) | 73 (16.3) | 1.1 (0.8, 1.5) | 271 (60.0) | |
Percentages may not total 100 due to missing data.
Adjusted for all other factors in the table
Table 3. Asthma Symptom Control According to Selected Factors Among 502 Mothers with Provider-diagnosed Asthma.
| Poorly-controlled | Not Well-controlled | Well-controlled | ||||
|---|---|---|---|---|---|---|
| N (%) | Adj OR | N (%) | Adj OR | N (%) | ||
| Age | ||||||
| ≤24 | 55 (43.7) | Ref | 29 (23.0) | Ref | 42 (33.3) | |
| 25-29 | 32 (26.2) | 1.5 (0.7,3.4) | 54 (44.3) | 3.7 (1.6,8.7) | 36 (29.5) | |
| 30-34 | 34 (22.8) | 2.0 (0.8,5.0) | 69 (46.3) | 5.2 (2.2,12) | 46 (30.9) | |
| ≥35 | 26 (25.2) | 1.7 (0.7,4.2) | 48 (46.6) | 4.8 (1.9,12) | 29 (28.2) | |
| Race/Ethnicity | ||||||
| White | 110 (26.3) | Ref | 178 (42.6) | Ref | 130 (31.0) | |
| Black | 12 (52.2) | 2.2 (0.6,7.3) | 6 (26.1) | 1.1 (0.3,4.3) | 5 (21.7) | |
| Hispanic | 21 (43.8) | 0.6 (0.2,1.6) | 12 (25.0) | 0.5 (0.2,1.3) | 15 (31.3) | |
| Asian | 1 (16.7) | 2.3 (0.1,47) | 4 (66.7) | 1.5 (0.1,16) | 1 (16.7) | |
| Other | 3 (42.9) | 0.5 (0.1,4.8) | 2 (28.6) | 0.9 (0.1,7.6) | 2 (28.6) | |
| Body Mass Index | ||||||
| Underweight | 6 (30.0) | 1.3 (0.3,5.3) | 9 (45.0) | 1.4 (0.4,5.3) | 5 (25.0) | |
| Normal weight | 80 (27.9) | Ref | 109 (38.0) | Ref | 98 (34.1) | |
| Overweight | 37 (35.6) | 1.5 (0.8,3.0) | 40 (38.5) | 1.4 (0.6,2.2) | 27 (26.0) | |
| Obese | 21 (25.0) | 0.8 (0.4,1.8) | 41 (48.8) | 1.1 (0.6,2.2) | 22 (26.2) | |
| Education | ||||||
| Less than high school | 20 (39.2) | 1.3 (0.6,3.6) | 19 (37.3) | 1.9 (0.6,6.1) | 12 (23.5) | |
| High school | 77 (33.9) | Ref | 85 (37.4) | Ref | 65 (28.6) | |
| Some college | 50 (22.3) | 0.9 (0.5,1.7) | 98 (43.8) | 0.9 (0.5,1.6) | 78 (33.9) | |
| Smoking | ||||||
| Never | 74 (29.8) | Ref | 89 (35.9) | Ref | 85 (34.3) | |
| Before pregnancy | 30 (22.7) | 1.0 (0.5,1.9) | 62 (47.0) | 1.3 (0.7,2.2) | 40 (30.3) | |
| During pregnancy | 43 (35.2) | 1.5 (0.7,3.3) | 51 (41.8) | 1.7 (0.8,3.6) | 28 (23.0) | |
| Coffee consumption | ||||||
| Never | 57 (33.1) | Ref | 63 (36.6) | Ref | 52 (30.2) | |
| Before pregnancy | 47 (33.3) | 1.3 (0.7,2.6) | 48 (34.0) | 1.1 (0.6,2.2) | 46 (32.6) | |
| During pregnancy | 17 (21.0) | 0.6 (0.2,1.3) | 42 (51.9) | 1.3 (0.6,2.4) | 22 (27.2) | |
| Alcohol | ||||||
| Never | 68 (31.1) | Ref | 103 (47.0) | Ref | 48 (21.9) | |
| Before pregnancy | 75 (28.5) | 0.7 (0.4,1.3) | 89 (33.8) | 0.3 (0.2,0.6) | 99 (37.6) | |
| During pregnancy | 4 (20.0) | 1.0 (0.2,4.3) | 10 (50.0) | 0.5 (0.1,1.6) | 6 (30.0) | |
| Income* | ||||||
| <25,000 | 29 (42.6) | 6.8 (2.3,20) | 29 (42.6) | 2.8 (1.0,8.0) | 10 (14.7) | |
| 25,000-45,000 | 20 (28.6) | 1.0 (0.5,2.2) | 25 (35.7) | 0.7 (0.2,1.5) | 25 (35.7) | |
| >45,000 | 71 (23.3) | Ref | 133 (43.6) | Ref | 101 (33.1) | |
| Household size | ||||||
| 1-2 | 36 (20.2) | Ref | 76 (42.7) | Ref | 66 (37.1) | |
| 3-4 | 68 (29.6) | 1.7 (1.0,3.1) | 93 (40.4) | 1.0 (0.6,1.7) | 69 (30.0) | |
| 5+ | 43 (46.7) | 3.6 (1.6,8.3) | 32 (34.8) | 1.1 (0.5,2.7) | 17 (18.5) | |
Percentages may not total 100 due to missing data.
Adjusted for all other factors in the table
There was no significant trend over time in the prevalence of physician-diagnosed asthma, but the proportion of women with possible asthma declined from 21% to 10% (p<.05) between 1997 and 2005 (data not shown).
The relationship between pregnancy and asthma symptoms varied considerably according to degree of symptom control (Table 4). While, overall, about half (53%) of women with provider-diagnosed asthma reported no change in symptoms, the proportion varied from 41% among poorly-controlled to 69% among well-controlled subjects. Those whose symptoms changed were almost equally divided between those who improved (24.6%) and those who worsened (22.4%). Women with poorly-controlled asthma were more likely to report improvement (34.9%) than women with not well-controlled (23%) or well-controlled asthma (16.7%). Worsening of symptoms was almost equally likely to occur in any trimester (6.7%-7.9%), whereas improvement was noted somewhat more often in the first trimester (11.8% vs. 8.3% or 4.5%).
Table 4.
Changes in Asthma Symptoms during Pregnancy.
| Asthma Control | ||||
|---|---|---|---|---|
| Poorly Controlled | Not Well Controlled | Well Controlled | Total | |
| No Change | 60 (41.0) | 97 (49.5) | 104 (69.3) | 261 (53.0) |
| Symptoms Improved | ||||
| Trimester 1 | 17 (11.6) | 25 (12.8) | 16 (10.7) | 58 (11.8) |
| Trimester 2 | 25 (17.1) | 8 (4.1) | 8 (5.3) | 41 (8.3) |
| Trimester 3 | 9 (6.2) | 12 (6.1) | 1 (0.7) | 22 (4.5) |
| Total | 51 (34.9) | 45 (23.0) | 25 (16.7) | 121 (24.6) |
| Symptoms Worsened | ||||
| Trimester 1 | 9 (6.2) | 24 (12.2) | 6 (4.0) | 39 (7.9) |
| Trimester 2 | 17 (11.6) | 15 (7.7) | 6 (4.0) | 38 (7.7) |
| Trimester 3 | 9 (6.2) | 15 (7.7) | 9 (6.0) | 33 (6.7) |
| Total | 35 (24.0) | 54 (27.6) | 21 (14.0) | 110 (22.4) |
| Total | 146 | 196 | 150 | 492 |
To consider rates of asthma medication use according to asthma status, we examined drugs reported by study subjects to have been used for asthma (indication-based medication use); to capture use among women who had undiagnosed or unknown asthma (and therefore would not report medications use “for asthma”), we also examined drugs known to be used in the treatment of asthma (class-based medication use) (Table 5). For both drug groups, rates were highest among those with provider-diagnosed asthma and within that category use declined with better symptom control; there was very little use among subjects with possible or past asthma. Of note, 63.3% of women whose asthma symptoms were poorly controlled did not use a controller medication during pregnancy. As expected, among women classified as non-asthmatics, none reported using a medication to treat asthma, although 4.1% reported use of a recognized asthma medication for other indications, such as colds or allergy.
Table 5.
Use of Asthma Drugs Among 3609 Mothers of Normal Newborns According to Asthma Status.
| Asthma Status | Indication-based asthma medication use | Indication-or Class-based asthma medication use | |
|---|---|---|---|
| Medication used to treat asthma | Use of at least 1 Asthma Medication | Use of 2 or more known Asthma Medications | |
| Provider-diagnosed | 292 (58.2) | 321 (63.9) | 286 (57.0) |
| Poorly-controlled | 109 (74.1) | 111 (75.5) | 105 (71.4) |
| Not well-controlled | 121 (59.5) | 140(69.3) | 122 (60.4) |
| Well-controlled | 62 (40.5) | 70 (45.8) | 59 (38.6) |
| Possible | 0 (0.0) | 50 (8.7) | 19 (3.3) |
| Past | 1 (0.7) | 6 (4.4) | 6 (4.4) |
| No Asthma | 0 (0.0) | 97 (4.1) | 21 (0.9) |
To explore possible trends in the use of asthma medications over time, we restricted our analyses to women with provider-diagnosed asthma and investigated use according to year of LMP. Among the drug classes, there were no significant trends in use of any asthma drug, any steroid, any beta-2 agonist, or any other asthma medication (Figure 1), nor did use of steroids by specific route change significantly (Figure 2). Virtually all beta-2 agonist use was via inhalation.
Figure 1.
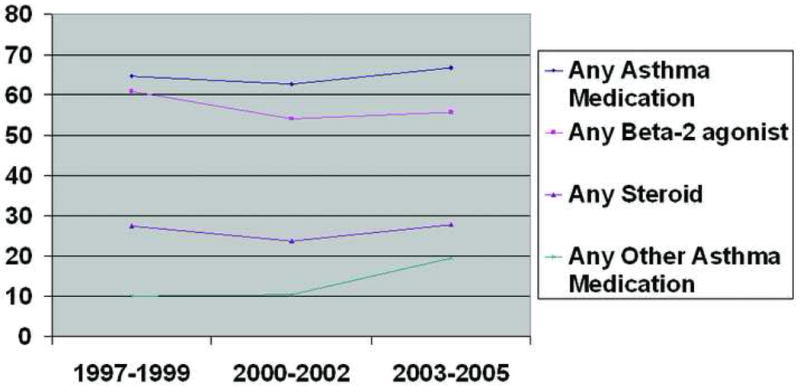
Trends in the Use of Asthma Medications During Pregnancy Among 502 Mothers with Provider-Diagnosed Asthma.
Figure 2.
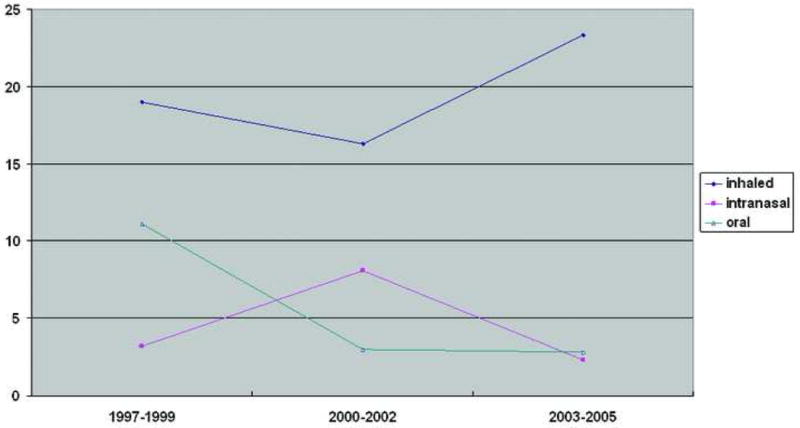
Trends in the Use of Corticosteroids During Pregnancy Among 502 Mothers with Provider-Diagnosed Asthma.
According to lunar month of pregnancy, rates of use of inhaled steroids and beta-2 agonists were nearly constant for the period beginning 2 months prior to the LMP through the 4th lunar month. Inhaled steroids declined from 5% before pregnancy to 4.5%, and for beta-2 agonists the corresponding rates were 16% and 14% (data not shown).
Use of other asthma medications, including leukotriene modifiers and combination products (e.g., Advair®), was generally low, but use of leukotriene modifiers increased consistently over time, and use of combination products increased significantly (p<.01), consistent with their recent introduction to the market (Figure 3).
Figure 3.
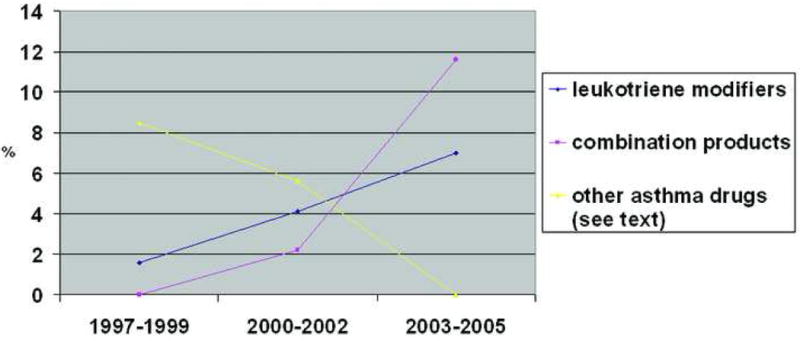
Trends in the Use of Selected Asthma Medications Among 502 Mothers with Provider-Diagnosed Asthma.
The most commonly reported specific asthma medications were albuterol (53%), fluticasone (9.4%), beclomethasone (5.2%), and budesonide (3.0%). Over the study years, only use of fluticasone changed significantly, from 2.4% to 8.4% (p<.05), perhaps due to its relatively recent introduction in 1997 (data not shown).
Discussion
Asthma is recognized as one of the most common serious chronic conditions to complicate pregnancy1, with a prevalence estimated to range up to 8%, although one Australian study among women who had recently given birth reported a rate as high as 12.4%14. We found that over 14% of women in our study reported provider-diagnosed asthma. While this is higher than previously reported2, 15, 16, it may not be inconsistent with other observations. It is known that the prevalence of asthma increased through the 1990's 3, 17, 18 and since our data are derived from 1998-2006, the increasing prevalence may account in part for our higher rates. Also, the northeast region of the United States, and Massachusetts in particular, have among the highest reported rates in the general U.S. population; in 2001, the Massachusetts rate of current asthma was 9.5, compared to the national average of 7.219
One novel aspect of this description of asthma in pregnancy is the attempt to include undiagnosed asthma. To do this, we relied not only on patient reports of physician diagnosed asthma but also used self-reported symptoms to assess asthma status. Using responses to the questions developed to elicit information on symptoms, we estimated the overall prevalence of both provider-diagnosed and possible asthma. Responses to questions were also used to develop a scale of symptom control, and we believe the data on drug treatment for asthma provide indirect support for the validity of this scale. Although drug treatment (other than systemic steroids) was not an element of the symptom control classification, we found a strong inverse trend for increasing medication use according to degree of control. Although our questions measured control more than severity, the two constructs are related, and there is an inverse relationship between severity and control20. Thus, we saw medication use increase as control declined or severity increased, as would be expected. However, among women whose symptoms were poorly-controlled, we found almost 2/3 did not use controller medications, suggesting that asthma symptoms in pregnancy may be inadequately treated. We also found a strong relation between use of multiple drugs and level of asthma symptom control. As would be expected, among women with unrecognized asthma (“possible asthma”), none reported use of a medication to treat asthma; however, these women did use asthma medications for what they considered to be other respiratory indications.
Many of the characteristics that we found to be positively associated with physician-diagnosed asthma among these pregnant women have been previously reported among asthmatics in general: race3, 21, education21, income21, and obesity21-23; we also found asthma to be more common in larger households, a variable associated with socioeconomic status. The higher prevalence of smoking among asthmatics with poorly-controlled disease (35%) was disturbing, given that the 2000 joint position statement of the American College of Obstetrics and Gynecology, the American College of Asthma, Allergy, and Immunology and the American Academy of Asthma, Allergy, and Immunology specifically identifies cigarettes as one of the asthma triggers to be avoided during pregnancy24. Higher smoking rates among asthmatics compared to non-asthmatics have been reported by others14, 25, suggesting that this may be an area for further education and intervention.
Several studies have reported that asthma symptoms remain stable during pregnancy for about a third of women, another third worsen, and the final third improve26, 27. Others have found that asthma is more likely to worsen in women with severe asthma28, 29. Our results may differ slightly due to the fact that we considered only asthma symptoms, while others compared physiologic measurements or exacerbations.
One potential limitation of the current study is that objective measures of asthma status, such as pulmonary function, could not be used to confirm the self-reports. However, self-reported physician-diagnosed asthma has been shown to be a method of identifying patients with asthma in epidemiologic studies.30
This study did not evaluate the risks to the fetus of asthma itself, but there appears to be general acceptance that uncontrolled asthma in pregnancy poses greater risks to the fetus than currently recommended asthma treatments15, 31, 32. It is also widely recognized that there are insufficient data on the risks and safety of asthma medications in pregnancy32-34. In order to provide guidance to health care providers treating pregnant women with asthma, the NAEPP reviewed existing research and recommended what are considered to be effective and relatively safe treatments. Guidelines specifically for pregnancy were published in 19937, followed by a general update in 199735 and a pregnancy-specific update in 20051. Since 1997, inhaled corticosteroids have been recommended as first-line therapy in pregnancy, yet we observed no substantial increase in inhaled corticosteroid use. This finding is consistent with data from the Slone Survey, a population-based survey of medication use in the U.S.36, 37; among women of child-bearing age interviewed between 1998 and 2005, use of inhaled steroids ranged from 1.9% in 1999 to 1.3% in 2006 (personal communication, Judy Kelly). It is possible that women might discontinue their use of inhaled steroids upon learning that they are pregnant because of concerns for the fetus, and discontinuation of these medications in early pregnancy has been reported38. However, we saw little evidence of such a change when we compared use in the two months prior to pregnancy with use through the fourth lunar month of pregnancy.
In summary, this study confirms that asthma is a common condition in pregnant women in Massachusetts; we found that nearly 14% of mothers of normal newborns reported having been told by a health care provider that they had asthma within the five years prior to the pregnancy, and 50% of these reported using at least one medication for their asthma during pregnancy. An additional 16% reported symptoms consistent with asthma, and almost 10% of those were treated with an asthma medication. Given the NAEPP guidelines and the introduction of new medications, we expected to see appreciable changes in the drug management of asthma. Although we observed a small increase in use of inhaled steroids to 23%, it remains well below that of beta-2 agonists (50-60%).
Leukotriene modifiers and combination drugs appear to have had little impact on the pharmacologic treatment of asthma during pregnancy in the current study. Of concern, among women whose symptoms were poorly controlled, only about 37% reported use of controller medications, suggesting inadequate treatment of potentially severe asthma symptoms. These rates of asthma, asthma symptoms and asthma medication use underscore the need to better understand the risks and safety of these medications in pregnancy, as well as the risks of asthma itself.
Acknowledgments
We thank Dawn Jacobs, RN, MPH, Fiona Rice, MPH, Rita Krolak, RN, Kathleen Sheehan, RN, Karen Bennett Mark, RN, Clare Coughlin, RN, Nastia Dynkin, Nancy Rodriguez-Sheridan, and Meghan Malone-Moses, MPH for their assistance in data collection and computer programming and the staff of the Massachusetts Department of Public Health for providing data on Massachusetts births; we also thank all the mothers who participated in the study.
This work was supported by Sanofi-Aventis, Inc; additional support was provided by the National Heart, Lung, and Blood Institutes grant HL 50763 and GlaxoSmithKline.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NAEPP expert panel report. Managing asthma during pregnancy: recommendations for pharmacologic treatment-2004 update. J Allergy Clin Immunol. 2005;115(1):34–46. doi: 10.1016/j.jaci.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Kwon HL, Belanger K, Bracken MB. Asthma prevalence among pregnant and childbearing-aged women in the United States: estimates from national health surveys. Ann Epidemiol. 2003;13(5):317–24. doi: 10.1016/s1047-2797(03)00008-5. [DOI] [PubMed] [Google Scholar]
- 3.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma--United States, 1980-1999. MMWR Surveill Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 4.Stafford RS, Ma J, Finkelstein SN, Haver K, Cockburn I. National trends in asthma visits and asthma pharmacotherapy, 1978-2002. J Allergy Clin Immunol. 2003;111(4):729–35. doi: 10.1067/mai.2003.177. [DOI] [PubMed] [Google Scholar]
- 5.Syed RZ, Zubairi AB, Zafar MA, Qureshi R. Perinatal outcomes in pregnancy with asthma. J Pak Med Assoc. 2008;58(9):525–7. [PubMed] [Google Scholar]
- 6.Wen SW, Demissie K, Liu S. Adverse outcomes in pregnancies of asthmatic women: results from a Canadian population. Ann Epidemiol. 2001;11(1):7–12. doi: 10.1016/s1047-2797(00)00077-6. [DOI] [PubMed] [Google Scholar]
- 7.Pregnancy NAEPRotWGoAa. Management of asthma during pregnancy. In: National Heart L; Blood Institute, editor. NIH publication 93-3279A. NIH; 1993. [Google Scholar]
- 8.Mitchell AA, Rosenberg L, Shapiro S, Slone D. Birth defects related to Bendectin use in pregnancy. I. Oral clefts and cardiac defects. JAMA. 1981;245(22):2311–4. [PubMed] [Google Scholar]
- 9.Werler MM, Hayes C, Louik C, Shapiro S, Mitchell AA. Multivitamin supplementation and risk of birth defects. Am J Epidemiol. 1999;150(7):675–82. doi: 10.1093/oxfordjournals.aje.a010070. [DOI] [PubMed] [Google Scholar]
- 10.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 11.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–7. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 12.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Vollmer WM, Markson LE, O'Connor E, et al. Association of asthma control with health care utilization and quality of life. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1647–52. doi: 10.1164/ajrccm.160.5.9902098. [DOI] [PubMed] [Google Scholar]
- 14.Kurinczuk JJ, Parsons DE, Dawes V, Burton PR. The relationship between asthma and smoking during pregnancy. Women Health. 1999;29(3):31–47. doi: 10.1300/J013v29n03_03. [DOI] [PubMed] [Google Scholar]
- 15.Gluck JC, Gluck PA. Asthma controller therapy during pregnancy. Am J Obstet Gynecol. 2005;192(2):369–80. doi: 10.1016/j.ajog.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 16.Venkataraman MT, Shanies HM. Pregnancy and asthma. J Asthma. 1997;34(4):265–71. doi: 10.3109/02770909709067216. [DOI] [PubMed] [Google Scholar]
- 17.Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol. 2006;6(11):869–74. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]
- 18.Weiss KB, Gergen PJ, Wagener DK. Breathing better or wheezing worse? The changing epidemiology of asthma morbidity and mortality. Annu Rev Public Health. 1993;14:491–513. doi: 10.1146/annurev.pu.14.050193.002423. [DOI] [PubMed] [Google Scholar]
- 19.Self-reported asthma prevalence and control among adults--United States, 2001. MMWR Morb Mortal Wkly Rep. 2003;52(17):381–4. [PubMed] [Google Scholar]
- 20.Schatz M, Zeiger RS, Vollmer WM, Mosen D, Cook EF. Determinants of future long-term asthma control. J Allergy Clin Immunol. 2006;118(5):1048–53. doi: 10.1016/j.jaci.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 21.Apter AJ. Advances in adult asthma 2006: its risk factors, course, and management. J Allergy Clin Immunol. 2007;119(3):563–6. doi: 10.1016/j.jaci.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115(5):897–909. doi: 10.1016/j.jaci.2004.11.050. quiz 10. [DOI] [PubMed] [Google Scholar]
- 23.Kwon HL, Triche EW, Belanger K, Bracken MB. The epidemiology of asthma during pregnancy: prevalence, diagnosis, and symptoms. Immunol Allergy Clin North Am. 2006;26(1):29–62. doi: 10.1016/j.iac.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 24.The use of newer asthma and allergy medications during pregnancy. The American College of Obstetricians and Gynecologists (ACOG) and The American College of Allergy, Asthma and Immunology (ACAAI) Ann Allergy Asthma Immunol. 2000;84(5):475–80. [PubMed] [Google Scholar]
- 25.Alexander S, Dodds L, Armson BA. Perinatal outcomes in women with asthma during pregnancy. Obstet Gynecol. 1998;92(3):435–40. doi: 10.1016/s0029-7844(98)00191-4. [DOI] [PubMed] [Google Scholar]
- 26.Lao TT, Huengsburg M. Labour and delivery in mothers with asthma. Eur J Obstet Gynecol Reprod Biol. 1990;35(2-3):183–90. doi: 10.1016/0028-2243(90)90160-3. [DOI] [PubMed] [Google Scholar]
- 27.Schatz M, Harden K, Forsythe A, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol. 1988;81(3):509–17. [PubMed] [Google Scholar]
- 28.Murphy VE, Gibson P, Talbot PI, Clifton VL. Severe asthma exacerbations during pregnancy. Obstet Gynecol. 2005;106(5 Pt 1):1046–54. doi: 10.1097/01.AOG.0000185281.21716.02. [DOI] [PubMed] [Google Scholar]
- 29.Schatz M, Dombrowski MP, Wise R, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112(2):283–8. doi: 10.1067/mai.2003.1516. [DOI] [PubMed] [Google Scholar]
- 30.Toren K, Brisman J, Jarvholm B. Asthma and asthma-like symptoms in adults assessed by questionnaires. A literature review. Chest. 1993;104(2):600–8. doi: 10.1378/chest.104.2.600. [DOI] [PubMed] [Google Scholar]
- 31.Murphy VE, Gibson PG, Smith R, Clifton VL. Asthma during pregnancy: mechanisms and treatment implications. Eur Respir J. 2005;25(4):731–50. doi: 10.1183/09031936.05.00085704. [DOI] [PubMed] [Google Scholar]
- 32.Rey E, Boulet LP. Asthma in pregnancy. Bmj. 2007;334(7593):582–5. doi: 10.1136/bmj.39112.717674.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schatz M. Asthma during pregnancy: interrelationships and management. Ann Allergy. 1992;68(2):123–33. [PubMed] [Google Scholar]
- 34.Schatz M. The efficacy and safety of asthma medications during pregnancy. Semin Perinatol. 2001;25(3):145–52. doi: 10.1053/sper.2001.24569. [DOI] [PubMed] [Google Scholar]
- 35.National Heart L; Blood Institute, editor. 2 NAEaPPEPR. Guidelines for the diagnosis and management of asthma. NIH; 1997. [PubMed] [Google Scholar]
- 36.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. Jama. 2002;287(3):337–44. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 37.Slone Survey. Slone Epidemiology Center at Boston University. [April 8, 2008]; http://www.bu.edu/slone/SloneSurvey/SloneSurvey.htm.
- 38.Enriquez R, Wu P, Griffin MR, et al. Cessation of asthma medication in early pregnancy. Am J Obstet Gynecol. 2006;195(1):149–53. doi: 10.1016/j.ajog.2006.01.065. [DOI] [PubMed] [Google Scholar]


