Abstract
Forged in the early 1960s, the paradigm for pharmaceutical innovation has remained virtually unchanged for nearly 50 years. During a period when most other research-based industries have made frequent and often sweeping modifications to their R&D processes, the pharmaceutical sector continues to utilize a drug development process that is slow, inefficient, risky, and expensive. Few who work in or follow the activities of the pharmaceutical industry question whether change is coming. They know that the pharmaceutical sector, as currently structured, is unable to deliver enough new products to market to generate revenues sufficient to sustain its own growth. Nearly all major drug developers are critically examining current R&D practices and, in some cases, considering a radical overhaul of their R&D models. But key questions remain. What will the landscape for pharmaceutical innovation look like in the future? And, who will develop tomorrow’s medicines?
CURRENT CHALLENGES FOR THE RESEARCH-BASED INDUSTRY
To understand why “business as usual” is no longer an option for the research-based drug industry, it is worth considering some of the myriad challenges that drug companies currently face. At the top of the list is the upcoming onslaught of patent expirations of many high-revenue-generating branded medicines. Between 2009 and 2012, worldwide sales for these products will exceed $112 billion (Table 1). Included in this list are 36 blockbusters (drugs with annual sales of $1 billion or more). Some important examples include Singulair (montelukast), with more than $4 billion in annual sales (patent expiration in 2012); Plavix (clopidogrel), with more than $8 billion in annual sales (patent expiration in 2011); and Lipitor (atorvastatin), with an industry-leading $13.7 billion in annual sales (patent expiration in 2010). Given that only 3 in 10 new products, on average, generate revenues equal to or greater than average industry R&D costs,1 the loss of patent protection on these blockbusters represents a very real threat to the industry’s ability to sustain its own growth. Without question, many of the large pharma mergers and acquisitions announced in 2009 reflect the industry’s desire to avoid the imminent danger of the patent cliff, rather than an interest in enhancing R&D capabilities or scope.
Table 1.
Patent expirations for 10 top-selling drugs each year
| 2009 | 2010 | 2011 | 2012 | ||||
|---|---|---|---|---|---|---|---|
| Product | 2007 Sales ($MM) | Product | 2007 Sales ($MM) | Product | 2007 Sales ($MM) | Product | 2007 Sales ($MM) |
| Prevacid | 3,962 | Protonix | 4,221 | Lipitor | 13,652 | Diovan | 5,012 |
| Topamax | 2,453 | Cozaar/Hyzaar | 3,350 | Plavix | 8,079 | Singulair | 4,266 |
| Lamictal | 2,194 | Aricept | 3,311 | Advair | 6,998 | Lexapro | 3,044 |
| Valtrex | 1,868 | Levaquin | 2,862 | Zyprexa | 4,661 | Viagra | 1,764 |
| Cellcept | 1,677 | Effexor XR | 2,657a | Actos | 4,333 | Avandia | 1,754 |
| Keppra | 1,407 | Taxotere | 2,569 | Seroquel | 4,219 | Symbicort | 1,575 |
| Flomax | 1,399 | Arimidex | 1,730 | Avapro | 2,685 | Zometa | 1,297 |
| Imitrex | 1,370 | Gemzar | 1,592 | Xalatan | 1,604 | Detrol | 1,190 |
| Adderall XR | 1,031 | Coreg | 1,174 | Avelox | 1,013 | Geodon | 854 |
| Suboxone | 531a | NovoSeven | 1,078 | Xeloda | 959 | Provigil | 852 |
| Total | $17,892 | Total | $24,544 | Total | $48,203 | Total | $21,608 |
US sales only.
Data from Tufts center for the study of Drug Development, 2010; MedNews 27(7), 2008; http://www.drugs.com/top200.
Another major challenge for the research-based industry comes from payers and other third-party providers, who, in their efforts to stem rising health-care costs, are increasingly demanding that drug companies demonstrate that their products offer therapeutic or cost advantages over competitors’ products and nonpharmaceutical treatment options. This movement is being accelerated in the United States by the creation of a comparative effectiveness research process for marketed drugs. Even in therapeutic areas that in the past were considered immune to cost pressures, such as antineoplastic agents, payers have become increasingly restrictive about the products that they will cover and the uses for which such coverage will be available. For example, in the United Kingdom, the National Institute for Health and Clinical Excellence, which uses health technology assessments to determine whether a product should be reimbursed by the National Health Service, initially recommended that coverage for Fludara (fludarabine) for chronic lymphocytic leukemia and Avastin (bevacizumab) and Erbitux (cetuximab) for colorectal cancer be denied.2 The bottom line is that, in many cases, if companies are to ensure a place on drug formularies and obtain reasonable reimbursement rates, they can no longer simply demonstrate that their drug is safe and effective—they must also show that their product offers significant economic or therapeutic value. One approach has been for companies to meet with groups such as the UK’s National Institute for Health and Clinical Excellence early in the clinical development process to help plan late-stage clinical trials that will generate the necessary evidence to support reimbursement of treatment costs to the patient.
The regulatory environment has also become more stringent in recent years, leading to new and more demanding hurdles that a new drug must clear in order to enter the market. Following the well-publicized market withdrawals of Vioxx (rofecoxib) and several other high-profile pharmaceutical products for safety reasons, the US Food and Drug Administration (FDA) and its counterparts in other major markets have put greater focus on preapproval safety evaluations and increased their reliance on postapproval mechanisms to monitor product safety and use. For example, with the passage in the United States of the Food and Drug Administration Amendments Act of 2007,3 the FDA was given new authority to demand submission of risk evaluation and mitigation strategies along with application for regulatory approval, to require postmarket clinical studies on approved products if safety questions arise, to mandate changes to a drug’s approved labeling, and to impose new distribution and use restrictions on marketed drugs. An unpublished analysis by the Tufts Center for the Study of Drug Development (Tufts CSDD) shows that, since the withdrawal of Vioxx from the market in 2004, submission of risk-minimization action plans in the United States have surged by 75%, as has the number of risk evaluation and mitigation strategies subsequently approved.4,5
At the end of the day, however, the greatest challenges confronting the research-based industry involve bringing promising new drug candidates out of discovery and into development, and managing the very cumbersome drug development process. In the area of drug discovery, new technologies such as high-throughput screening, combinatorial chemistry, and a host of “omics” tools (including pharmacogenomics, proteomics, and metabolomics) were supposed to usher in a new era of innovative drug discovery, leading to newer and better medicines for many diseases for which treatment was inadequate or lacking. For many years, however, in the absence of appropriate validation tools that would allow researchers to identify molecules having the greatest likelihood of successful development, these discovery technologies merely added time and cost to the R&D process without providing any appreciable benefits. Despite the industry’s concerted efforts over the past two decades to effect substantive improvements in performance and efficiency in the area of drug development, the metrics suggest that few or no gains have been made.
One hopes, however, that ongoing efforts within industry, government, and academic institutions to identify and validate new biomarkers will ultimately increase the power and utility of the discovery technologies.
R&D METRICS: THE TIME, RISK, AND COST OF NEW DRUG DEVELOPMENT
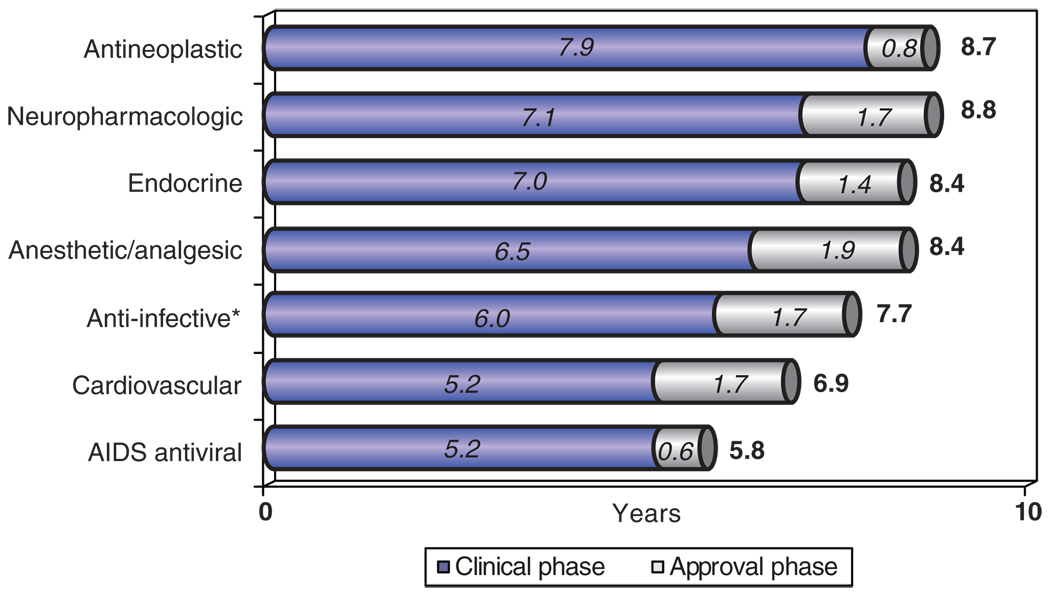
For more than 30 years, Tufts CSDD has documented the growing challenge of bringing new pharmaceutical products to market. Recent analyses of the development path for new molecular and biological entities approved by the FDA show that the average time required to take a product from the start of clinical testing to regulatory approval is 7.2 years. This mean, however, masks considerable variation among different therapeutic areas. For example, as shown in Figure 1, clinical development times range from a relatively short period of 5.2 years for AIDS antiviral agents to the extraordinarily long period of 7.9 years for antineoplastic agents. In the case of neuropharmacologic and cancer drugs, if one adds the average time to obtain regulatory approval (1.7 and 0.8 years, respectively), the total time to bring a candidate drug from the start of human testing to market is nearly 9 years (this excludes the preclinical, animal testing phase, as well as discovery and research). Given that these products are intended to meet critical therapeutic needs and large market demands, this is an extremely long time.
Figure 1.
Clinical development times (from IND filing to NDA submission) and regulatory approval times (from NDA submission to approval) for new molecular entities approved by the US Food and Drug Administration during the 5-year period 2003–2007, grouped by therapeutic area. Analysis by the Tufts Center for the Study of Drug Development, based on data included in its approved products database. *Note that the anti-infectives category excludes AIDS antiviral agents. IND, investigational new drug application; NDA, new drug application.
High attrition rates are another major concern for drug developers. Tufts CSDD has shown that, for candidate drugs that entered the clinical testing phase during 1999–2004, the clinical approval success rate—i.e., the likelihood that a compound entering clinical testing will eventually reach the marketplace—was 16%.6 It is noteworthy that, despite industry’s efforts over the years to address low success rates, this level actually represents a decline from the 21.5% average for drugs that began clinical testing in the early 1990s. As in the case of drug development times, the 16% average masks considerable differences across therapeutic areas. Clinical success rates range from 27% for systemic anti-infectives to dismal rates of 8 and 7% for neuropharmacologic and cardiovascular agents, respectively.
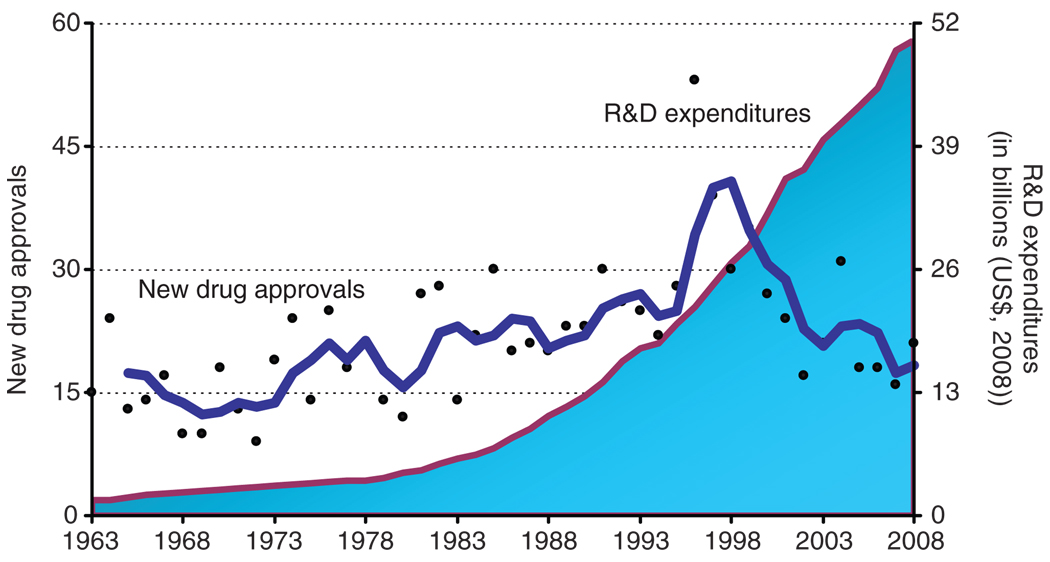
Not surprisingly, long development times coupled with very low success rates translate into high overall R&D costs for the research-based industry. A recent Tufts CSDD study showed that the average capitalized cost to bring one new biopharmaceutical product to market, including the cost of failures, is $1.24 billion, in 2005 dollars.7 For conventional pharmaceutical products, the corresponding figure is $1.32 billion. These high drug development costs are causing overall R&D spending across the pharmaceutical sector to spiral out of control, exceeding $50 billion in the United States in 2008 (see Figure 2).
Figure 2.
New drug approvals (dots), represented on the left vertical axis, and pharmaceutical R&D expenditures (shaded area), represented on the right vertical axis, in the United States from 1963 to 2008. R&D expenditures are presented in terms of constant 2008 dollar value. The trend line is a 3-year moving average. The source of drug approval data is the Tufts Center for the Study of Drug Development (CSDD). The source of R&D expenditure data is the Pharmaceutical Research and Manufacturers of America; Industry Profile 2009; conversion of actual expenses to constant dollars was performed by Tufts CSDD.
EXPLAINING CURRENT DRUG DEVELOPMENT METRICS
The factors contributing to the onerous time, cost, and risk burdens associated with new drug development are multifaceted and interrelated. For one, drug sponsors are increasingly focusing on drugs to treat chronic and complex indications, such as those for psychiatric and neurologic disorders and cancer. Clinical programs for these indications tend to be larger, longer, and more complex than those for others, resulting in, among other things, the need for greater patient enrollment in clinical studies. Patient recruitment and retention, however, are major bottlenecks in drug development programs, often leading to significant delays in study initiation and higher trial costs.
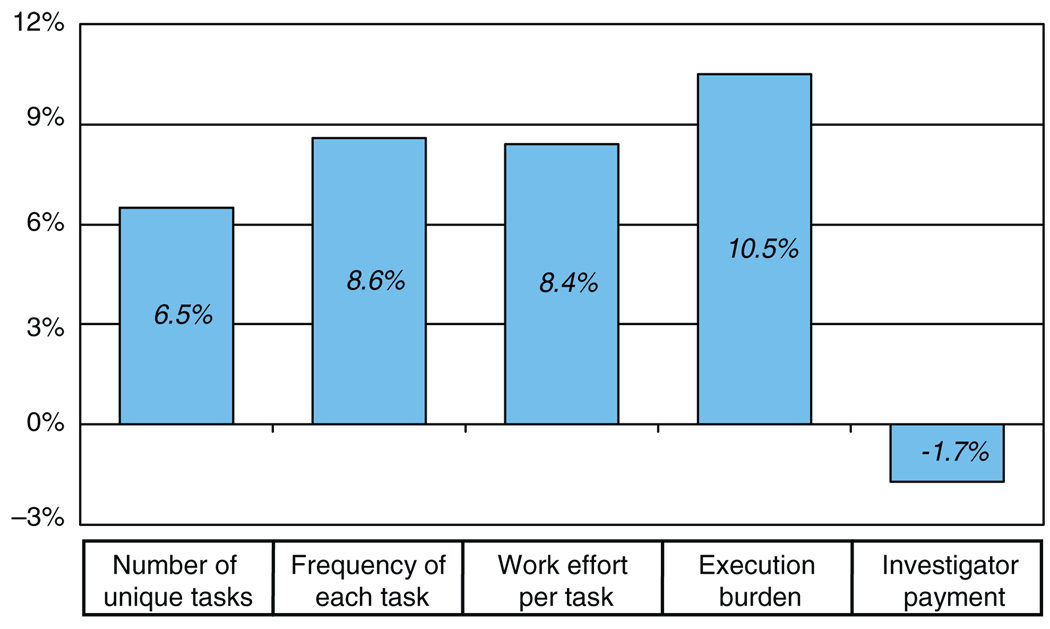
Protocol complexity is another factor that contributes to escalating drug development times and costs. A 2008 Tufts CSDD study on protocol complexity in new drug development found that, in clinical studies conducted between 1999 and 2005, there were substantial increases in (i) the number of unique procedures, (ii) the overall frequency of procedures, (iii) the eligibility criteria for enrollment, and (iv) the investigative site work burden over the 6-year period (results summarized in Figure 3).8 Correlated with these increases were an observed increase in clinical development cycle times, a decline in patient enrollment and retention rates, a drop in grant funding per procedure per protocol, and a more than threefold increase in the overall number of case report form pages.
Figure 3.
Summary of clinical protocol design trends as presented in Getz et al.8 See the original article for a description of categories and periods of measurement.
Blockbuster R&D strategies (those that focus on large patient populations and therefore high sales potential) have also contributed to high development costs by increasing the likelihood of late-stage clinical failures. These models, adopted by many drug sponsors in the 1990s, typically focus on chronic diseases, such as hypertension, arthritis, hypercholesterolemia, and depression. Unfortunately, these indications often represent crowded pharmaceutical markets, and therefore a greater likelihood of termination of the development process at the clinical stage for economic or market-related reasons. Tufts CSDD analyses have shown that these economic failures tend to occur late in the clinical development process, at an average of 3.7 years after the start of clinical studies, when R&D costs and resource demands are at their peak.9
Other factors that contribute to the increasing challenge of bringing new drugs to market include the growing demands on sponsors from regulatory agencies, as well as the need to conduct more preapproval, market-oriented studies to ensure a competitive drug label and increase the likelihood of obtaining formulary coverage and a favorable reimbursement decision by health plans and other insurers.
Regardless of the reasons, the dramatic rise in R&D spending by drug developers, combined with the relatively low number of new product approvals over the past 10 years, has created an unsustainable situation for the research-based industry. This dire state of affairs, which is graphically depicted in Figure 2, is particularly vexing for the large-cap sector of the industry. Despite having relatively large cash reserves and low debt—which has enabled many recent mega acquisitions, such as Pfizer’s acquisition of Wyeth for $68 billion, Merck’s acquisition of Schering-Plough for $41 billion, and Roche’s purchase of the remaining shares of Genentech for $46 billion—many large pharmaceutical and biotechnology companies are posting very low earnings.
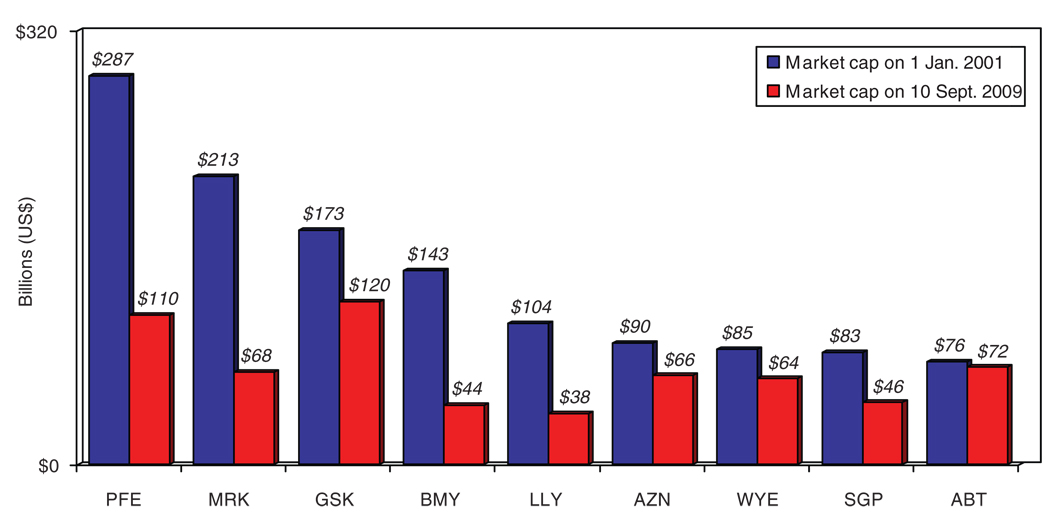
Poor earnings by the large companies in the pharmaceutical industry are reflected in declining stock market capitalizations (i.e., the aggregate value of a company, obtained by multiplying the number of outstanding shares by the current price per share). A Tufts CSDD analysis of the stock market values of nine top-tier pharmaceutical companies indicates that there was a nearly $650 billion loss in value overall in these companies between January 2001 and September 2009 (see Figure 4). How does one explain the counterintuitive finding that these top-tier firms have large cash reserves and low debt but poor earnings? Why are these companies not performing well on the stock market? A likely answer is that investors are concerned about pipeline viability; that is, they are questioning the ability of large pharmaceutical companies to successfully bring enough new products to market to generate and sustain growth. This sentiment is reflected in investment advice offered by a chief investment officer of a major European investment firm in 2007: “A cheap stock is no argument to buy when there’s no growth on the horizon. I don’t see pharmaceutical shares outperforming. Buying a drug stock is buying a pretty big risk these days” (Bloomberg.com, 16 August 2007).
Figure 4.
Market capitalization of top-tier pharmaceutical companies in January 2001 and September 2009. Cumulative loss in market capitalization for these companies over the period is $626 billion. Ticker symbols are as follows: ABT, Abbott; AZN, AstraZeneca; BMY, Bristol-Myers Squibb; GSK, GlaxoSmithKline; LLY, Lilly; MRK, Merck; PFE, Pfizer; SGP, Schering-Plough; WYE, Wyeth. Data from http://www.valueline.com; Tufts Center for the Study of Drug Development analysis.
IS IT CORE COMPETENCY OR CORE COMPLACENCY?
For the research-based pharmaceutical industry, the prescription for success is clear. To be competitive in today’s challenging economic, regulatory, and political environment, drug sponsors must reduce product development times, terminate unpromising candidates sooner, improve patient-recruitment capabilities, enhance the protocol design process, control development costs, maintain quality, focus on areas of high therapeutic need, and dramatically boost productivity. This list of goals is not new to the drug industry; nearly all major drug companies have been attempting to address these performance issues for well over a decade—but, regrettably, with limited success.
So, what makes the likelihood of success any greater now than in the past? Are companies currently capable of achieving the type of substantive improvements in drug development efficiency and performance that have proven to be so elusive over the past decade? The answer lies in the ability of the industry to transform an outdated and entrenched R&D paradigm.
The most promising bit of evidence that this change is occurring is the merging of operational and strategic performance objectives within the industry. Drug sponsors are increasingly aware that efforts to boost productivity by focusing solely on operational performance or corporate strategy are doomed to failure. Across the sector, companies are attempting to align the goals of operations functions with those of the strategic planners.
This is particularly apparent in the way many companies are now utilizing contract research organizations (CROs) and other service providers. The relationship between sponsor and CRO is evolving from one in which CROs were merely ad hoc providers of transactional services to one in which CROs serve as functional service providers (FSPs) or alliance partners to the industry. Such FSP and alliance relationships are characterized by formal agreements between the drug sponsor and the CRO through which the sponsor sheds the particular capability that is provided by the CRO. This avoids the type of “shadow R&D” expense that is typical of transactional relationships. The shift to this new model of outsourcing is highlighted by Eli Lilly’s decision in 2008 to outsource its entire preclinical animal toxicology function, having entered into an agreement with the full-service CRO Covance to conduct these analyses for the company.10
For drug sponsors, FSP and alliance relationships offer distinct advantages over transactional arrangements. They allow sponsors to predict and manage resource needs better, maintain leaner operations, engage with the CRO at a senior management level, and coordinate standard operating procedures with their CRO partner. Not surprisingly, in a comparative analysis across a spectrum of pharmaceutical companies, Tufts CSDD found that a commonly reported “best practice” among companies that consistently performed better than industry averages on a series of performance measures was a focus on core competencies and a high level of strategic FSP outsourcing that allowed these companies to better allocate and prioritize resources.11,12 This shift is reflected in a growing demand for outsourced clinical services. For example, Getz has recently shown that the total spending on contract clinical services has outpaced that for total global clinical development spending by 4.3% (13.4 vs. 9.1%, respectively).13
The key to establishing effective FSP relationships for industry is the ability of individual companies to identify their own “core competencies” and to avoid “core complacency,” that is, the tendency to maintain a function simply because that is the way the company has operated in the past. It is instructive to consider how many functions that were once unquestioned core competencies and “owned” by the drug industry are now being either routinely outsourced or critically reassessed. Examples include discovery chemistry, manufacturing, the work of central laboratories, and data management. In addition, in the areas of research, discovery, and early development, some large pharmaceutical companies are establishing new outsourcing models. These include Lilly’s arrangement with its independently operating Chorus unit14 to conduct proof-of-concept studies, and the novel agreement among Johnson & Johnson, Lilly, Merck, Novartis, and Pfizer to co-found Enlight Biosciences,15 a newly created organization that identifies promising leads, arranges for early R&D, and then licenses or sells the drug candidates to the member companies.
MOVING TO A NEW BUSINESS MODEL FOR PHARMACEUTICAL R&D
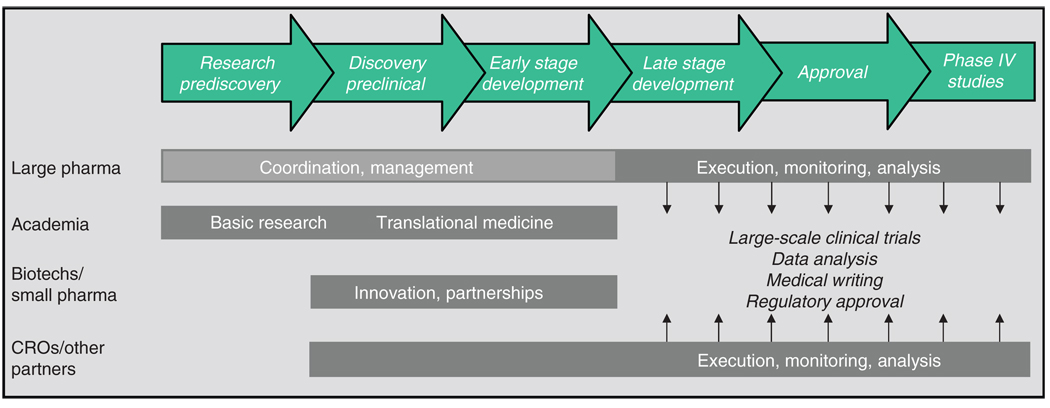
The drug industry’s gradual embrace of FSP relationships and alliances heralds the shift from a fully integrated pharmaceutical company model of R&D, in which a sponsor “owns” the entire drug development process from synthesis to marketing, toward a networked model of innovation, sometimes referred to as a fully integrated pharmaceutical network, or FIPNet. FIPNets engage all the major stakeholders in the drug development process, melding the core competencies of each component to leverage capabilities, enhance efficiency, and boost output. Figure 5 shows an example of a FIPNet model. As seen in the figure, each stakeholder plays a complementary and integrated role in the R&D process.
Figure 5.
A FIPNet (fully integrated pharmaceutical network) model of drug development, in which the core capabilities of different stakeholders in the development process are leveraged. CRO, contract research organization.
Academia
Academic research centers provide both basic research, which is the foundation of pharmaceutical and biopharmaceutical innovation, and translational medicine, which promotes the transition from basic research to applied clinical research and commercialization. The growth of translational research and the evolving role of academic research centers in pharmaceutical innovation were catalyzed in 2004 by the National Institutes of Health Roadmap,16 the goal of which is to invest in new pathways to discovery, support research teams of the future, and re-engineer the clinical research enterprise.17 Efforts included the creation of centers of translational research at each of the National Institutes of Health institutes, as well as the launch, in 2006, of the Clinical and Translational Research Awards program. With nearly 50 of these awards distributed to date, and a goal of 60 centers to be funded by the year 2012, academic research centers in the United States are increasingly being viewed as essential players—and potential partners—in pharmaceutical innovation.
Small pharmaceutical and biotechnology companies
Another critical component of the FIPNet model is the small-tier pharmaceutical and biotechnology sector. These small companies, often saddled with high debt and few, if any, marketed products to generate revenue, are heavily dependent on larger companies, venture capitalists, and other investors to sustain their R&D activities. The large pharmaceutical companies, however, view this sector as more flexible and less risk averse than larger companies. Moreover, they are less encumbered by functional silos, making them better able to focus on emerging technologies and on developing highly innovative therapeutics. In today’s environment of icy capital markets and dwindling large pharmaceutical pipelines, there is an obvious synergy between the large and small pharmaceutical sectors. This is evidenced by the growing number of partnerships and alliances between small and large companies, as well as by the acquisitions of small firms by large companies. Examples include GlaxoSmithKline’s relationship with Concert Pharmaceuticals and Sanofi-Aventis’s relationship with Regeneron, as well as the acquisitions of MedImmune by AstraZeneca, of Mederex by Bristol-Myers Squibb, and of ImClone by Lilly. In the FIPNet model, small pharmaceutical and biotech companies provide highly innovative R&D and early development capabilities.
CROs and other partners
As discussed above, pharmaceutical companies are increasingly entering into FSP and alliance relationships with CROs to reduce overhead costs, increase efficiency, and bolster output. Other R&D partners include nongovernmental organizations and public–private partnerships, such as the Medicines for Malaria Venture, the TB Alliance, and the International AIDS Vaccine Initiative, which, in partnership with industry, are playing an increasingly important role in the development of new medicines, especially those for neglected diseases. Another public–private partnership is the European Union’s Innovative Medicines Initiative, established in December 2007 (ref. 18), which represents a unique partnership between the European Federation of Pharmaceutical Industries and Associations and the European Community. The initiative is intended to pool the competencies of the public and private sectors in the European Union. Finally, patient-advocacy groups and other disease-specific organizations, such as the Multiple Myeloma Research Foundation and the Juvenile Diabetes Research Foundation, have become increasingly proactive in funding academic and industry research and furthering specific research agendas.
Large pharmaceutical companies
For many of the larger companies that have established a reputation for being able to take a molecule from the laboratory bench to the pharmacy shelf, the move to innovation networks is particularly challenging. It is reasonable to ask what role large pharmaceutical companies will play in the new innovation landscape, especially in research and discovery and early development. The likely answer is that in the early R&D stages, large companies will function as coordinators and managers of the activities of their academic, small pharma, and other partners, providing resources, investment, and insights into the product development process. In the later stages of development, large pharmaceutical companies, utilizing their FSP and alliance relationships with CROs, will conduct the large, multicenter phase III clinical trials, oversee the regulatory affairs function, manage scale-up and manufacturing, and handle marketing and sales.
FINAL THOUGHTS
The current environment for innovation presents formidable economic, regulatory, and political challenges for the research-based pharmaceutical industry. In particular, the growing time, cost, and risk related to drug development are stubborn obstacles to filling industry pipelines and boosting the output of new pharmaceutical and biological products. Presented here is a model of an innovation network. Although structures may vary, the innovation network offers the best mechanism to ensure viability and economic success for all sectors of the pharmaceutical and biotechnology industry, as well as the uninterrupted flow of innovative lifesaving and life-improving medicines for waiting patients.
Acknowledgments
The author is director of the Tufts Center for the Study of Drug Development, a nonprofit, academic research center at Tufts University, Boston, MA. The Tufts Center is funded in part by unrestricted grants from pharmaceutical and biotechnology firms, as well as companies that provide related services (e.g., contract research, consulting, and technology firms) to the research-based industry. The author is also supported in part by grant UL1 RR025752 from the National Center for Research Resources. No other potential conflict of interest relevant to this article is reported.
Footnotes
CONFLICT OF INTEREST
No other potential conflict of interest relevant to this article is reported.
References
- 1.Grabowski HG, Vernon JM, DiMasi JA. Returns on research and development for 1990s new drug introductions. Pharmacoeconomics. 2002;20 suppl. 3:11–29. doi: 10.2165/00019053-200220003-00002. [DOI] [PubMed] [Google Scholar]
- 2.Resisting NICE. BioCentury: The Bernstein Report on BioBusiness. 2007 October 8;vol. 15(no. 45):A1–A11. [Google Scholar]
- 3.FDA Amendments Act. Pub. L. No. 110-85, 121 Stat. 823. [Google Scholar]
- 4.Jenkins J. Washington, DC: American Course on Drug Development and Regulatory Sciences; 2009. Sep 30, Development of Drugs in the Safety Environment Post FDAAA. [Google Scholar]
- 5.A tale of two drugs: atrial fibrillation reviews draw focus on RiskMAPS. The Pink Sheet. 2008;70:8. [Google Scholar]
- 6.DiMasi JA, Feldman L, Seckler A, Wilson A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin Pharmacol Ther. doi: 10.1038/clpt.2009.295. this issue. [DOI] [PubMed] [Google Scholar]
- 7.DiMasi JA, Grabowski HG. The cost of biopharmaceutical R&D: is biotech different? Managerial Decision Econ. 2007;28:469–479. [Google Scholar]
- 8.Getz KA, Wenger J, Campo RA, Seguine ES, Kaitin KI. Assessing the impact of protocol design changes on clinical trial performance. Am. J. Ther. 2008;15:450–457. doi: 10.1097/MJT.0b013e31816b9027. [DOI] [PubMed] [Google Scholar]
- 9.DiMasi JA. Risks in new drug development: approval success rates for investigational drugs. Clin Pharmacol Ther. 2001;69:297–307. doi: 10.1067/mcp.2001.115446. [DOI] [PubMed] [Google Scholar]
- 10.Lilly sells its Greenfield, Indiana, operations to Covance; expands existing collaboration between the two companies. 2008 [press release] < http://newsroom.lilly.com/ReleaseDetail.cfm?ReleaseID=326599>.
- 11.Kaitin KI, editor. Fastest Drug Developers Consistently Best Peers on Key Performance Metrics. no. 5. vol. 8. Boston: Tufts Center for the Study of Drug Development Impact Report; 2006. September/October, [Google Scholar]
- 12.Kaitin KI, editor. Best R&D Practices of Top Pharma/Biotech Performers. no. 4. vol. 4. Boston: Tufts Center for the Study of Drug Development R&D Management Report; 2009. Oct, [Google Scholar]
- 13.Getz K. FIPNet: Pharma’s New, Sexy, But Not Yet Ready For Prime-Time Model. < http://www.nxtbook.com/nxtbooks/advanstar/insideoutsourcing_200911/#/12>.
- 14.Lilly sings a new tune: Chorus unit brings high efficiency note to early R&D. The Pink Sheet. 2007 February 26;vol. 69(no. 009):26. < http://www.choruspharma.com/p070226_26.pdf>.
- 15.Enlight Biosciences. 2008 [press release] < http://www.enlightbio.com/news/enlight-biosciences-launched-in-collaboration-with-merck-and-co-inc-pfizer-and-eli-lilly-pharmaceutical-and-academic-luminaries-combineforces-to-address-innovation-bottleneck-33>.
- 16.The NIH Common Fund. Common Fund Initiatives. < http://nihroadmap.nih.gov/initiatives.asp>.
- 17.Milne CP, Kaitin KI. Translational medicine: An engine of change for bringing new technology to community health. Sci. Transl. Med. 2009;1:5cm5. doi: 10.1126/scitranslmed.3000222. < http://stm.sciencemag.org/content/1/5/5cm5.full.pdf>. [DOI] [PubMed]
- 18.Council of the European Union. Regulation setting up the Joint Undertaking for the implementation of the Joint Technology Initiative on Innovative Medicines. 2007 < http://register.consilium.europa.eu/pdf/en/07/st15/st15977.en07.pdf>.