Abstract
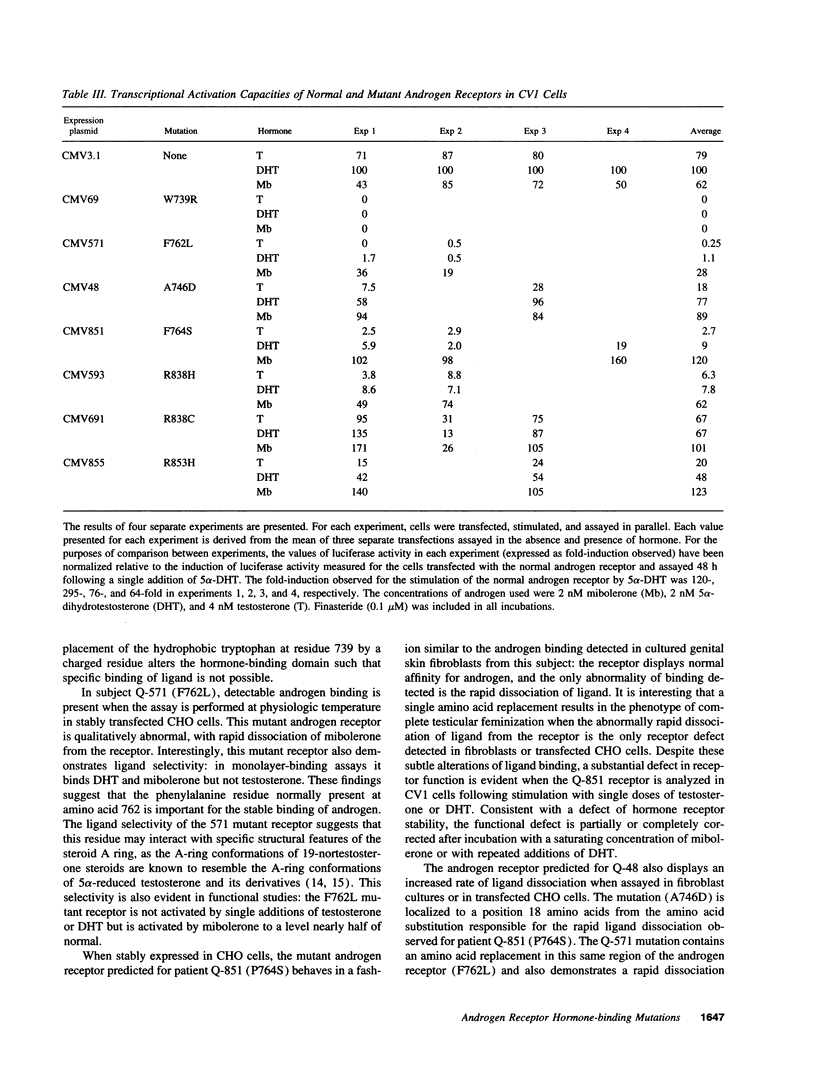
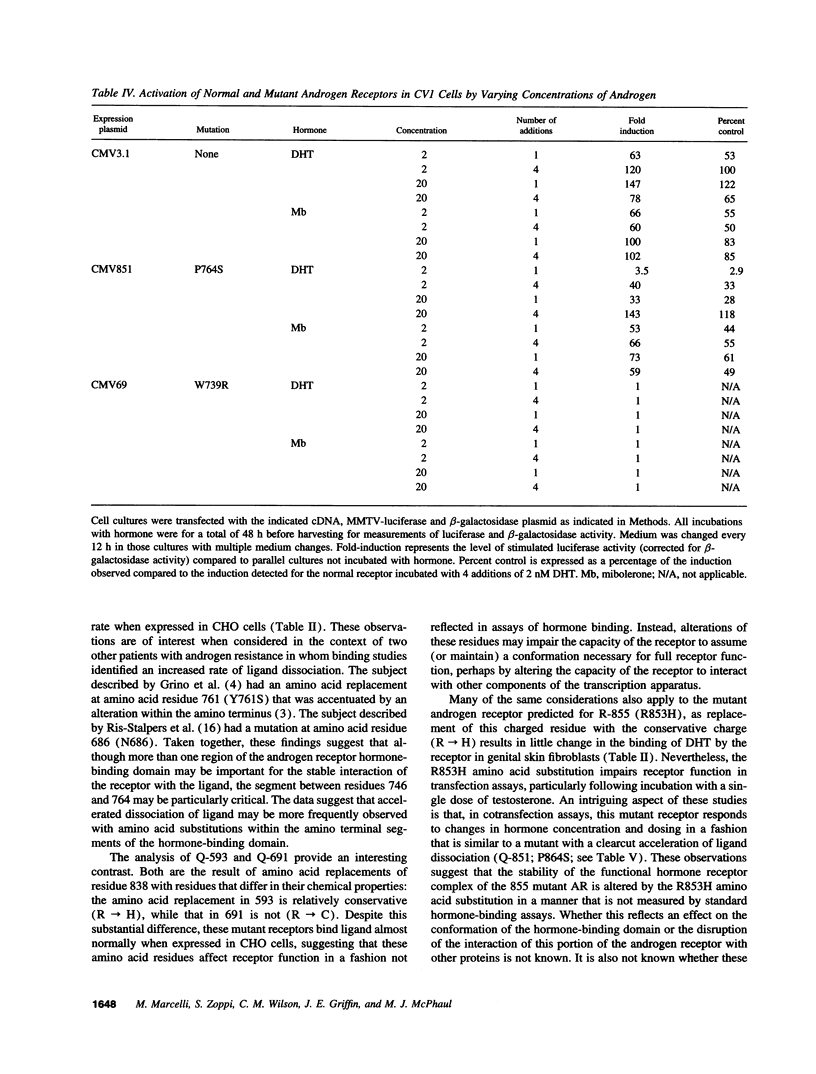
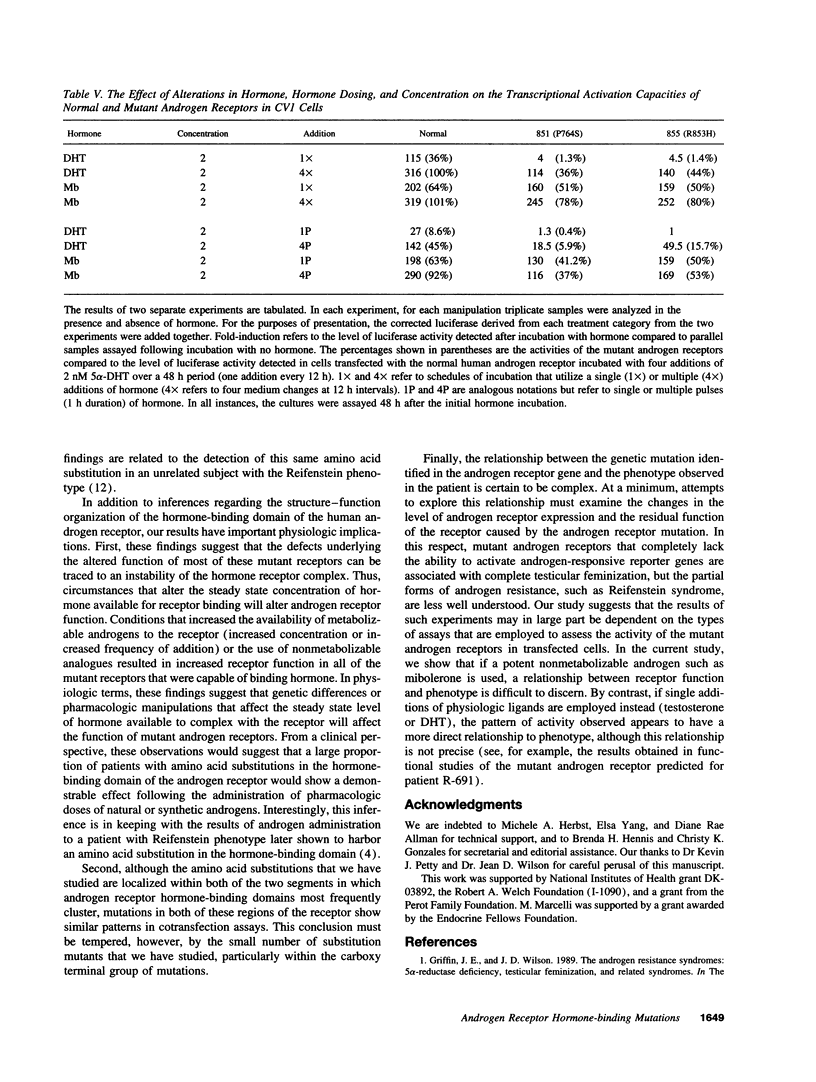
We have investigated the basis of androgen resistance in seven unrelated individuals with complete testicular feminization or Reifenstein syndrome caused by single amino acid substitutions in the hormone-binding domain of the androgen receptor. Monolayer-binding assays of cultured genital skin fibroblasts demonstrated absent ligand binding, qualitative abnormalities of androgen binding, or a decreased amount of qualitatively normal receptor. The consequences of these mutations were examined by introducing the mutations by site-directed mutagenesis into the androgen receptor cDNA sequence and expressing the mutant cDNAs in mammalian cells. The effects of the amino acid substitutions on the binding of different androgens and on the capacity of the ligand-bound receptors to activate a reporter gene were investigated. Substantial differences were found in the responses of the mutant androgen receptors to incubation with testosterone, 5 alpha-dihydrotestosterone, and mibolerone. In several instances, increased doses of hormone or increased frequency of hormone addition to the incubation medium resulted in normal or near normal activation of a reporter gene by cells expressing the mutant androgen receptors. These studies suggest that the stability of the hormone receptor complex is a major determinant of receptor function in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deslypere J. P., Young M., Wilson J. D., McPhaul M. J. Testosterone and 5 alpha-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol Cell Endocrinol. 1992 Oct;88(1-3):15–22. doi: 10.1016/0303-7207(92)90004-p. [DOI] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Griffin J. E., Durrant J. L. Qualitative receptor defects in families with androgen resistance: failure of stabilization of the fibroblast cytosol androgen receptor. J Clin Endocrinol Metab. 1982 Sep;55(3):465–474. doi: 10.1210/jcem-55-3-465. [DOI] [PubMed] [Google Scholar]
- Grino P. B., Isidro-Gutierrez R. F., Griffin J. E., Wilson J. D. Androgen resistance associated with a qualitative abnormality of the androgen receptor and responsive to high dose androgen therapy. J Clin Endocrinol Metab. 1989 Mar;68(3):578–584. doi: 10.1210/jcem-68-3-578. [DOI] [PubMed] [Google Scholar]
- Liao S., Liang T., Fang S., Castañeda E., Shao T. C. Steroid structure and androgenic activity. Specificities involved in the receptor binding and nuclear retention of various androgens. J Biol Chem. 1973 Sep 10;248(17):6154–6162. [PubMed] [Google Scholar]
- Marcelli M., Tilley W. D., Wilson C. M., Griffin J. E., Wilson J. D., McPhaul M. J. Definition of the human androgen receptor gene structure permits the identification of mutations that cause androgen resistance: premature termination of the receptor protein at amino acid residue 588 causes complete androgen resistance. Mol Endocrinol. 1990 Aug;4(8):1105–1116. doi: 10.1210/mend-4-8-1105. [DOI] [PubMed] [Google Scholar]
- Marcelli M., Tilley W. D., Wilson C. M., Wilson J. D., Griffin J. E., McPhaul M. J. A single nucleotide substitution introduces a premature termination codon into the androgen receptor gene of a patient with receptor-negative androgen resistance. J Clin Invest. 1990 May;85(5):1522–1528. doi: 10.1172/JCI114599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhaul M. J., Marcelli M., Tilley W. D., Griffin J. E., Isidro-Gutierrez R. F., Wilson J. D. Molecular basis of androgen resistance in a family with a qualitative abnormality of the androgen receptor and responsive to high-dose androgen therapy. J Clin Invest. 1991 Apr;87(4):1413–1421. doi: 10.1172/JCI115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhaul M. J., Marcelli M., Zoppi S., Wilson C. M., Griffin J. E., Wilson J. D. Mutations in the ligand-binding domain of the androgen receptor gene cluster in two regions of the gene. J Clin Invest. 1992 Nov;90(5):2097–2101. doi: 10.1172/JCI116093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris-Stalpers C., Trifiro M. A., Kuiper G. G., Jenster G., Romalo G., Sai T., van Rooij H. C., Kaufman M., Rosenfield R. L., Liao S. Substitution of aspartic acid-686 by histidine or asparagine in the human androgen receptor leads to a functionally inactive protein with altered hormone-binding characteristics. Mol Endocrinol. 1991 Oct;5(10):1562–1569. doi: 10.1210/mend-5-10-1562. [DOI] [PubMed] [Google Scholar]
- Saartok T., Dahlberg E., Gustafsson J. A. Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin. Endocrinology. 1984 Jun;114(6):2100–2106. doi: 10.1210/endo-114-6-2100. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tilley W. D., Marcelli M., Wilson J. D., McPhaul M. J. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci U S A. 1989 Jan;86(1):327–331. doi: 10.1073/pnas.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Griffin J. E., Wilson J. D., Marcelli M., Zoppi S., McPhaul M. J. Immunoreactive androgen receptor expression in subjects with androgen resistance. J Clin Endocrinol Metab. 1992 Dec;75(6):1474–1478. doi: 10.1210/jcem.75.6.1464650. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]