Abstract
Context: Enlargement of the vestibular aqueduct (EVA) is a commonly detected inner ear anomaly related to hearing loss and often associated with mutations of SLC26A4 encoding pendrin, a transmembrane exchanger of Cl−, I−, and HCO3−. Here we describe the phenotypes of 27 Korean EVA subjects and their SLC26A4 genotypes determined by bidirectional nucleotide sequencing. Results: The detected variants include two novel missense substitutions (p.V138L and p.P542R). We characterized the ability of p.V138L and p.P542R pendrin products to traffic to the plasma membrane in COS-7 cells and to transport Cl−, I−, and HCO3− in Xenopus oocytes. The results indicate that p.P542R is a benign polymorphic variant, whereas p.V138L is a pathogenic mutation. Since this and other studies of East Asian EVA cohorts show that the majority of SLC26A4 mutations affect either or both of two amplicons (exons 7–8 and 19), we developed a hierarchical protocol that integrates direct sequencing with denaturing high-performance liquid chromatography analyses for detection of SLC26A4 mutations in these populations. We validated the cost efficiency of the integrated protocol by a simulated screen of published East Asian EVA cohorts with known SLC26A4 genotypes. Conclusions: Our study further defines the spectrum of SLC26A4 mutations among East Asians and demonstrates a rapid and efficient protocol for their detection.
Introduction
Enlargement of the vestibular aqueduct (EVA; MIM 600709) is a common radiological inner ear malformation associated with sensorineural hearing loss (Valvassori and Clemis, 1978). EVA is the radiologic finding of highest penetrance in the syndrome of recessive sensorineural hearing loss with goiter (Pendred syndrome [PDS]; MIM 274600) but can also be observed without goiter in nonsyndromic EVA (NSEVA) (DFNB4; MIM 600791) (Phelps et al., 1998). Mutations of SLC26A4, which encodes pendrin, can cause either PDS or NSEVA (Everett et al., 1997; Li et al., 1998; Usami et al., 1999). Pendrin, a polytopic transmembrane protein, exchanges Cl− and I− across the apical membrane of thyroid follicular cells (Royaux et al., 2000; Gillam et al., 2004). It is also expressed in the inner ear, where it has been proposed to exchange Cl− and HCO3− (Wangemann et al., 2007). Pendrin is also expressed in type B intercalated cells of the renal collecting duct (Royaux et al., 2001), and in respiratory and mammary epithelial cells (Rillema and Hill, 2003; Pedemonte et al., 2007), although its absence in these tissues is not known to contribute to the phenotype of PDS.
The overlap of SLC26A4 genotypes and phenotypes in PDS and NSEVA has led to uncertainty in their nosology. This uncertainty is compounded by the detection of only one recessive mutant allele of SLC26A4 in many EVA patients and by the uncertain pathogenic potential of many SLC26A4 variants (Choi et al., 2009). Many of these variants are missense substitutions whose pathogenic potential may be clarified by evaluation of their genotypic and phenotypic context in the EVA patient as well as by functional characterization of their intracellular trafficking and anion exchange properties (Choi et al., 2009).
The expense of bidirectional DNA sequencing of all 21 exons of SLC26A4 and their adjacent splice sites is an obstacle to widespread molecular diagnostic testing of EVA patients. Alternative methods include denaturing high-performance liquid chromatography (DHPLC) (Prasad et al., 2004; Albert et al., 2006) and exon-specific strategies (Park et al., 2003). The latter can be incorporated into hierarchical screens that account for different identities and distributions of mutations of SLC26A4 among different ethnic populations (Tsukamoto et al., 2003; Park et al., 2005; Pryor et al., 2005; Wu et al., 2005b; Albert et al., 2006; Wang et al., 2007). This type of strategy has been proposed for the comparatively restricted distribution of SLC26A4 mutations prevalent in East Asian EVA subjects (Park et al., 2005; Wu et al., 2005b; Dai et al., 2008; Guo et al., 2008).
The purpose of this study was to refine the known SLC26A4 mutation spectrum in Korean EVA patients and to develop a DHPLC screen to detect these mutations. We show that a DHPLC screen can be integrated with direct nucleotide sequencing into a cost-effective hierarchical protocol for the detection of SLC26A4 mutations in Korean EVA patients. We validate the protocol by a simulated analysis of published EVA cohorts with known SLC26A4 genotypes from a variety of East Asian populations. We also demonstrate an evaluation of pathogenicity of two interesting novel missense substitutions, one of which is the second most frequent missense variant from our Korean cohort in this study.
Materials and Methods
Subjects
This study was approved by the institutional review board of the Seoul National University Hospital (IRB00002177 and FWA00008721). Written informed consent was obtained from the subjects or their parents. Twenty-seven unrelated subjects (probands) had a computed tomography scan showing a vestibular aqueduct diameter greater than 1.5 mm at the midpoint between the common crus and the external aperture. All subjects had bilateral severe to profound hearing loss (≥70 dB HL in the better-hearing ear) by serial audiological examinations, including pure tone audiometry, auditory brainstem response analysis, or both. Subjects with syndromic features other than goiter were excluded. Of the 27 subjects, 11 had a family history of hearing loss with EVA, whereas the other 16 subjects were sporadic cases. Fifty normal Koreans were control subjects for genotype analyses.
SLC26A4 genotype analysis
Genomic DNA was isolated from peripheral blood leukocytes using Gentra PureGene (Qiagen, Valencia, CA). All 21 exons and adjacent intronic sequences of SLC26A4 were amplified by polymerase chain reaction (PCR) and sequenced as described (Park et al., 2003). DNA from parents and siblings, when available, was evaluated to determine segregation and meiotic phase configuration of mutant alleles of SLC26A4 in probands with two mutations.
Functional analysis of novel missense variants
We expressed wild-type or variant pendrin (p.V138L and p.P542R), fused at its C-terminus to enhanced green fluorescent protein, in COS-7 cells and evaluated its trafficking as described (Choi et al., 2009). Wild-type and variant pendrin products were expressed in Xenopus oocytes for measurement of Cl−/I− and Cl−/HCO3− exchange activity as described (Stewart et al., 2004, 2007; Choi et al., 2009).
DHPLC
DHPLC primers and amplicon lengths were selected as described (Prasad et al., 2004). We also designed a new primer pair for amplicon 7–8: 5′-GTG GGA AGA TTC ATA TGA GAA TTG (forward) and 5′-GTT TCT TCC AGA TCA CAC ACA A (reverse). PCR amplifications were performed in 50 μL containing 100 μM dNTPs, 100 ng template DNA, 10 pmol of each primer, and 1.25 U Ace-Taq DNA Polymerase (Genenmed, Seoul, Korea) in 1 × reaction buffer (30 mM Tris-HCl, pH 9.1; 50 mM KCl; 2 mM MgCl2). The amplifications were performed using a PTC-100 Thermal Cycler (Bio-Rad, Hercules, CA) with an initial denaturation of 5 min at 95°C followed by 30-step cycles (10 s at 94°C, 20 s at 57°C, and 30 s at 72°C) and a final extension for 10 min at 72°C.
DHPLC was performed using the Wave System (Transgenomic, Omaha, NE). PCR products were mixed with sequence-confirmed wild-type products at a 2:1 ratio to detect homozygous mutations. The mixture was denatured at 95°C for 5 min followed by a reduction in temperature from 95°C to 25°C over 45 min (Prasad et al., 2004). Five microliters of each mixture was loaded onto a DNASep-HT column (Transgenomic), and amplicons were eluted in 0.1 M triethylammonium acetate, pH 7, using a linear acetonitrile gradient at a flow rate of 1.5 mL/min. Heteroduplex mismatches were detected by the appearance of extra peaks or a distortion of peak shape. Temperatures were calculated using Navigator Software (Transgenomic) and a Web site tool at http://insertion.stanford.edu/melt.html. Amplicons for exons 14, 16, 18, and 21 required one DHPLC temperature, whereas all other amplicons required two assays at two different temperatures (Table 1).
Table 1.
Denaturing High-Performance Liquid Chromatography Amplicons and Temperatures
| Exon | Size (bp) | DHPLC temperature (°C) |
|---|---|---|
| 1 | 403 | 61.7, 65.2 |
| 2 | 292 | 64.0, 68.0 |
| 3 | 242 | 59.2, 60.9 |
| 4 | 243 | 54.9, 60.5 |
| 5 | 373 | 53.6, 59.5 |
| 6 | 288 | 55.0, 60.0 |
| 7–8 | 576 | 54.7, 56.7 |
| 9 | 254 | 57.5, 59.0 |
| 10 | 288 | 58.2, 64.7 |
| 11–12 | 485 | 56.1, 59.6 |
| 13 | 264 | 53.0, 61.9 |
| 14 | 387 | 56.7 |
| 15 | 254 | 55.0, 56.0 |
| 16 | 241 | 56.2 |
| 17 | 374 | 55.7, 59.0 |
| 18 | 190 | 56.1 |
| 19 | 312 | 55.3, 57.7 |
| 20 | 246 | 53.3, 56.8 |
| 21 | 296 | 59.0 |
DHPLC, denaturing high-performance liquid chromatography.
Simulated mutation screening
To compare the cost of different screening strategies, we assembled a composite cohort of 247 EVA subjects with known SLC26A4 genotypes from seven independent studies. The cohort included 58 Koreans from this and two other studies (Park et al., 2005; Lee et al., 2008), 42 Japanese (Tsukamoto et al., 2003), and 147 Chinese (mainland Chinese +Han Chinese) (Wu et al., 2005b; Hu et al., 2007; Wang et al., 2007). Owing to the possibility of overlap of some EVA subjects between two reported Japanese cohorts (Tsukamoto et al., 2003; Suzuki et al., 2007), we included only one study with a larger cohort in our composite cohort (Tsukamoto et al., 2003). The estimated reagent cost per amplicon for sequencing and DHPLC screening at a single temperature was US$11.00 and US$1.60, respectively.
Results
SLC26A4 genotypes
The SLC26A4 genotypes of our Korean EVA subjects are shown in Table 2. Sixteen (59.3%) of 27 subjects had two likely pathogenic variants, 7 (25.9%) had one variant, while 4 (14.8%) had no detectable pathogenic variants. p.H723R and c.919-2A>G constituted 30 (76.9%) of 39 potentially pathogenic alleles. Eight (50%) of 16 subjects carrying two SLC26A4 variants were homozygous for either of these two prevalent founder mutations (Park et al., 2003; Wu et al., 2005b).
Table 2.
SLC26A4 Phenotypes and Genotypes of Korean Subjects with Enlargement of the Vestibular Aqueduct
| |
|
|
Hearing (dB)a |
SLC26A4 genotype |
||
|---|---|---|---|---|---|---|
| Subject | Sex | Age (yearmonth) | Right | Left | Allele 1 | Allele 2 |
| 1 | F | 12 | 100 | 100 | p.H723R | Wild type |
| 2 | F | 17 | 110 | 70 | Wild type | Wild type |
| 3 | M | 9 | 110 | 110 | p.H723R | p.M147V |
| 4 | F | 12 | 110 | 110 | p.H723R | p.V138L |
| 5 | M | 16 | 110 | 110 | p.H723R | c.919-2A>G |
| 6 | M | 11 | 100 | 100 | p.H723R | p.L676Q |
| 7 | M | 8 | 100 | 100 | p.P542R | Wild type |
| 8 | M | 12 | 110 | 90 | p.H723R | c.919-2A>G |
| 9 | F | 4 | NR | NR | p.H723R | p.H723R |
| 10 | M | 21 | 110 | 110 | p.H723R | Wild type |
| 11 | M | 24/12 | NR | NR | p.H723R | p.H723R |
| 12 | M | 110/12 | NR | NR | c.919-2A>G | p.V659L |
| 13 | F | 7 | 85 | 85 | c.919-2A>G | c.919-2A>G |
| 14 | F | 12 | NR | NR | p.H723R | p.H723R |
| 15 | M | 7 | NR | NR | p.H723R | p.H723R |
| 16 | F | 8 | NR | NR | p.H723R | p.H723R |
| 17 | F | 3 | 75 | 85 | p.H723R | p.H723R |
| 18 | M | 24/12 | NR | NR | p.H723Rb | p.V138Lb |
| c.165-13T>Gb | ||||||
| 19 | M | 2 | NR | NR | p.H723R | p.H723R |
| 20 | M | 12/12 | NR | NR | Wild type | Wild type |
| 21 | M | 34 | 100 | 100 | Wild type | Wild type |
| 22 | F | 8 | 90 | 90 | p.H723R | Wild type |
| 23 | F | 2 | NR | NR | c.919-2A>G | Wild type |
| 24 | M | 3 | 100 | 100 | Wild type | Wild type |
| 25 | F | 12 | 70 | 70 | c.1707 + 5G>A | Wild type |
| 26 | M | 21 | NR | NR | p.H723R | Wild type |
| 27 | M | 27 | 100 | 100 | p.V138L | c.1149+3A>G |
c.1341 + 47T>C (rs17154326) were detected in subjects 7 and 21. In subject 7, c.1341 + 47T>C was in trans with p.P542R.
Novel variants are in bold.
Pure tone averages (dB HL) at 500 Hz, 1 kHz, and 2 kHz or auditory brainstem response threshold.
Meiotic configuration could not be determined.
NR, no response.
Novel missense variants
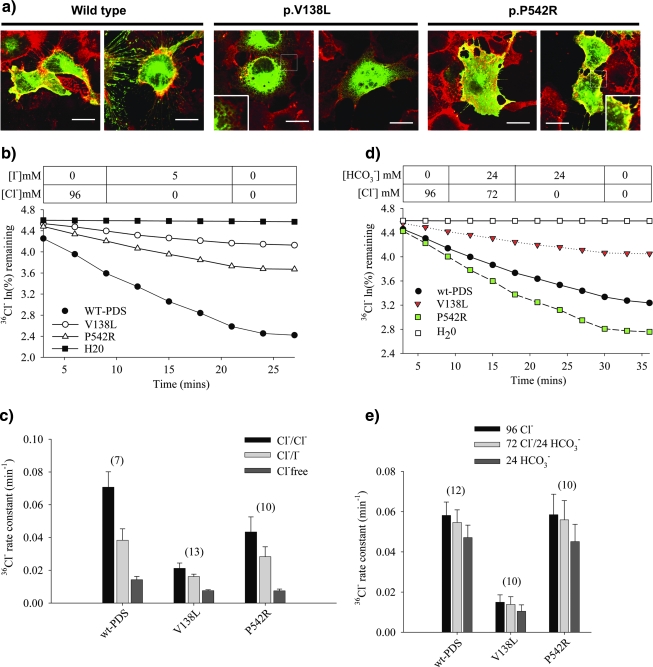
We detected two novel missense variants, p.V138L (c.412G>C) and p.P542R (c.1625C>G), that segregated with deafness within each family. p.V138L was the second most frequent missense variant in this study (Table 2). p.P542R was detected in a sporadic EVA case (subject 7), and the subject had more severe inner ear anomalies in addition to atypical EVA (Fig. 1). These residues are conserved in human, rat, and mouse SLC26A4 orthologs. Neither variant was detected in 100 normal Korean control chromosomes. The p.V138L pendrin product displayed an intracellular reticular pattern with no detectable localization at the plasma membrane in COS-7 cells, whereas the wild-type and p.P542R products colocalized with concanavalin A at the cell surface (Fig. 2a). The p.V138L pendrin rate constants for Cl−/Cl−, Cl−/I−, and Cl−/HCO3− exchange, respectively, were 26–30%, 42%, and 22% of those for wild-type pendrin. The p.P542R pendrin product exhibited respective rate constants that were 61–100%, 74%, and 95% of the corresponding wild-type rate constants, respectively (Fig. 2c, e).
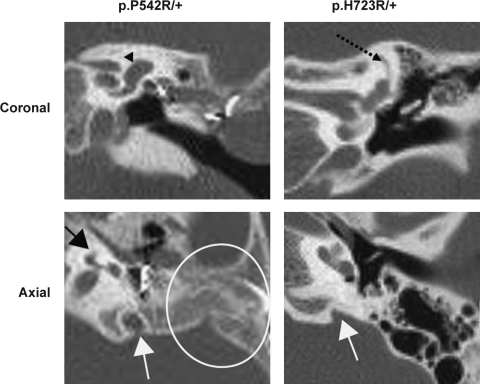
FIG. 1.
Temporal bone computed tomography scans. A coronal section of subject 7 (p.P542R/+) shows an enlarged vestibule (black arrowhead) and absent superior semicircular canal. An axial section demonstrates severe cochlear hypoplasia (black arrow) and poor mastoid pneumatization (white circle) in addition to enlargement of the vestibular aqueduct (EVA) (white arrow). Examination of adjacent sections confirmed this latter structure as the vestibular aqueduct and not the jugular bulb. Another subject (p.H723R/+) with a typical EVA has a superior semicircular canal (black dotted arrow), relatively normal cochlea, a well-pneumatized and aerated mastoid, and EVA (white arrow).
FIG. 2.
(a) Intracellular trafficking of pendrin variants in COS-7 cells. Merged representative images show green fluorescent protein–tagged wild-type or missense allele products (green) and concanavalin A staining (red) of plasma membrane. Colocalization (yellow) demonstrates targeting of pendrin to the plasma membrane. Scale bars = 20 μm. (b) Time course of 36Cl− efflux from representative oocytes and (c) rate constants (mean ± SE) for each group of (n) oocytes expressing wild-type or variant pendrin in extracellular medium containing 96 mM Cl−, 0 Cl−/5 mM I−, or 0 Cl−/0 I−, with gluconate as equimolar substituent anion. (d) Time course of 36Cl− efflux from representative oocytes and (e) rate constants (mean ± SE) for each group of (n) oocytes expressing wild-type or variant pendrin in extracellular medium containing 96 mM Cl−, 72 mM Cl−/24 mM HCO3−, or 0 Cl−/24 mM HCO3−/72 mM gluconate. Color images available online at www.liebertonline.com/gtmb.
Novel splice site variant
The c.165-13T>G intronic variant in subject 18 may be pathogenic because this variant was not detected in 100 normal Korean control chromosomes, and it is located in a highly conserved region among human, rat, mouse, dog, and chicken. Two different programs (http://rulai.cshl.edu/cgi-in/tools/ESE3/esefinder.cgi?process=home and www.fruitfly.org) predict that this nucleotide change creates a cryptic splice acceptor site whose utilization would disrupt the open reading frame. Samples from family members were unavailable to determine if c.165-13T>G is in cis configuration with either of two other mutations, p.V138L or p.H723R, in subject 18.
DHPLC detection of Korean variants of SLC26A4
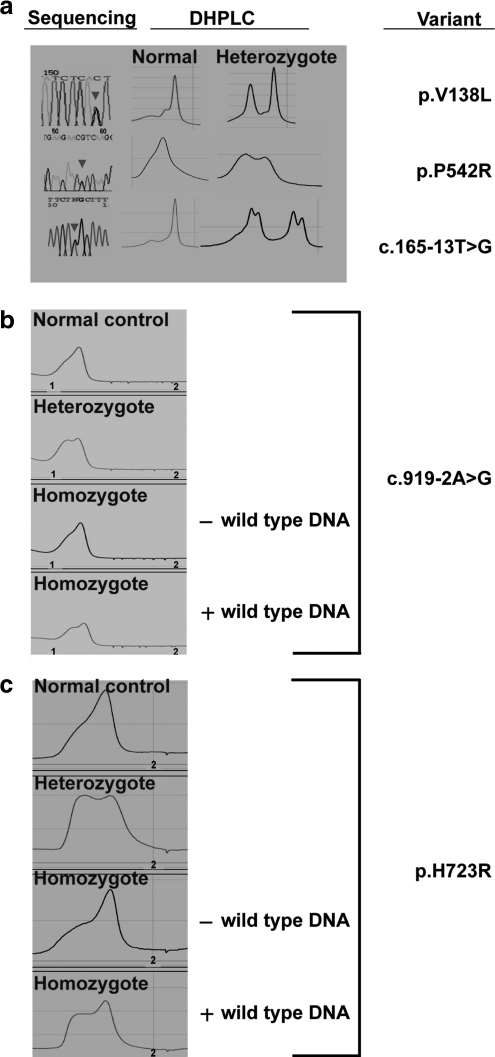
To evaluate the sensitivity and specificity of DHPLC for detecting SLC26A4 variants in our EVA cohort, we analyzed 513 amplicons (27 EVA subjects vs. 19 amplicons). Forty-two amplicons showed an aberrant elution profile (EP), yielding a detection rate of 1.55 (42/27) abnormal EPs per subject. Thirty-four (81%) of 42 aberrant EPs represented true nucleotide variations. These 34 EPs included two from amplicons with a known single-nucleotide polymorphism (SNP), c.1341+47T>C (rs17154326). All of the variants among the 27 subjects could be identified by abnormal EP, yielding a sensitivity of 100% for DHPLC (Fig. 3a). All of the eight false-positive EPs were for wild-type amplicon 7–8 amplified with primers originally reported by Prasad et al. (2004) (data not shown). Therefore, we designed a new primer pair for amplicon 7–8 that produces distinguishable EP for the wild-type allele and c.919-2A>G homozygotes (Fig. 3b).
FIG. 3.
(a) Electropherograms and denaturing high-performance liquid chromatography (DHPLC) elution profiles (EP) for novel SLC26A4 sequence variants (red arrowheads). (b, c) DHPLC EP of normal control, heterozygous, or homozygous c.919-2A>G (b) or p.H723R (c) variants with or without sequence-verified wild-type DNA added at a ratio of 2:1. EP differences for homozygotes are less prominent than for heterozygotes.
SLC26A4 mutation distribution
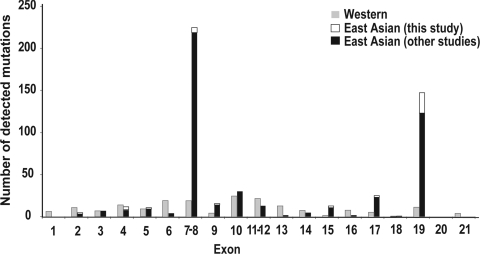
The exonic distribution of SLC26A4 mutations among East Asian EVA subjects from this and seven other studies is shown (Fig. 4). Japanese cohorts showed a similar spectrum with that from our Korean cohort (Tsukamoto et al., 2003; Suzuki et al., 2007). Although Han Chinese population showed a distinctive mutation spectrum where c.919-2A>G alone accounted for most of the mutant alleles of SLC26A4 (Wu et al., 2005b), two largest studies with mainland Chinese population (Wang et al., 2007; Guo et al., 2008) indicated that p.H723R is the second most frequent mutation following c.919-2A>G. Resultantly, 319 (73%) of 532 East Asian EVA mutations are located in DHPLC amplicons 7–8 and 19. Among these eight studies, individual SLC26A4 genotypes were available for 247 subjects from this and six independent studies. Ninety-seven (39%) of 247 subjects had two mutations located in either or both of these amplicons. This was true for 36%, 31%, and 43% of Korean, Japanese, and Chinese (mainland Chinese + Han Chinese) subjects, respectively.
FIG. 4.
Exonic distribution of SLC26A4 mutations in East Asian EVA subjects from this and seven other studies (Tsukamoto et al., 2003; Park et al., 2005; Wu et al., 2005b; Hu et al., 2007; Wang et al., 2007; Guo et al., 2008; Lee et al., 2008) and Caucasian (western) EVA subjects from four studies (Prasad et al., 2004; Pryor et al., 2005; Albert et al., 2006; Pera et al., 2008).
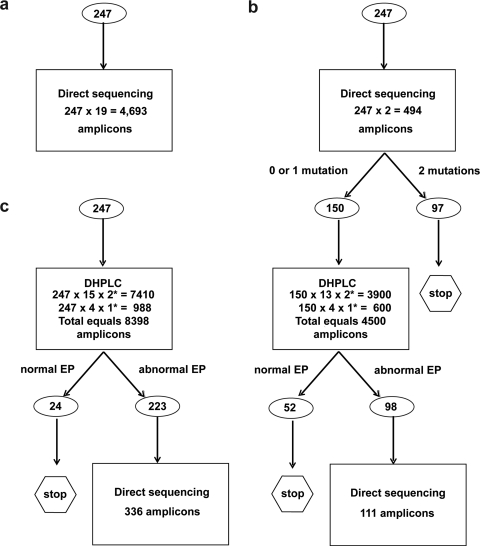
Integrated DHPLC sequencing protocol
We designed an integrated protocol for East Asian SLC26A4 mutation detection in which the first step is bidirectional sequencing of two amplicons (exons 7–8 and 19). If this does not reveal two pathogenic alleles, the other 17 amplicons are screened by DHPLC and directly sequenced if they show abnormal EPs (Fig. 5). We sought to compare the estimated costs of this integrated protocol with those of nonhierarchical direct sequencing or DHPLC analyses of all amplicons. We calculated the estimated costs for each of these strategies in simulated screens based upon published genotype results for a composite cohort of 247 East Asian EVA subjects from this and six other studies. The costs per subject for the integrated, nonhierarchical DHPLC, and sequencing protocols were $56.00, $69.36, and $209.00, respectively. Our integrated protocol is predicted to reduce the overall cost by 19% and 73% compared with the latter two protocols, respectively (Fig. 5).
FIG. 5.
Schematic diagrams illustrating (a) nonhierarchical sequencing, (b) hierarchical integrated sequencing-DHPLC, and (c) nonhierarchical DHPLC simulated screens of a composite cohort of 247 East Asian EVA subjects from this and six other studies (Tsukamoto et al., 2003; Park et al., 2005; Wu et al., 2005b; Hu et al., 2007; Wang et al., 2007; Lee et al., 2008). Numbers of subjects are in circles. Asterisks indicate numbers of DHPLC analysis temperatures.
Discussion
Novel variants
The missense substitution c.412G>C (p.V138L) affects the same amino acid as the well-characterized pathogenic mutation c.412G>T (p.V138F) (Van Hauwe et al., 1998; Borck et al., 2003). The three subjects with p.V138L all had at least one other pathogenic variant. We confirmed trans configuration of p.V138L with the other mutations in two subjects, but family members were not available to determine meiotic configuration in the third subject (subject 18) (Table 2). The retention of p.V138L pendrin in the cytoplasm, most likely endoplasmic reticulum, of COS-7 cells (Fig. 2a), as previously described for other pathogenic SLC26A4 variants including missense variants that were also detected in this Korean cohort (p.H723R, p.M147V, and p.L676Q) (Table 2) (Rotman-Pikielny et al., 2002; Taylor et al., 2002; Yoon et al., 2008), confirms its pathogenic potential. Nearly no residual hearing in these subjects 4, 18, and 27 (Table 2) is compatible with this abnormal trafficking. However, interestingly, p.V138L retains some residual exchange activity in nonmammalian Xenopus oocytes (Fig. 2b–e). This contrasts with the complete loss of iodide efflux activity previously described for p.V138F pendrin expressed in mammalian cells (Taylor et al., 2002). This difference may arise from differences in expression temperature, choice of heterologous expression system (Karniski, 2001, 2004), or intrinsic properties of the mutant residue side chains. Since this substantial residual exchange activity of p.V138L is also in contrast with almost complete loss of functions recorded from p.L236P, p.E384G, and p.T416P in an identical expression system at the same temperature (Choi et al., 2009), p.V138L might resemble p.H723R in its potential susceptibility to therapeutic strategies to facilitate plasmalemmal trafficking of potentially functional pendrin from the endoplasmic reticulum (Kim and Arvan, 1998; Gillam et al., 2004; Yoon et al., 2008).
The intracellular trafficking and anion exchange properties of p.P542R pendrin suggest that it is a fortuitously detected benign polymorphic variant, although this rare variant is conserved in human, rat, and mouse SLC26A4 orthologs and was not detected in 100 normal Korean control chromosomes. In this study, this conclusion is supported by the genotypic and phenotypic context in which it was detected (Taylor et al., 2002; Pfarr et al., 2006; Choi et al., 2009). It was detected once as a single heterozygote (subject 7) (Table 2) in a subject with EVA accompanied by superior semicircular canal agenesis, severe cochlear hypoplasia, and severe hypopneumatization of the mastoid (Fig. 1). This constellation of severe anomalies probably reflects an etiology other than SLC26A4 mutations in this subject (subject 7) (Wu et al., 2005a; Fitoz et al., 2007). However, a pathogenic contribution of p.P542R pendrin to EVA via a complex or oligogenic mechanism in this patient cannot be completely ruled out. In contrast, the single mutant alleles with known or probable pathogenicity in subjects 1, 10, 22, 23, 25, and 26 (Table 2) (Park et al., 2005; Wu et al., 2005b; Yoon et al., 2008) are highly likely to contribute to the pathogenesis of EVA as supported by much more frequent detection of single mutations of SLC26A4 in nonsyndromic EVA patients than in normal Western controls (Campbell et al., 2001; Park et al., 2003; Pryor et al., 2005; Albert et al., 2006; Azaiez et al., 2007; Pera et al., 2008).
Integrated DHPLC sequencing protocol
A definitive molecular diagnosis could be made with the first step of this integrated protocol for approximately 40% of East Asian EVA subjects. DHPLC analyses detect not only pathogenic variants but also nonpathogenic variants or SNPs. Most of the missense variants detected in East Asians to date have proven to be pathogenic (Yoon et al., 2008), and we also show that p.V138L, a second most frequent missense variant in this study, is clearly pathogenic. However, because of lack of information about the frequency of SNP detection in the other East Asian subjects in our composite cohort, our simulation could not count for those unreported possible SNPs. Our Korean cohort showed only one known SNP twice in addition to one splice site (c.165-13T>G) and one missense (p.P542R) variants of unknown pathogenicity among 513 amplicons (Table 2), indicating that SNPs are not frequent in SLC26A4 in Korean EVA subjects. If SNPs to be confirmed by the sequencing are not rare in the other East Asian cohorts unlike in our Korean cohort, then cost effectiveness of DHPLC screening compared with a sequencing protocol might not be as high as predicted in our simulation. Even in this situation, our integrated protocol will have a substantial advantage in the cost reduction over the nonhierarchical DHPLC screening because a molecular diagnosis of about 40% of East Asian EVA subjects does not require further DHPLC screening in our integrated protocol.
Another advantage of our hierarchical integrated protocol is that it circumvents DHPLC screening of all, or nearly all, homozygous variants that are common potential sources of false-positives or false-negatives in this population. Although our conditions and primers permit the detection of homozygous mutations, EP differences for homozygotes are less prominent than for heterozygotes (Fig. 3b, c).
Better cost effectiveness can be achieved by substituting PCR-based allele-specific detection methods (Dai et al., 2008) or direct sequencing of only one amplicon for the first step of our integrated protocol, direct sequencing of both exons 7–8 and 19, in some geographic regions, such as Taiwan, with distinct features in exonic distribution of SLC26A4 mutations (Wu et al., 2005b).
This type of hierarchical protocol will be most useful in populations with one or a few prevalent mutations with a proven pathogenicity and a high proportion of EVA patients with two mutant alleles of SLC26A4. The protocol's advantages are unlikely to be as significant in European and other mixed populations characterized by broader mutation distributions and fewer EVA patients with two mutant alleles (Fig. 4) (Prasad et al., 2004; Pryor et al., 2005; Albert et al., 2006; Pera et al., 2008; Choi et al., 2009). Future studies to define the identities and distributions of SLC26A4 mutations among different ethnic populations are justified by their comparatively high prevalence as a cause of deafness (Park et al., 2003; Dai et al., 2008; Guo et al., 2008).
Acknowledgments
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (Project No. A080588) and also by Grant No. 21-2005-016 from the Seoul National University Hospital research fund, NIDCD/NIH intramural research funds Z01-DC-00060-07 (A.J.G.), Z01-DC00039-10 (T.B.F.), and NIH grants DK43495 (S.L.A.) and DK34854 (Harvard Digestive Diseases Center support to S.L.A.). We thank Dr. Thomas Friedman for support and critical review of the manuscript and the NIH Fellows Editorial Board for editorial assistance.
Disclosure Statement
No competing financial interests exist.
References
- Albert S. Blons H. Jonard L, et al. SLC26A4 gene is frequently involved in nonsyndromic hearing impairment with enlarged vestibular aqueduct in Caucasian populations. Eur J Hum Genet. 2006;14:773–779. doi: 10.1038/sj.ejhg.5201611. [DOI] [PubMed] [Google Scholar]
- Azaiez H. Yang T. Prasad S, et al. Genotype-phenotype correlations for SLC26A4-related deafness. Hum Genet. 2007;122:451–457. doi: 10.1007/s00439-007-0415-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck G. Roth C. Martine U, et al. Mutations in the PDS gene in German families with Pendred's syndrome: V138F is a founder mutation. J Clin Endocrinol Metab. 2003;88:2916–2921. doi: 10.1210/jc.2002-021334. [DOI] [PubMed] [Google Scholar]
- Campbell C. Cucci RA. Prasad S, et al. Pendred syndrome, DFNB4, and PDS/SLC26A4 identification of eight novel mutations and possible genotype-phenotype correlations. Hum Mutat. 2001;17:403–411. doi: 10.1002/humu.1116. [DOI] [PubMed] [Google Scholar]
- Choi BY. Stewart AK. Madeo AC, et al. Hypo-functional SLC26A4 variants associated with nonsyndromic hearing loss and enlargement of the vestibular aqueduct: genotype-phenotype correlation or coincidental polymorphism? Hum Mutat. 2009;30:599–608. doi: 10.1002/humu.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai P. Li Q. Huang D, et al. SLC26A4 c.919-2A>G varies among Chinese ethnic groups as a cause of hearing loss. Genet Med. 2008;10:586–592. doi: 10.1097/gim.0b013e31817d2ef1. [DOI] [PubMed] [Google Scholar]
- Everett LA. Glaser B. Beck JC, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nat Genet. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- Fitoz S. Sennaroglu L. Incesulu A, et al. SLC26A4 mutations are associated with a specific inner ear malformation. Int J Pediatr Otorhinolaryngol. 2007;71:479–486. doi: 10.1016/j.ijporl.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Gillam MP. Sidhaye AR. Lee EJ, et al. Functional characterization of pendrin in a polarized cell system. Evidence for pendrin-mediated apical iodide efflux. J Biol Chem. 2004;279:13004–13010. doi: 10.1074/jbc.M313648200. [DOI] [PubMed] [Google Scholar]
- Guo YF. Liu XW. Guan J, et al. GJB2, SLC26A4 and mitochondrial DNA A1555G mutations in prelingual deafness in Northern Chinese subjects. Acta Otolaryngol. 2008;128:297–303. doi: 10.1080/00016480701767382. [DOI] [PubMed] [Google Scholar]
- Hu H. Wu L. Feng Y, et al. Molecular analysis of hearing loss associated with enlarged vestibular aqueduct in the mainland Chinese: a unique SLC26A4 mutation spectrum. J Hum Genet. 2007;52:492–497. doi: 10.1007/s10038-007-0139-0. [DOI] [PubMed] [Google Scholar]
- Karniski LP. Mutations in the diastrophic dysplasia sulfate transporter (DTDST) gene: correlation between sulfate transport activity and chondrodysplasia phenotype. Hum Mol Genet. 2001;10:1485–1490. doi: 10.1093/hmg/10.14.1485. [DOI] [PubMed] [Google Scholar]
- Karniski LP. Functional expression and cellular distribution of diastrophic dysplasia sulfate transporter (DTDST) gene mutations in HEK cells. Hum Mol Genet. 2004;13:2165–2171. doi: 10.1093/hmg/ddh242. [DOI] [PubMed] [Google Scholar]
- Kim PS. Arvan P. Endocrinopathies in the family of endoplasmic reticulum (ER) storage diseases: disorders of protein trafficking and the role of ER molecular chaperones. Endocr Rev. 1998;19:173–202. doi: 10.1210/edrv.19.2.0327. [DOI] [PubMed] [Google Scholar]
- Lee KY. Choi SY. Bae JW, et al. Molecular analysis of the GJB2, GJB6 and SLC26A4 genes in Korean deafness patients. Int J Pediatr Otorhinolaryngol. 2008;72:1301–1309. doi: 10.1016/j.ijporl.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XC. Everett LA. Lalwani AK, et al. A mutation in PDS causes non-syndromic recessive deafness. Nat Genet. 1998;18:215–217. doi: 10.1038/ng0398-215. [DOI] [PubMed] [Google Scholar]
- Park HJ. Lee SJ. Jin HS, et al. Genetic basis of hearing loss associated with enlarged vestibular aqueducts in Koreans. Clin Genet. 2005;67:160–165. doi: 10.1111/j.1399-0004.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- Park HJ. Shaukat S. Liu XZ, et al. Origins and frequencies of SLC26A4 (PDS) mutations in East and South Asians: global implications for the epidemiology of deafness. J Med Genet. 2003;40:242–248. doi: 10.1136/jmg.40.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedemonte N. Caci E. Sondo E, et al. Thiocyanate transport in resting and IL-4-stimulated human bronchial epithelial cells: role of pendrin and anion channels. J Immunol. 2007;178:5144–5153. doi: 10.4049/jimmunol.178.8.5144. [DOI] [PubMed] [Google Scholar]
- Pera A. Villamar M. Vinuela A, et al. A mutational analysis of the SLC26A4 gene in Spanish hearing-impaired families provides new insights into the genetic causes of Pendred syndrome and DFNB4 hearing loss. Eur J Hum Genet. 2008;16:888–896. doi: 10.1038/ejhg.2008.30. [DOI] [PubMed] [Google Scholar]
- Pfarr N. Borck G. Turk A, et al. Goitrous congenital hypothyroidism and hearing impairment associated with mutations in the TPO and SLC26A4/PDS genes. J Clin Endocrinol Metab. 2006;91:2678–2681. doi: 10.1210/jc.2006-0142. [DOI] [PubMed] [Google Scholar]
- Phelps PD. Coffey RA. Trembath RC, et al. Radiological malformations of the ear in Pendred syndrome. Clin Radiol. 1998;53:268–273. doi: 10.1016/s0009-9260(98)80125-6. [DOI] [PubMed] [Google Scholar]
- Prasad S. Kolln KA. Cucci RA, et al. Pendred syndrome and DFNB4-mutation screening of SLC26A4 by denaturing high-performance liquid chromatography and the identification of eleven novel mutations. Am J Med Genet A. 2004;124:1–9. doi: 10.1002/ajmg.a.20272. [DOI] [PubMed] [Google Scholar]
- Pryor SP. Madeo AC. Reynolds JC, et al. SLC26A4/PDS genotype-phenotype correlation in hearing loss with enlargement of the vestibular aqueduct (EVA): evidence that Pendred syndrome and non-syndromic EVA are distinct clinical and genetic entities. J Med Genet. 2005;42:159–165. doi: 10.1136/jmg.2004.024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rillema JA. Hill MA. Prolactin regulation of the pendrin-iodide transporter in the mammary gland. Am J Physiol Endocrinol Metab. 2003;284:E25–E28. doi: 10.1152/ajpendo.00383.2002. [DOI] [PubMed] [Google Scholar]
- Rotman-Pikielny P. Hirschberg K. Maruvada P, et al. Retention of pendrin in the endoplasmic reticulum is a major mechanism for Pendred syndrome. Hum Mol Genet. 2002;11:2625–2633. doi: 10.1093/hmg/11.21.2625. [DOI] [PubMed] [Google Scholar]
- Royaux IE. Suzuki K. Mori A, et al. Pendrin, the protein encoded by the Pendred syndrome gene (PDS), is an apical porter of iodide in the thyroid and is regulated by thyroglobulin in FRTL-5 cells. Endocrinology. 2000;141:839–845. doi: 10.1210/endo.141.2.7303. [DOI] [PubMed] [Google Scholar]
- Royaux IE. Wall SM. Karniski LP, et al. Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci USA. 2001;98:4221–4226. doi: 10.1073/pnas.071516798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AK. Kerr N. Chernova MN, et al. Acute pH-dependent regulation of AE2-mediated anion exchange involves discrete local surfaces of the NH2-terminal cytoplasmic domain. J Biol Chem. 2004;279:52664–52676. doi: 10.1074/jbc.M408108200. [DOI] [PubMed] [Google Scholar]
- Stewart AK. Kurschat CE. Vaughan-Jones RD, et al. Acute regulation of mouse AE2 anion exchanger requires isoform-specific amino acid residues from most of the transmembrane domain. J Physiol. 2007;584:59–73. doi: 10.1113/jphysiol.2007.136119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H. Oshima A. Tsukamoto K, et al. Clinical characteristics and genotype-phenotype correlation of hearing loss patients with SLC26A4 mutations. Acta Otolaryngol. 2007;127:1292–1297. doi: 10.1080/00016480701258739. [DOI] [PubMed] [Google Scholar]
- Taylor JP. Metcalfe RA. Watson PF, et al. Mutations of the PDS gene, encoding pendrin, are associated with protein mislocalization and loss of iodide efflux: implications for thyroid dysfunction in Pendred syndrome. J Clin Endocrinol Metab. 2002;87:1778–1784. doi: 10.1210/jcem.87.4.8435. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K. Suzuki H. Harada D, et al. Distribution and frequencies of PDS (SLC26A4) mutations in Pendred syndrome and nonsyndromic hearing loss associated with enlarged vestibular aqueduct: a unique spectrum of mutations in Japanese. Eur J Hum Genet. 2003;11:916–922. doi: 10.1038/sj.ejhg.5201073. [DOI] [PubMed] [Google Scholar]
- Usami S. Abe S. Weston MD, et al. Non-syndromic hearing loss associated with enlarged vestibular aqueduct is caused by PDS mutations. Hum Genet. 1999;104:188–192. doi: 10.1007/s004390050933. [DOI] [PubMed] [Google Scholar]
- Valvassori GE. Clemis JD. The large vestibular aqueduct syndrome. Laryngoscope. 1978;88:723–728. doi: 10.1002/lary.1978.88.5.723. [DOI] [PubMed] [Google Scholar]
- Van Hauwe P. Everett LA. Coucke P, et al. Two frequent missense mutations in Pendred syndrome. Hum Mol Genet. 1998;7:1099–1104. doi: 10.1093/hmg/7.7.1099. [DOI] [PubMed] [Google Scholar]
- Wang QJ. Zhao YL. Rao SQ, et al. A distinct spectrum of SLC26A4 mutations in patients with enlarged vestibular aqueduct in China. Clin Genet. 2007;72:245–254. doi: 10.1111/j.1399-0004.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- Wangemann P. Nakaya K. Wu T, et al. Loss of cochlear HCO3-secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol. 2007;292:F1345–F1353. doi: 10.1152/ajprenal.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC. Chen PJ. Hsu CJ. Specificity of SLC26A4 mutations in the pathogenesis of inner ear malformations. Audiol Neurootol. 2005a;10:234–242. doi: 10.1159/000085825. [DOI] [PubMed] [Google Scholar]
- Wu CC. Yeh TH. Chen PJ, et al. Prevalent SLC26A4 mutations in patients with enlarged vestibular aqueduct and/or Mondini dysplasia: a unique spectrum of mutations in Taiwan, including a frequent founder mutation. Laryngoscope. 2005b;115:1060–1064. doi: 10.1097/01.MLG.0000163339.61909.D0. [DOI] [PubMed] [Google Scholar]
- Yoon JS. Park HJ. Yoo SY, et al. Heterogeneity in the processing defect of SLC26A4 mutants. J Med Genet. 2008;45:411–419. doi: 10.1136/jmg.2007.054635. [DOI] [PubMed] [Google Scholar]