FK-506 (FK) is a novel immunosuppressant isolated from Streptomyces tsukubaensis (Fig 1). It is nearly 500 times more potent than cyclosporine (CyA) in inhibiting lymphocyte proliferation in mixed lymphocyte cultures.1 In vivo, it significantly prolongs the graft survival in rats receiving heterotopic heart transplants2 and dogs receiving renal3 or liver transplants.4
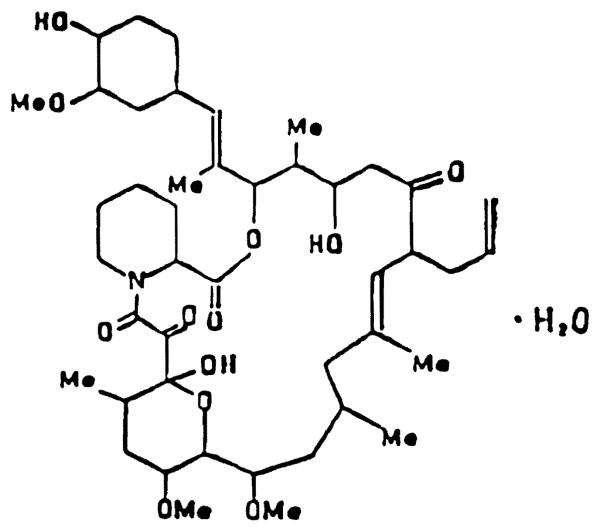
Fig 1.
Chemical structure of FK-506.
PHYSICOCHEMICAL PROPERTIES
FK has a unique chemical structure with a molecular weight of 822.05. FK is fairly lipid soluble and is similar to CyA in its solubility properties. It is poorly soluble in water and hexane but very soluble in methanol, chloroform, ethyl acetate, ether, and acetone. In the solid state FK is stable for at least 6 months at 24°C and 40°C. A methanolic solution of the drug appears to be stable for at least 4 weeks at −20°C, for 2 weeks at 5°C, and about 1 week at 24°C. A methanolic solution of the drug decomposes into multiple components in presence of 6 N HCI. However, FK is stable in the presence of very dilute acid.
DOSAGE FORM
Several dosage forms of FK were used in our studies. The intramuscular dosage form contains 50 mg of the drug, 10 mg of the surfactant HCD-60, and 125 mg of mannitol. This preparation is used after the addition of a required amount of normal saline and sonication for 15 minutes at room temperature. The oral formulation is a solid dispersion of the drug (20%) that is either suspended in water for studies in rats or filled in a soft gelatin capsule for studies in dogs. For intravenous studies, the intramuscular dosage form is diluted in a mixture of alcohol, Cremophor EL (BASF Wyandotte Corp, Parsippany, NJ), and normal saline.
DRUG ANALYSIS
FK does not contain any chromophores. A methanolic solution of the drug absorbs UV light to the maximum extent (λmax) at 192 nm. The compound also does not volatilize readily and has no intrinsic fluorescence. Therefore, traditional analytic methods such as gas chromatography or gas chromatography–mass spectrometry cannot readily be used to analyze intact FK in biologic fluids. FK can be measured by high-pressure liquid chromatography (HPLC) or by an enzyme immunoassay (EIA).
HPLC Analysis
HPLC assay can be used for FK measurement under certain circumstances. Because a number of components absorb UV light at 192 nm, a wave length of 210 or 214 nm has to be used for measuring FK by HPLC. This results in a low sensitivity for measuring the drug in biologic fluids. The HPLC assay is linear over a concentration range of 50 to 1,000 ng/mL and requires at least 1 mL of the biologic fluid. This assay can be used to test the stability of the dosage form of the drug.
Enzyme Immunoassay
In this assay, the drug is extracted from biologic fluids with benzene after the addition of 0.2 mol/L phosphate buffered saline (PBS, pH 7.0). The benzene extract is reconstituted in a mixture of 1:4 peroxidase–FK conjugate (FK-POD) and 0.05% Tween 20 and 0.5% bovine serum albumin in PBS (T-BSA-PBS). Before this, 200 μL of the polyclonal FK-antibody solution in PBS is added to each well of a 96-well flat tissue culture plate and incubated at 4°C overnight. The plates are then washed multiple times with 0.05% Tween 20 in PBS. Approximately 300 μL of T-BSA-PBS is added to all the wells and incubated at room temperature for two hours to suppress any nonspecific binding of FK-POD to the plastic wells. The plates are washed with 0.05% Tween in PBS and filled with PBS. This solution is aspirated, the reconstituted extract is added to each well, and the plates are incubated on a plate rotor overnight at 4°C. The solution in the plates is aspirated, the substrate solution (1.0 mg/mL of O-phenylenediamine dihydrochloride and 0.015% H2O2 in phosphate-citrate buffer, pH 5.4) is added, and the plates are incubated at room temperature for 20 minutes in the dark. Fifty microliters of 4 N H2SO4 is added to stop the reaction of the peroxidase enzyme with H2O2 and O-phenylenediamine. The optical density is measured at 492 nm. The concentration of FK in the sample is calculated from a standard curve developed by using known amounts of FK.
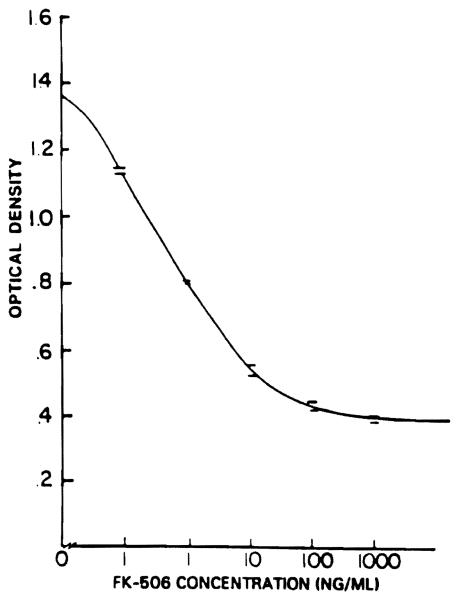
The minimum detectable concentration of FK when using EIA is 0.1 ng/mL. A standard curve is constructed and used over a concentration range of 0.1 to 100 ng/mL (Fig 2). Samples with a concentration of greater than 100 ng/mL are diluted in PBS and then analyzed.
Fig 2.
Standard curve of plasma FK concentration v optical density at 492 nm.
PHARMACOKINETICS
Absorption
Oral administration
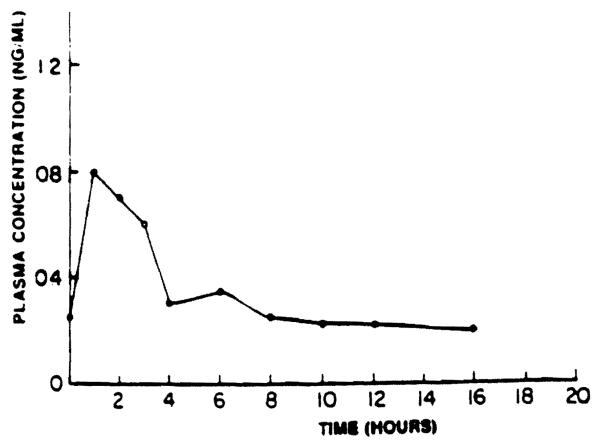
Oral absorption studies of FK were carried out in male beagle dogs eight days after daily oral administration of FK in a soft gelatin capsule. Blood samples were obtained from the femoral vein at 0, 1, 2, 3, 4, 6, 8, 10, 12, 16, and 24 hours after drug administration. Figure 3 shows the plasma concentration–off-time profile in a dog after oral administration of 1 mg/kg of FK. After oral administration of 1, 2, or 4 mg/kg of FK, the trough plasma concentrations were 0.48, 1.4, and 6.8 ng/mL, respectively. There was a wide variation in the trough plasma concentrations of FK in animals receiving the same oral dose,(0.25 to 0.8 ng/mL after a 1-mg/kg dose and 0.2 to 2.3 ng/mL after a 2-mg/kg dose). The drug was rapidly absorbed, with the time for maximum blood concentration (tmax) of one to three hours. This indicates that the drug is absorbed from the proximal part of the small intestine. However, after 4 mg/kg, there was prolonged absorption of the drug, and peak plasma concentrations were observed only six to ten hours after drug administration. The specific reasons for this observation are not clear at this point but may be related to potential gastric motility changes mediated by the drug. The maximal plasma concentrations (Cmax) ranged from 0.8 to 1.8 ng/mL after a 1-mg/kg dose and from 3.2 to 5.3 ng/mL after a 2-mg/kg dose. When absorption studies were conducted on day 15 after daily FK administration, a general tendency for Cmax and Tmax to increase was observed, which indicated changes in the rate and the extent of FK absorption with time.
Fig 3.
Plasma concentration of FK in a dog (no. 2) on day 8 aftar daily oral administration of 1 mg/kg of FK.
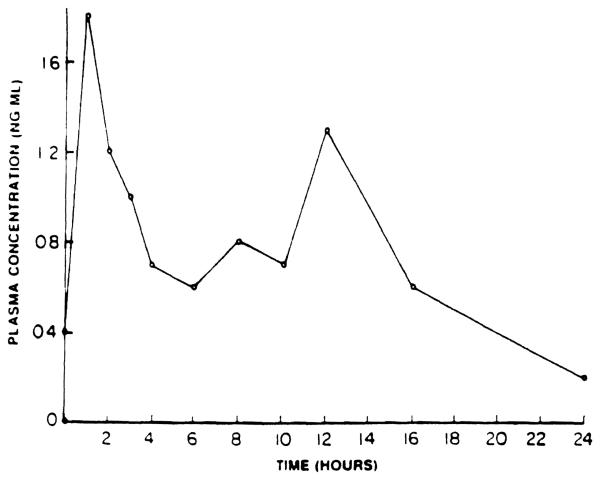
In some dogs, secondary peaks in plasma concentrations were observed (Fig 4). The specific reason(s) for this phenomenon is not clear at this time. In theory, this may be due to enterohepatic recirculation of the drug or biliary excretion of a conjugated metabolite of the drug that is hydrolyzed in the gut and is reabsorbed. Precipitation of the drug due to the local environment, such as pH changes, and subsequent reabsorption can also be responsible for such an observation. Differential absorption from different segments of the gut could also contribute to secondary peaks. Studies in animals where bile can be diverted from the gut5 and further studies of the pH-solubility profile of the drug are needed to characterize this process.
Fig 4.
Plasma concentration of FK in a dog. (no. 3) on day 8 aftar daily oral administration of 1 mg/kg of FK.
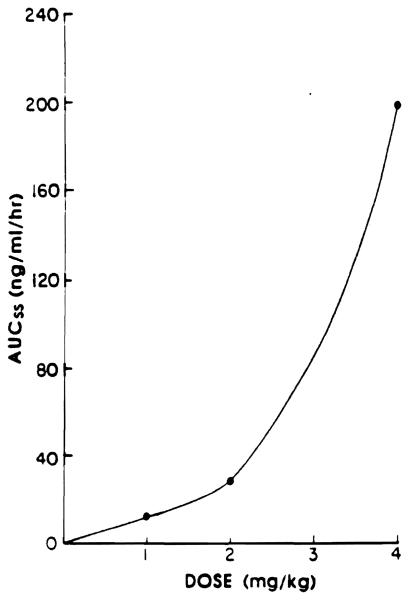
The area under the plasma concentration–v-time curve (AUC) as measured on day 8 after oral administration of 1, 2, or 4 mg/kg of FK is shown in Fig 5. This figure indicates a disproportionate increase in AUC with a small increase in the dose. This may be due to saturation of the drug-metabolizing enzymes responsible for FK elimination, saturation of the tissue uptake of the drug, or prolonged or sustained presence of the drug at the site of absorption, thereby increasing the fraction of the dose that is absorbed after a 4-mg/kg dose.
Fig 5.
Area under tha plasma FK concentration–-v-time curve after oral administration of 1, 2, and 4 mg/kg of FK.
Intramuscular administration
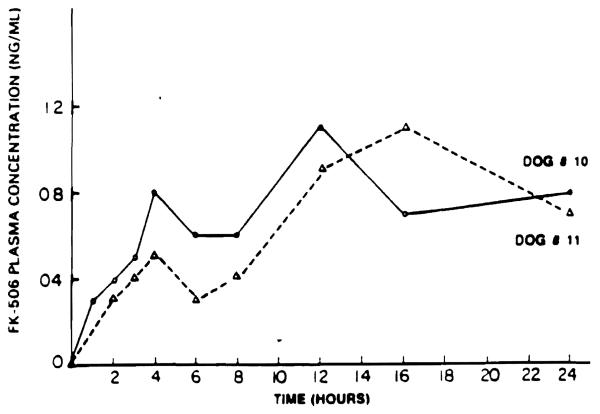
FK was administered intramuscularly (0.49 mg/kg) into the thigh muscle of two male beagle dogs. Blood samples were obtained at 0, 1, 2, 3, 4, 6, 8, 12, 16, and 24 hours after drug administration. FK was slowly absorbed from the injection site, as indicated by a slow and continuous increase in the plasma concentration of the drug over the entire study period in both dogs (Fig 6).
Fig 6.
Plasma concentration of FK in dog no. 10(Δ) and dog no. 11 (O) after a single intramuscular injection of 0.49 mg/kg of FK.
Distribution
Preliminary studies indicate that FK is distributed into RBCs. FK, however, does not alter the uptake of CyA by human RBCs. This is indicated by a blood-to-plasma ratio of 1.5 for CyA in the presence of 500 ng/mL of FK and in the absence of FK. Studies are currently under way to examine the binding of FK to various components of blood and to tissues.
Elimination
Studies have been carried out in rats and dogs after various routes of administration to determine the urinary excretion of FK. Independent of the route of administration, less than 1% of the dose of the drug appears in the urine, as determined by HPLC or EIA in both species over a 48-hour time period. This observation suggests that the drug is extensively metabolized in the body before elimination. After intramuscular administration in dogs (0.49 mg/kg), less than 1% of the drug is excreted in the bile and urine. These observations indicate that the kinetics of FK are not likely to be altered in renal impairment. However, impairment in liver function is likely to result in drug accumulation, thereby necessitating adjustments in the FK dosing regimen. The half-life of FK ranges from 4.4 to 8.0 hours, with a mean of 6.1 hours.
The metabolic fate of FK has not yet been characterized. The drug may undergo hydrolytic cleavage and may also be conjugated and demethylated. If the drug is conjugated and the conjugate is excreted in the bile, there is a potential for enterohepatic recirculation of the drug. This may explain the secondary peaks observed in the plasma concentration–v-time curves in dogs after FK administration.
Drug Interaction
Because immunosuppressants are commonly used in combination, drug interactions are of major concern in transplant patients. Although CyA has been reported to inhibit drug-metabolizing enzyme systems,6 steroids are known to induce the hepatic mixed-function oxidases.7 We studied the effect of FK on drug-metabolizing enzyme systems.
Male Wistar rats weighing between 230 and 250 g were used in this study. The animals were treated with placebo or 0.12 or 1.2 mg/kg of FK intramuscularly for 14 days. On day 15, the animals were anesthetized by ether, and the livers were excised and washed with Tris-KCl buffer (0.02 mol/L Tris-KCl buffer, pH 7.4, 1.15% KCl). Approximately 4 g of the liver was homogenized in 2 vol of Tris-KCl buffer in a polytron homogenizer, and microsomes were prepared according to standard techniques. The protein content of the microsomes were determined by the biuret method8 with BSA as the standard. Cytochrome P-450 and Cytochrome b5 contents of the microsomal suspensions were determined by the method of Omura and Sato.9 NADPH cytochrome c reductase activity was determined by the method of Phillips and Langden10 as modified by Gigon et al.11 The N-demethylation of ethylmorphine (5 mmol/L) was determined in an incubation mixture consisting of 5 mmol/L MgCl2 0.43 mmol/L NADP, 10 mmol/L glucose-6-phosphate, 1.5 enzyme units of glucose-6-phosphate dehydrogenase, 50 mmol/L Tris-HCl (pH 7.4), and 2 mg of microsomal protein according to the method of Nash.12
Table 1 summarizes the result of this study. At a dose of 0.128 mg/kg of FK, there is a significant decrease in the activity of ethylmorphine N-demethylase activity. At a dose of 1.28 mg/kg, in addition to ethylmorphine N-demethylase activity, cytochrome P-450 and cytochrome c reductase activity was also decreased. This indicates that FK has a dose-dependent effect on different components of the hepatic mixed-function oxidase system. CyA also similarly affects the mixed-function oxidase system in the liver.6 These observations suggest that the elimination of coadministered drugs may be altered in the presence of FR. Therefore, dosage adjustment of other drugs may be necessary. On the other hand, lower doses of other drugs may be effective in patients who receive FK, and this may result in some cost saving.
Table 1.
Effect of Chronic intramuscular Administration of FK on Hepatic Drug-Metabolizing Enzyme Systems
| Group | Microsomal Protein (mg/g of Liver) |
Cytochrome P-450 (nmol/mg Protein) |
Cytochrome b5 (nmol/mg Protein) |
Cytochrome c Reductase (nmol/Reduced mm/mg Protein |
Ethyl Morphine N-Demethylase (nmol HCHO/ mm/mg Protein |
|---|---|---|---|---|---|
| Placebo | 24.0 ± 4.0 | 0.902 ± 0.019 | 0.499 ± 0.021 | 291 ± 65 | 19.29 ± 2.74 |
| 0.128 mg/kg | 25.8 ± 1.8 | 0.825 ± 0.074 | 0.398 ± 0.044 | 267 ± 31 | 13.69* ± 2.92 |
| 1.28 mg/kg | 24.2 ± 1.0 | 0.675 ± 0.026* | 0.412 ± 0.022 | 181 ± 17* | 6.35* ± 1.81 |
Significantly different at p ≤ .05.
SUMMARY
FK is a potent immunosuppressive agent. FK can be analyzed in biologic fluids by EIA. The oral absorption of FK is rapid but variable in dogs. After intramuscular administration, FK is slowly and continuously absorbed. FK is primarily eliminated by metabolism. Less than 1% of the administered dose is excreted in the bile or the urine. After chronic intramuscular administration FK inhibits drug metabolism. Monitoring of FK levels in plasma is essential for the proper interpretation of efficacy and toxicity studies.
REFERENCES
- 1.Zeevi A. Transplant Proc. this issue. [Google Scholar]
- 2.Todo S. Transplant Proc. this issue. [Google Scholar]
- 3.Todo S. Transplant Proc. this issue. [Google Scholar]
- 4.Todo S. Transplant Proc. this issue. [Google Scholar]
- 5.Ericzon BG, Todo S, Lynch S, et al. Transplant Proc. 1987;19:1248. [PMC free article] [PubMed] [Google Scholar]
- 6.Augustine JA, Zemaitis MA. Drug Metab Dispos. 1986;14:73. [PubMed] [Google Scholar]
- 7.Shukla V, Garg S, Mathur W. Pharmacology. 1984;29:117. doi: 10.1159/000138000. [DOI] [PubMed] [Google Scholar]
- 8.Gornall AG, Bardawill CS, David MM. J Biol Chem. 1949;177:751. [PubMed] [Google Scholar]
- 9.Omura T, Sato R. J Biol Chem. 1964;239:2370. [PubMed] [Google Scholar]
- 10.Phillips AH, Langden RG. J Biol Chem. 1962;237:2652. [PubMed] [Google Scholar]
- 11.Gigon PL, Gram TE, Gillette JR. Mol Pharmacol. 1969;5:109. [PubMed] [Google Scholar]
- 12.Nash I. Biochem J. 1953;55:416. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]