Abstract
Under FK506-based immunosuppression, 13 abdominal multivisceral transplantations were performed in 6 children and 7 adults. Of the 13 recipients, 7 (53.8%) are alive and well with functioning grafts after 9 to 31 months. Six recipients died: three from PTLD, one from rejection, one from sepsis, and one from respiratory failure. In addition to rejection, postoperative complications occurring in more than isolated cases included PTLD (n=6), abdominal abscess formation (n=5), pancreatitis (n=3), and ampullary dysfunction (n=2). In addition, infection by enteric microorganisms was common during the early postoperative period. Currently, all 7 survivors are on an oral diet and have normal liver function. Two recipients (one insulin-dependent) require antidiabetes treatment, in one case following distal pancreatectomy and in the other after two episodes of pancreatic rejection. Thus, abdominal multivisceral transplantation is a difficult but feasible operation that demands complex and prolonged posttransplantation management. It is not yet ready for application and awaits a better strategy of immune modulation.
Abdominal multivisceral transplantation was developed experimentally more than 30 years ago (1, 2). The procedure was extremely difficult, yielding >5-day survival in only 5 of 39 untreated animals. No clinical application of multivisceral transplantation could be envisioned at that time. In 1989, we first reported multivisceral transplantation in two children who had short bowel syndrome and total parenteral nutrition (TPN)* induced liver failure (3). The first patient died during the immediate postoperative period, but the second patient survived under cyclosporine-based immunosuppression with well-functioning organs for 192 days. Including these two cases, eight attempts (4-6) at abdominal multivisceral transplantation under cyclosporine-based immunosuppression have been reported. All eight patients died. with the longest surviving 320 days.
With the advent of a new immunosuppressive agent, FK506, a clinical trial of intestinal transplantation was instituted at our center in May 1990 (7). Promising results with these initial cases prompted us once again to attempt abdominal multivisceral transplantation (8, 9). From October 1991 to the end of 1993, 13 patients underwent multivisceral transplantation at our center, 10 as a primary procedure and 3 for the attempted rescue of recipients of less-complex multivisceral grafts that had failed. Seven recipients are currently alive for 8 to 30 months.
MATERIALS AND METHODS
Patients
The 13 patients included 6 children who were 5.4±3.9 years old and 7 adults with a mean age of 32.0±7.1 years (age range 1.6 years to 44.8 years). Ten were primary recipients, and the other 3 had already failed a lesser intraabdominal transplant procedure. The procedure had to be abandoned in 2 additional candidates because of excessive bleeding during preliminary dissection of the native organs; both died. The indications for the 10 primary recipients included short bowel syndrome with (n=4) or without (n=3) TPN-related liver failure, mesenteric venous thrombosis with end-stage liver failure (n=l), juvenile polyposes (n=l), and malignant endocrine tumor (n=1). Although isolated intestine or combined intestine and liver grafting was considered for the short bowel patients, multivisceral transplantation was chosen because of the existence of thromboses of both the celiac axis and the superior mesenteric artery (n=3). TPN-related liver and pancreas failure (n=3), and pseudo-obstruction affecting the entire gastrointestinal tract (n=1). The 3 patients undergoing rescue multivisceral transplantation had undergone graft removal 2 months previously due to rejection of an isolated intestinal graft (n=1) or were bearing a rejecting intestine-liver (n=1) or liver (n=1) graft. The last patient also had intestinal pseudoobstruction since birth and should have undergone a multivisceral transplantation on the first occasion. The features of the 13 recipients are summarized in Table 1.
Table 1.
Demographics of the 13 abdominal multivisceral transplant recipients
| Patient | Age | Indicationa | Preoperative TPNb |
Graftc | Hospitalization |
Survivald (days) |
Cause of death | ||
|---|---|---|---|---|---|---|---|---|---|
| Duration (months) |
Total bilirubin (mg/dl) |
ICU (days) |
Discharge (days) |
||||||
| 1 | 32.6 | CA & SMA thrombosis | 36 | 0.5 | MV without colon | 60 | 196 | >944 | |
| 2 | 24.3 | CA & SMA thrombosis | 3 | 1.3 | MV without colon | 11 | 119 | >717 | |
| 3 | 4.2 | Failed liver/intestine transplantation |
50 | 23 | MV with kidney | 23 | 57 | PTLD | |
| 4 | 31.5 | Volvulus | 15 | 4.6 | MV without colon | 43 | 111 | >641 | |
| 5 | 4.5 | Pseudoobstruction | 54 | 26.1 | MV | 30 | 151 | >559 | |
| 6 | 44.8 | Malignant neuroendocrine tumor |
0 | 0.7 | MV | 21 | 49 | PTLD | |
| 7 | 1.8 | Juvenile polyposes | 22 | 0.5 | MV without liver | 17 | 87 | >420 | |
| 8 | 32.6 | Budd-Chiari syndrome | 0 | 3.7 | MV | 120 | 197 | Respiratory failure | |
| 9 | 10.9 | Pseudoobstruction (after liver transplantation) |
19 | 0.4 | MV | 7 | 76 | >355 | |
| 10 | 9.3 | Volvulus | 24 | 8.3 | MV | 26 | 110 | 198 | PTLD |
| 11 | 34.9 | Gardner’s syndrome | 26 | 18.1 | MV | 41 | 70 | >281 | |
| 12 | 23.4 | Failed intestine transplantation |
163 | 1.2 | MV | 91 | 147 | Sepsis | |
| 13 | 1.6 | Intestinal atresia | 19 | 58.2 | MV | 58 | 58 | Rejection | |
CA: celiac axis; SMA: superior mesenteric artery.
TPN: total parenteral nutrition.
MV: multivisceral grafts include the stomach, liver, pancreas, intestine, and colon.
As of May 15, 1994.
Donor operation
The grafts were obtained for ABO-blood type identical cadaveric donors with a mean age of 3.3±3.4 years for the children and 21.6±l0.2 for the adults, ranging from 0.6 to 39 years. Lymphocytotoxic crossmatch was strongly positive in 2 recipients; one survives after 14 months and the other died of intractable cellular rejection after 58 days. HLA matching was random. Immediately after the donors were accepted, selective bacterial decontamination was initiated by the method described previously (8). Immunomodulation of donors or grafts by irradiation. ALG, or anti–T cell monoclonal antibody (OKT3) administration was not attempted.
The techniques for multivisceral graft harvesting have been described before (8, 10). En bloc retrieval of the multivisceral grafts includes four steps: dissection from the retroperitoneum of the stomach, liver, pancreas, spleen, duodenum, small bowel, and varying lengths of the colon; transection of the vena cava above and below the liver; removal of the celiac axis and the superior mesenteric artery in continuity with the anterior wall of the abdominal aorta; and transection of the gastrointestinal tract at the abdominal esophagus or proximal stomach and at the descending colon distally. University of Wisconsin solution (1–2 L) was flushed through the abdominal aorta for graft preservation. Splenectomy, Heineke-Mikulicz pyloroplasty, and lumenal irrigation with 1–2 L cold lactated Ringer’s solution were performed on the back table. Cold ischemia time from clamping of the donor aorta until graft revascularization in the recipient varied from 4.1 hr to 10.8 hr, with a median of 8.3 hr.
Recipient operation
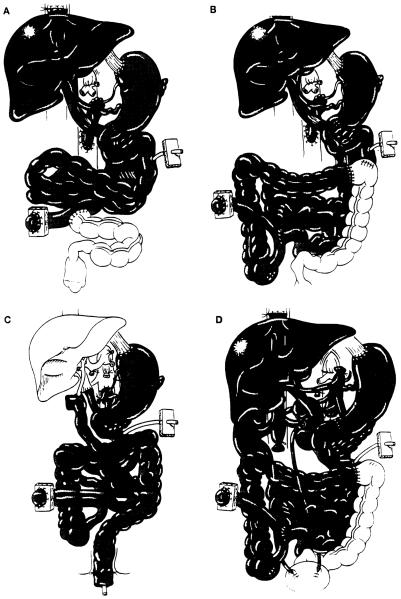
The operative procedure was similar to that used originally in experimental animals 30 years ago (1, 2) and in the early clinical cases (3). In brief, arterial reconstruction was performed by end-to-side anastomosis of the Carrel patch containing both the celiac axis and the superior mesenteric artery to the side of the host infrarenal abdominal aorta. The donor thoracic aorta was used as an arterial graft. Venous outflow from the graft was via the hepatic graft, which was transplanted using the piggy-back method (11). Proximal gastrointestinal continuity was reconstructed by anastomosing the distal end of the recipient esophagus or the gastric stump to the anterior wall of the graft stomach. The terminal ileum (n=3) (Fig. 1A), or ascending, transverse, or descending colon (n=10) of the grafts was anastomosed in a side-to-side or end-to-side fashion to the proximal end of the remaining recipient colon (Fig. 1B). Cholecystectomy was carried out in all cases, and a catheter was introduced via the cystic duct into the common bile duct for biliary decompression and postoperative cholangiogram. A tube jejunostomy, with terminal ileostomy (n=5), colostomy (n=2), or Bishop-Koop ileostomy (n=6), was made for enteric decompression, enteral feeding, and a route for endoscopic examination.
Figure 1.
Scheme of multivisceral transplantation: (A) Multiviscerai transplantation without colon; (B) Multivisceral transplantation with colon; (C) Multivisceral transplantation without liver and with rectal reconstruction by a pull-through technique; (D) Multivisceral transplantation with bilateral kidneys.
Modification of the procedure was required in some cases. Because of the profuse hemorrhage that precluded the operation in two patients with mesenteric venous thromboses, the celiac axis and the superior mesenteric artery were occluded in Case 8 by angiographic placement of intraaortic balloons immediately before starting the procedure. This adult patient tolerated this modification and survived the operation with a blood loss of 26 units. In a pediatric patient with juvenile polyposis and a normal liver, the liver was omitted from the multivisceral graft (Fig. 1C). Her rectum was reconstructed by a pull-through technique. Kidneys were included with the multivisceral grafts in another pediatric patient who had renal insufficiency (Fig. 1D).
Postoperative management
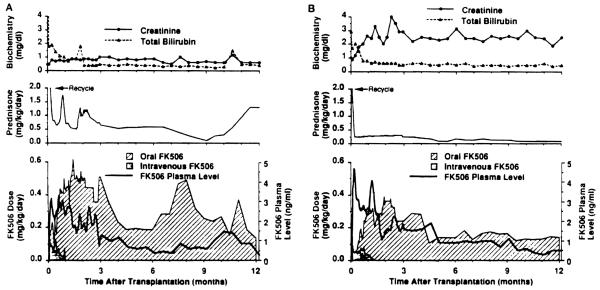
Management of multivisceral transplant recipients after transplantation was the same as for isolated intestinal recipients, including methods of nutritional management and prevention of infection. FK506, steroids, and prostaglandin E1 (Prostin) were used for immunosuppression. Intravenous FK506 (0.1 to 0.15 mg/kg/day) was started intraoperatively, and was then switched to oral FK506 (0.3 mg/kg/day or slightly less) when the patient became tolerant to enteral feeding. Trough plasma levels of FK506 were maintained at 2–3 ng/ml for the first month, 1–2 ng/ml until the third month. and 1 ng/ml thereafter (Fig. 2). Methylprednisolone 1 g in adults or hydrocortisone in children was given intravenously immediately after the grafts were revascularized and was followed by rapid tapering of predonisone over 5 days after transplantation. Maintenance prostaglandin El (0.6 to 0.8 μg/kg/hr) was continued until intravenous FK506 was stopped.
Figure 2.
Immunosuppression and liver and kidney function. Values expressed as median. (A) Pediatric patients; (B) Adult patients.
Monitoring of intestinal graft rejection was based on clinical findings, endoscopic examination, and histopathological study of endoscope-guided biopsies. Treatment of intestinal rejection depended upon its severity as described before (8). Routine liver function tests were used to monitor liver rejection, and liver biopsies were taken if needed. Frequent measurements of amylase and lipase levels in blood and/or fluid in Jackson-Pratt drains were used to monitor pancreas rejection.
Assessment of graft function
Body weight, volume of stool output, frequency and nature of the stool, and dependency on TPN, enteral feeding, and/or oral diet were repeatedly evaluated to assess intestinal graft function. In addition, absorptive function was directly measured by d-xylose test and by 72-hr fecal fat secretion. Measurements of gastric emptying by radiolabeled test meals, intestinal transit time by a barium follow-through, and contractile activity by manometry were performed periodically to determine the motility of the gastrointestinal tract. Graft function of the liver and the pancreas was determined by serial determination of blood chemistries. Pancreatic endocrine function was periodically studied in long-term survivors by measuring blood glucose and c-peptide levels after intravenous injection of 0.5 g/kg glucose.
RESULTS
Survival
Of the 13 patients, 6/10 given primary grafts and 1/3 in which the multivisceral procedure was secondary are currently alive and well with follow-up of 9 to 31 postoperative months (Tables 1 and 2). Six are at home while one pediatric patient is currently hospitalized for the treatment of posttransplant lymphoproliferative disease (PTLD); this last patient’s antidonor lymphocytotoxic crossmatch was positive. With an actual patient survival of 7/13 (53.8%), the actuarial patient survival rates by the life-table method at 3 months, 6 months, one year, and 2 years are 76.9%, 69.2%, 53.8%, and 53.8%, respectively.
Table 2.
Current status of 7 survivors
| Casea | Age | Survivalb (days) |
Location | Graft function |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrointestine |
Liver |
Pancreas |
|||||||||
| TPN | Stool | d-xylose (mg/dl) |
fecal fat excretion (%) |
GOT (U/L) |
T. bil (mg/dl) |
Blood sugar (mg/dl) |
Treatment | ||||
| 1 | 32.6 | 994 | Home | Free | Formed | 32.5 | 48.2 | 30 | 0.5 | 121 | |
| 2 | 24.3 | 717 | Home | Free | Formed | 23.7 | 62.6 | 23 | 0.3 | 225 | Anti-DM agent |
| 4 | 31.5 | 641 | Home | Free | Formed | 34.1 | 23.5 | 44 | 1.0 | 72 | |
| 5 | 4.5 | 559 | Home | Free | Formed | 26.9 | 15.9 | 85 | 0.5 | 78 | |
| 9 | 1.8 | 420 | Hospitalc | Free | Formed | 32.8 | 50 | 0.3 | 75 | ||
| 11 | 10.9 | 355 | Home | Free | Formed | 26.4 | 28 | 0.2 | 53 | ||
| 12 | 34.9 | 281 | Home | Free | Formed | 39 | 0.6 | 126 | Insulin (15 U/day) | ||
Causes of mortality
The 6 deaths occurred 1.5, 2, 2, 5, 6.5, and 6.5 months after the multivisceral transplantation (Table 1). Three of the 6 deaths were caused by PTLD, one by respiratory failure, one by rejection, and one by sepsis (Table 1). Two of the 3 deaths caused by PTLD occurred at 49 days and 198 days after primary transplantation; the third was 57 days after replacement of a rejected combined liver-intestine graft. In the last case, the rejection had followed reduction of immunosuppression at 10 months for the treatment of PTLD in the donor colon after the primary transplantation. At the time of the rescue multivisceral transplantation 15 months following the primary procedure, no PTLD lesions were detectable in the specimen or elsewhere.
Patient 8, who died from cytomegalovirus (CMV) infection, was CMV seronegative pretransplant and received grafts from a CMV-seropositive donor; although the infection was controlled, the lungs were destroyed. Patient 12, who was rescued from rejection of her isolated intestinal graft, was found to have a resistant intraabdominal infection with Torulopsis glabrata in the abdominal cavity at the time of retransplantation and died from this 5 months later.
Rejection
Ileal biopsies collected via the terminal ileostomy or the Bishop-Koop ileostomy showed rejection in 11 (84.6%) of the 13 recipients. On 162 occasions, the ileal biopsies were taken simultaneously with sampling from other sites: stomach (n=50), duodenum and/or jejunum (n=27), or colon (n=85). When the ileum had histological evidence of acute rejection, rejection was also found at the aforementioned sites at 0% (0/3), 25% (2/8), and 43.7% (7/16), respectively. Isolated rejection at these sites was found only once, at the jejunum in patient 7. Acute rejection of the stomach was never seen.
Rejection of the liver and the pancreas was less frequent and serious, with an incidence by clinical criteria of 46.2% (6/13) for the liver and 30.1% (4/13) for the pancreas. Increased immunosuppression for liver rejection was not required, but severe acute pancreatitis was caused by rejection in one patient after immunosuppression had been reduced.
No evidence of graft-versus-host disease (GVHD) was detected clinically or in the tissue samples (including 3 postmortems) in any of the cases.
Complications
All recipients, except patient 9, had significant or life-threatening complications (Table 3), of which the 6 examples of PTLD were the most common. Of the 3 patients who survived PTLD, 2 have resolved lesions and the third is improving. The 5 intraabdominal abscesses were caused by leakage from the gastrostomy site (n=1), enteric perforation (n=2), and necrotizing pancreatitis (n=2). The pancreatic abscesses were apparently caused by preservation; one patient underwent total pancreatectomy (patient 8) and the other distal pancreatectomy (patient 11). Patient 12, who had necrosis and scarring at the muscle layer of the intestinal wall, required 5 separate enteric resections after each of 5 perforations over a time span of 1.5 months. Two adult recipients required transient dialysis for renal failure to which FK506 and nephrotoxic antibiotics appeared to be contributory (Figure 2). A persistent elevation of cannulicular enzymes from ampullary dysfunction in patient 2 was relieved with endoscopic papillotomy, while another patient with this complication did not require intervention.
Table 3.
Complications
| Patients (%) | Comments (case)a | |
|---|---|---|
| Major: | ||
| PTLD | 6 (46.2%) | Died (cases 3, 7, & 12), resolved (cases 2 & 5), resolving (case 9) |
| Abdominal abscess | 5 (38.5%) | Due to intestinal perforation (cases 9 & 14), pancreatitis (cases 10 & 13), intestinal leakage (case 7) |
| Renal failure | 3 (23.1%) | Required transient dialysis (cases 1 & 4) |
| Pancreatitis | 3 (23.1%) | Due to preservation injury (cases 10 & 13), rejection (case 2) |
| Ampullary dysfunction | 2 (15.4%) | Required endoscopic papillotomy (case 2) |
| Infectious:b | ||
| Abdomen | 12 (92.3%) | Wound, J-P drain, or discharge |
| Lung | 9 (69.2%) | Sputum or bronchoalveolar lavage |
| Blood | 12 (92.3%) | Blood or lines |
| Bacterial overgrowth | 11 (84.6%) | >109 CFU/ml by stool culture |
Cases are shown in Table 1.
Positive culture of enteric microorganisms.
During the early postoperative period, enteric microorganisms, including Enterococcus faecium, Enterococcus faecalis, Escherichia coli, and Klebsiela, were cultured from the peritoneal fluid and/or wound discharge of 12 (92.3%) of the 13 patients, in the sputum and/or bronchoalveolar lavage of 9 (69.2%), and in the blood and/or catheters of 12 (92.3%). All of the recipients who survived for more than 2 months after surgery developed a normal bacterial flora of the stool with bacterial overgrowth with >109 colony forming units/ml.
Graft function
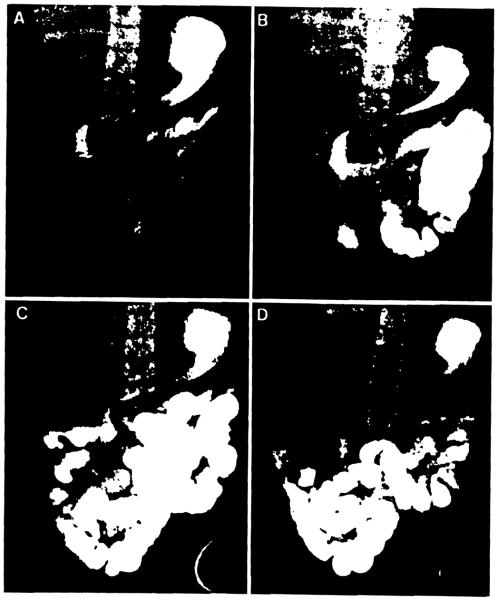
All 7 surviving recipients as well as patients who died after more than 3 months gained or maintained their body weight exclusively on an unrestricted oral diet (Table 2). Among the current survivors, the latest d-xylose absorption test was normal in all studied, but fecal fat excretion was universally elevated, especially in patient 2 who had 2 episodes of pancreas rejection. Motility function of the gastrointestinal tract was highly variable, and was influenced by the duration of follow-up. The trend in long survivors suggested recovery of normal gastric emptying and intestinal transit after one year (Fig. 3). However, manometric studies commonly showed that antral and intestinal contractions were hypoactive during fasting and after feeding, with absent migrating motor complex propagation, even later than one year.
Figure 3.
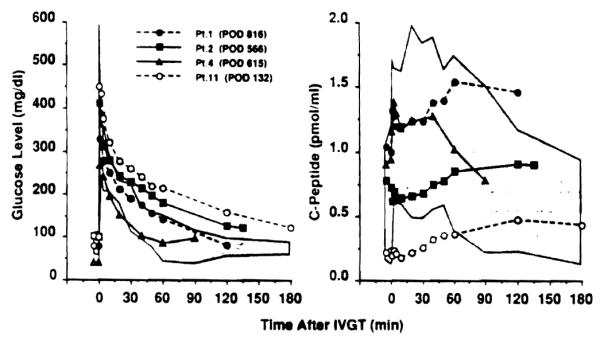
Blood level of glucose and C-peptide after intravenous glucose tolerance test. Glucose was given at a dose of 0.5 mg/kg. Shaded areas indicate range obtained from 5 normal controls. POD: postoperative days.
Liver function is normal or near-normal in all of the surviving patients. Two of them require treatment for hyperglycemia caused by distal pancreatectomy (patient 11) or pancreas rejection (patient 2)—one with insulin and the other without. Changes in blood glucose and C-peptide in these 2 patients during an intravenous glucose tolerance test are shown in Figure 4.
Figure 4.
Barium follow-through of multivisceral recipient (patient 10). (A) 30 min; (B) 2 hr; (C) 3 hr; (D) 4 hr.
DISCUSSION
Our experience and that of others with multivisceral (3-6, 8, 9), liver-intestinal (7, 8, 12), and isolated intestinal (13-15) transplantation have delineated what can and cannot be achieved with all 3 of these procedures using current management methods. As with liver transplantation through the 1970s, the procedures that include intestine are feasible but impractical means of therapy that are not yet ready for general use. Although half the multivisceral recipients were restored to near normal health, including relatively complete dietary rehabilitation, the early and late mortality was excessive, with a lengthy list of complications even for patients who successfully ran the gauntlet of the first postoperative year.
The problems were much the same as those still seen with conventional liver transplantation, but they have been far more frequent and serious: incomplete control of rejection with consequent bacterial translocation throughout the damaged intestinal graft, a high rate of lethal septic complications, and the extraordinary 46% incidence of lymphoproliferative disorders. The most encouraging notation was the total absence of GVHD, which was feared at one time to make the intestine a forbidden organ for transplantation unless there was a perfect MHC match (16).
The next large advance is predicted to turn on a strategy that would have been an inconceivable proposal with the previous paradigm of transplantation immunology. However, the freedom from GVHD of these and other kinds of human intestinal recipients (7-9) can now be explained by the mutually canceling interactions of the coexisting cell populations following the transplantation of any whole organ that we have postulated to be the seminal mechanism of graft acceptance (17, 18). Lymphoid depletion of the graft, which was the consensus of workers in the intestinal transplantation field until recently (19-22), unbalances the graft-host-immunologic relation and appears to us to be unnecessary and probably contraindicated. When T cell depletion of the intestine was attempted in earlier clinical cases, there was an almost universal incidence of B cell lymphomas (3, 4, 6).
Armed with the realization that persistent spontaneous chimerism begins within minutes by migration of donor non-parenchymal cells from all grafts, and most dramatically from organs with a large hematolymphopoietic constituency, this timing has been simulated by perioperative infusion of 3×108/kg unaltered bone marrow cells in 45 consecutive unconditioned human recipients of kidneys, livers, hearts, and lungs under conventional FK506-prednisone immunosuppression. The freedom of these patients from GVHD (trivial only in 2), their benignancy of recovery (all are well), and the demonstration of stable macrochimerism 10 the first 18 after 4 to 17 months (23) has generated plans to use the same treatment protocols for future intestinal recipients.
Footnotes
Presented at the 20th Annual Meeting of the American Society of Transplant Surgeons. May 18–20, 1994, Chicago, IL.
Aided by Project Grant DK-29961 from the National Institutes of Health, Bethesda, MD.
- GVHD
- graft-versus-host disease
- PTLD
- post-transplant lymphoproliferative disorder
- TPN
- total parenteral nutrition
REFERENCES
- 1.Starzl TE, Kaupp HA., Jr Mass homotransplantation of abdominal organs in dogs. Surg Forum. 1960;11:28. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Rowe M, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;26:1449. [PMC free article] [PubMed] [Google Scholar]
- 4.Williams JW, Sankary HN, Foster PF, Lowe J, Goldman GM. Splanchnic transplantation: an approach to the infant dependent on parenteral nutrition who develops irreversible liver disease. JAMA. 1989;261:1458. doi: 10.1001/jama.261.10.1458. [DOI] [PubMed] [Google Scholar]
- 5.Margreiter R, Konigsrainer A, Schmid T, et al. Successful multivisceral transplantation. Transplant Proc. 1992;24:1226. [PubMed] [Google Scholar]
- 6.Grant DR. Small bowel transplantation. Gastrointest J Club. 1992;1:3. [Google Scholar]
- 7.Todo S, Tzakis A, Abu-Elmagd K, et al. Cadaveric small bowel and small bowel–liver transplantation in humans. Transplantation. 1992;53:369. doi: 10.1097/00007890-199202010-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todo S, Tzakis A, Abu-Elmagd K, et al. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abu-Elmagd K, Todo S, Tzakis A, et al. Intestinal transplantation: three years experience. J Am Coll Surg. 1994;179:385. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335. [PMC free article] [PubMed] [Google Scholar]
- 11.Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:649. doi: 10.1097/00000658-198911000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant D, Wall W, Mimeault R, et al. Successful small bowel/liver transplantation. Lancet. 1990;335:181. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 13.Todo S, Tzakis A, Reyes J, et al. Small intestinal transplantation in humans with or without the colon. Transplantation. 1994;57:840. doi: 10.1097/00007890-199403270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deltz E, Schroeder P, Gebhardt H, et al. Successful clinical small bowel transplantation: report of a case. Clin Transplant. 1989;3:89. [Google Scholar]
- 15.Goulet O, Revillon Y, Brousse N, et al. Successful small bowel transplantation in an infant. Transplantation. 1992;53:940. doi: 10.1097/00007890-199204000-00046. [DOI] [PubMed] [Google Scholar]
- 16.Monchik GJ, Russell PS. Transplantation of small bowel in the rat: technical and immunological considerations. Surgery. 1971;70:693. [PubMed] [Google Scholar]
- 17.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 19.Pomposelli F, Maki T, Gaber L, Balogh K, Monaco AP. Induction of graft versus host disease by small intestinal allotransplantation. Transplantation. 1985;40:343. doi: 10.1097/00007890-198510000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Shaffer D, Maki T, DeMichele SJ, et al. Studies in small bowel transplantation: prevention of graft versus host disease with preservation of allograft function by donor pretreatment with antilymphocyte serum. Transplantation. 1988;45:262. [PubMed] [Google Scholar]
- 21.Shaffer D, Ubhl CS, Simpson MA, et al. Prevention of graft vs. host disease following small bowel transplantation with polyclonal and monoclonal antilymphocyte serum: effect of timing and route of administration. Transplantation. 1991;52:948. doi: 10.1097/00007890-199112000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Diflo T, Monaco AP, Balogh K, Maki T. The existence of graft-versus-host disease in fully allogeneic small bowel transplantation in the rat. Transplantation. 1989;47:7. doi: 10.1097/00007890-198901000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Fontes P, Rao AS, Demetris AJ, et al. Augmentation with bone marrow of donor leukocyte migration for kidney, liver, heart, and pancreas islet transplantation. Lancet. doi: 10.1016/s0140-6736(94)92756-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]