Abstract
In this article, the physiological impact of one form of stress – physical exercise – on the neuroendocrine system will be discussed. The specific intent of the review is to present an overview of stress endocrinology, the conceptual models associated with this area of study, and a discourse on the dual role of exercise as both a stressor and a modifier of stress within the neuroendocrine system. These points are addressed with respect to the current research literature dealing with exercise endocrinology in an adult population.
Keywords: disregulation, endocrinology, hormones, physical activity, sport
Stress is something experienced by all of us, no matter who we are, and it has both a positive and a negative effect on our lives. Modern society has created an environment where there are tremendous opportunities to experience both negative stresses (distress) as well as positive stress (eustress) on a daily basis [1,2]. Such stressful encounters have profound impacts upon the physiological workings of the human body, both in constructive and destructive fashions. One physiological system that is extremely reactive to stress is the neuroendocrine system [1,2]. In fact, many clinicians and researchers use the responses of the neuroendocrine system as a means of assessing the stress effects and reactivity of the human body.
Physical exercise is an activity that is known to provoke large and diverse stress responses within the neuroendocrine system. However, chronic exercise training is also known to cause abatement in the stress responses of the neuroendocrine system to certain forms of stress. The intent of this article is to provide an overview on these somewhat paradoxical effects of physical exercise upon the neuroendocrine system. Specifically, the intended purpose of this review is twofold: to provide a brief overview of stress endocrinology and the conceptual models associated with this area of study; and to address the dual role of exercise as both a stressor and a modifier of stress within the neuroendocrine system, both in acute and chronic exposure settings.
Background & overview
Stress endocrinology
The word stress is actually a very ambiguous term, as many different definitions can be applied, depending upon the scientific focus and discipline that is under study. This, unfortunately, has led to some confusion in the research literature as to exactly what is meant when the word is used. To avoid confusion, for the purposes of this article the definition put forth by McEwen will be used, where stress is defined as “a real or interpreted threat to the physiological or psychological integrity (i.e., homeostasis) of an individual that results in physiological and/or behavioral responses” [1–4].
Consequently, any activity, event, or stimulus that causes stress can be referred to as a stressor. When a stressor provokes a change in the body’s psychophysiological systems, the characterization of the response of an individual system can be referred to as a stress response and the signaling agents of a system as stress mediators [3,4]. The intent of the stress response mediators is to help the body accommodate and adjust to the stressor, thereby speeding the body’s re-establishment of a homeostatic equilibrium [3].
Conceptual models
Over the last 100 years, researchers have proposed different theoretical models to explain and characterize the dynamics of the human stress response. Beginning with Walter Cannon’s work on the fight or flight response, the dominant paradigm was later elaborated upon by Han Selye into his eloquent General Adaptation Syndrome model of stress [5,6]. Both of these iconic figures of the field have been viewed as belonging to the non-specific school of thought – that is, a stressor is a stressor and similar responses are provoked, regardless of the nature of the stimulus. More recent work by Mason [7] and Chrousos and Gold [8] has modified the doctrine of nonspecificity. These and other investigators have proposed that the human stress response has a more precise degree of specificity, depending upon (among other things) the particular type of challenge to homeostasis (i.e., the stressor), the organism’s perception of the stressor and the perceived ability to cope with the stressor.
Today, this Specificity Homeostatic Theory model of stress response has morphed into the Allostasis–Allostatic Load model, as originally put forth by Sterling and Eyer [9] and expanded upon by McEwen [1]. Understanding this model involves viewing homeostasis simplistically as representative of stability through consistency; mechanisms attempt to hold constant a controlled variable by sensing its deviation from a set point and feeding back to correct any error.
Conversely, allostasis is representative of stability through change; that is, mechanisms attempt to change the controlled variable by predicting what level will be needed and potentially overriding local feedback to meet anticipated demand. According to this model, allostatic load refers to the price the body pays for being forced to constantly adapt to adverse stressful (psychophysiological) events. Specifically, it represents either the presence of too much stress or the inefficient operation of the stress response. Thus, the stress-mediator responses are inappropriate to the stressor. In this conceptual model, as allostatic load is intensified and persists, the risk for the development of certain chronic illnesses/diseases potentially increases [3,4,10,11].
Regardless of which theoretical model of stress and stress response a researcher subscribes to, the neuroendocrine system is regarded by all as one of the most responsive of physiological systems to stressors. This responsiveness occurs since the neuroendocrine system is a key signaler and modulator of many of the other physiological systems attempting to accommodate stressors and re-establish homeostasis.
Exercise endocrinology
With respect to the neuroendocrine system, physical exercise (hereafter referred to as exercise) can provoke large and diverse changes in the concentration of many hormones from resting levels. BOX 1 illustrates the diversity of responses by demonstrating some of the hormones that are substantially affected by exercise. Almost all of these hormones could be considered, in some way, as stress response hormones (i.e., mediators); however, a majority of stress researchers and clinicians focus upon the hormones associated with the sympathetic nervous system and the hypthalamo–pituitary–adrenocortical–adrenomedullary systems as key responders when attempting to quantify and evaluate the neuroendocrine stress response. For this reason, this discussion will be limited to only what are considered by most scientists as the key hormones of these systems: norepinephrine, epinephrine, adrenocorticotropic hormone (ACTH) and cortisol [10,12,13,101].
Box 1. Major stress-related hormones typically affected substantially by an acute exercise session
Adrenocorticotropic hormone
Atrial natriuretic peptide
Arginine vasopressin
β-endorphin
Brain natriuretic peptide
Corticotropin-releasing hormone
Cortisol
Cytokines
Dynorphins
Enkephalins
Epinephrine
Growth hormone
Norepinephrine
Prolactin
Renin–angiotensin–aldosterone
Testosterone
In looking at these hormones as biomarkers of the stress response, there are many procedural (e.g., blood sample timing) and methodological (e.g., bioassays used for analysis) factors that can influence the accuracy, reliability and validity of information obtained (see BOX 2). These factors have not always been controlled adequately by all investigators and, unfortunately, have resulted in the existence of some confusing data in the stress research literature (see [14] for greater detail on these factors).
Box 2. Methodological and procedural factors that can influence the measurement and assessment of circulating hormone levels in the blood
Circadian rhythms (e.g., time of day of specimen collection)
Specimen collected (e.g., hormone concentrations differ in blood, urine or saliva)
Specimen collection procedure (e.g., catheter or venipuncture for blood)
Biochemical assay for detection (e.g., radioimmunoassay or chemiluminescent)
Subject dietary practices (e.g., consumption of meals prior to specimen collection)
Environmental factors (e.g., heat, humidity, barometric pressure)
Subject life stressors (e.g., relationships, finances and security)
The primary physiological factor that seems to determine the neuroendocrine stress response to a single acute session of exercise is the volume of exposure, where volume is comprised of the intensity and/or duration of the exercise session [15–19]. The greater the exercise volume, the greater the neuroendocrine response observed in most, but not all, cases (provided that other modifying factors, discussed later, do not come into play). To aid the reader, TABLE 1 presents a categorization and definition of exercise intensity and type typically found in exercise physiology literature.
Table 1.
Categorization and definition of exercise intensity and exercise type.
| Category of exercise | Intensity (percentage VO2max) | Dominant energy pathway | Typical duration period | Descriptive terms for exercise |
|---|---|---|---|---|
| Light or easy exercise | <35% | Aerobic | >30 min | Short-term, submaximal, steady-state |
| Moderate exercise | >35%, <70% | Aerobic | >30 min, <180 min | Submaximal-prolonged, steady-sate |
| Heavy exercise | >70% | Aerobic–anaerobic | <120 min | Submaximal-prolonged, high-intensity |
| Maximal exercise | 100% | Aerobic–anaerobic | <15 min | Maximal or max, high-intensity |
| Supramaximal exercise | >100% | Anaerobic | <1 min | Sprints, power |
Exercise intensity is typically quantified as a percentage of an individual’s maximal oxygen uptake (VO2max; i.e., maximal aerobic capacity). This information is based upon work by Bouchard and colleagues in Exercise, Fitness, and Health: A Consensus of Current Knowledge (Human Kinetics, Champaign, IL, USA, 1990), as reported and modified by Hackney [57].
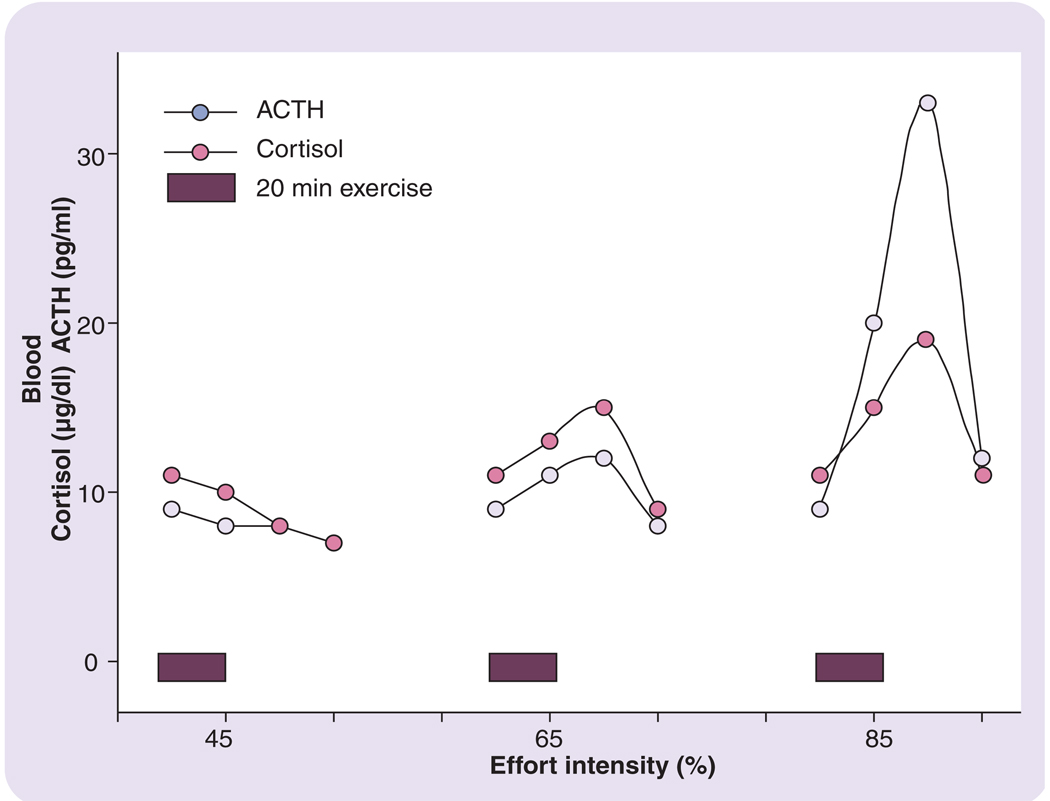
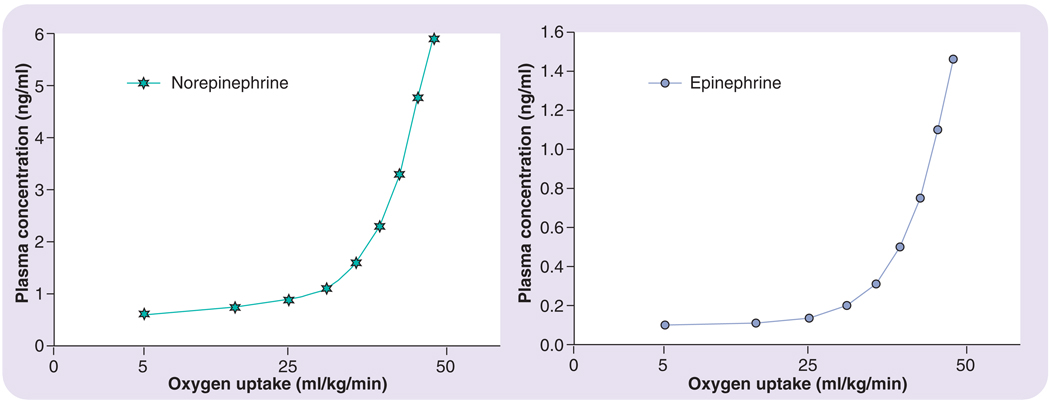
FIGURES 1 & 2 are composites of findings from several different research studies in adults, which illustrate aspects of the volume effect [16,17,20–22]. As seen in FIGURE 1, as exercise intensity is increased, there are approximately proportional increases in circulating concentrations of ACTH and cortisol; pre- to postexercise. It is important to note, however, that there is a critical threshold of exercise intensity that must be reached (~50–60% of maximal oxygen uptake [VO2max]) before circulating levels increase in response to exercise [23,24]. In a similar fashion, circulating norepinephrine and epinephrine demonstrate this intensity-dependent response to exercise, as illustrated in FIGURE 2 [22].
Figure 1. Pituitary–adrenal axis hormonal responses to exercise of increasing intensity.
Redrawn from part of the data presented in [15], [20] and [21]. Effort/intensity is represented as a percentage of maximal oxygen uptake.
ACTH: Adrenocorticotropic hormone.
Figure 2. Blood (plasma) catecholamine (norepinehrine and epinephrine) response to exercise of increasing intensity.
Oxygen consumption is oxygen uptake expressed relative to bodyweight. Redrawn from part of the data presented in [18] and [22].
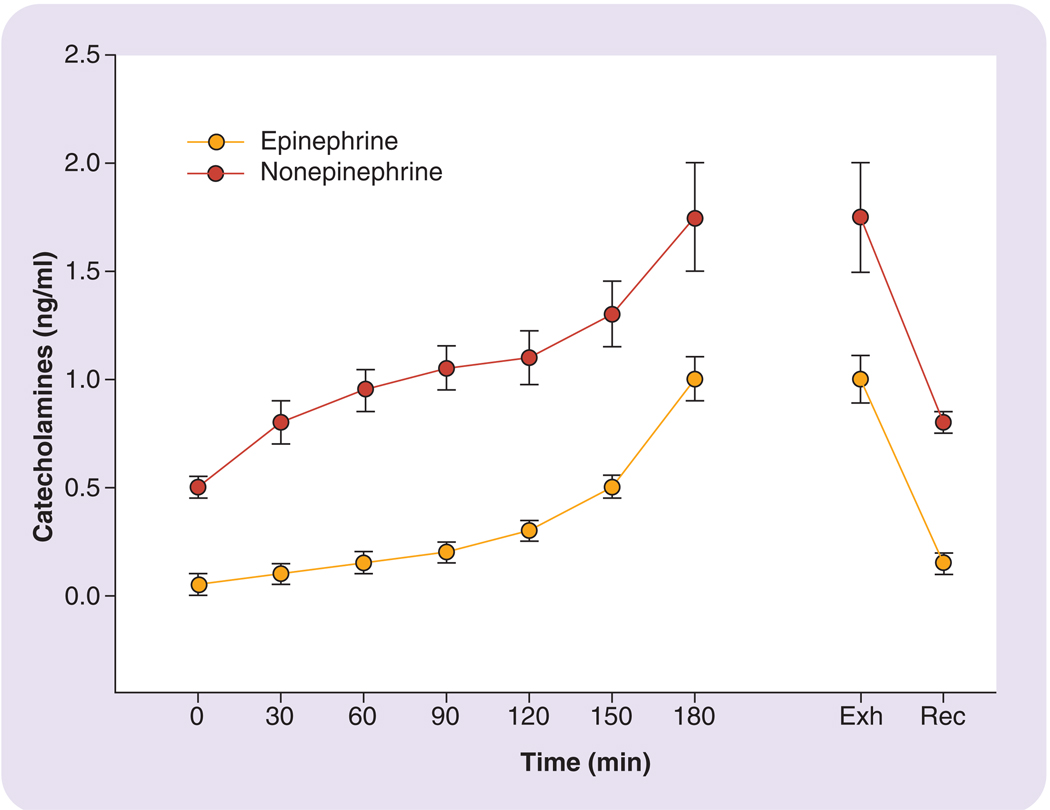
Interestingly, if the intensity of exercise is held constant and the duration of the exercise session is extended (i.e., steady-state exercise), the hormonal stress response still becomes further augmented. FIGURE 3 demonstrates some findings by Galbo and colleagues illustrating this point: the figure depicts the gradual increases in norepinephrine and epinephrine over a 3-h moderate constant-intensity exercise session [15]. A similar phenomenon concerning extending the duration of exercise has been demonstrated with cortisol by numerous studies [15–17,19,21,25]. The mechanism for these gradual increases seems to be a combination of events influencing the hormone levels such as, hemoconcentration, increased production and reduced metabolic clearance [19].
Figure 3. Blood (plasma) catecholamine (norepinehrine and epinephrine) response to a steady-state submaximal exercise session.
Redrawn from part of the data presented in [15] and [21]. Exh: Point of exhaustion for subjects; Rec: Recovery following exercise.
Studies suggest that the neuroendocrine stress response to an acute exercise session appears very transient in nature, and during the recovery from exercise, the hormonal levels return to baseline or slightly below basal values relatively rapidly. Hackney and associates have demonstrated this latter point in finding that, typically, prolonged endocrine disturbances only accompany extremely stressful exercise (e.g., intensive training or overreaching training [vide infra]) or excessively prolonged exercise (e.g., hours to days) in normal healthy adults [26–28]. For example, Hackney and Viru found that when athletes performed two intense training sessions in the course of a single day, at night, their circulating cortisol levels were significantly depressed. Furthermore, the more intense these daytime exercise sessions (i.e., higher intensity of workload), the greater the magnitude of the night-time suppression observed [28]. While rare, such exercise training practices do occur in the training regimes of elite athletes [27,28].
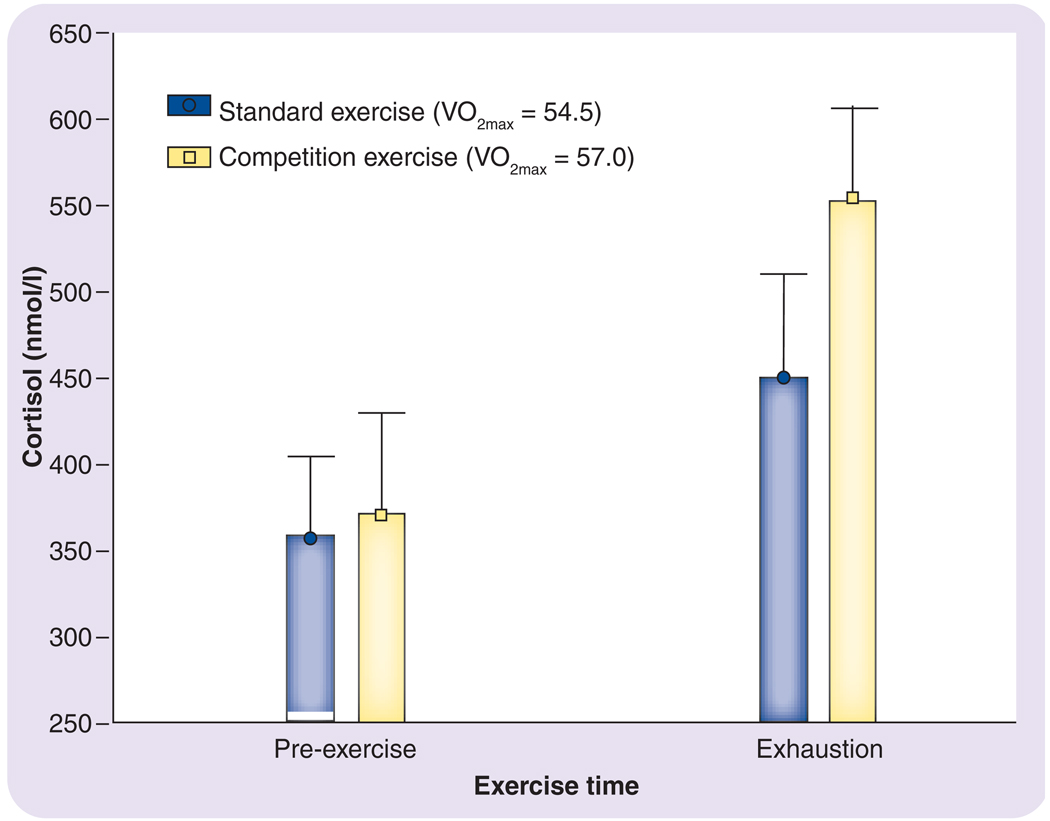
The studies discussed above demonstrate that exercise provokes a neuroendocrine stress response in adults. It is important to note, however, that not all exercise is necessarily the same. For example, the stress hormonal response can be further heightened when exercise is performed in competitive situations. FIGURE 4 demonstrates cortisol response to two identical incremental treadmill runs to exhaustion in the same adult subjects on separate days. The postexercise cortisol is substantially greater when the subjects performed their exercise in a simulated competitive environment, even though the maximal oxygen uptake responses did not differ substantially between the two conditions [29]. These subjects were all highly trained, experienced competitive athletes; consequently, exercise in a competitive environment was not a novel situtaion.
Figure 4. Cortisol levels in the blood of men pre- and postexercise involving a strenuous maximal exercise treadmill running test.
The testing was performed in a normal setting and in a simulated competitive exercise situation. The VO2max values did not differ significantly between the two exercise sessions. Redrawn from part of the data presented in [29].
V02max: Maximal oxygen uptake.
Moreover, competitions seem to vary in the magnitude of the stress response that they provoke. Obminiski and associates examined resting morning cortisol profiles in international-level competitive ice skaters [30]. The skaters were followed from their precompetitive season training, through their national championships and the European Championships, and finally to the 2002 Nagano Winter Olympics. There was a progressive increase in the cortisol levels of the skaters as the competitions became of increasing international importance.
However, there are modifying factors that can influence the neuroendocrine stress response to exercise. BOX 3 presents a list of some of the most typical factors known to influence hormonal responses to exercise. These factors, depending upon their manipulation, can augment or attenuate the hormonal response rather substantially; these effects are summarized in BOX 3. An in-depth discussion of these factors is beyond the scope of this review, but the reader is directed to [16] and [31] for further details.
Box 3. Factors that can influence the neuroendocrine system response to an acute exercise session
Mode of activity – smaller muscle mass activities can induce greater sympathetic nervous system activity during exercise
Anaerobiosis of the exercise – the greater the degree of anaerobic metabolism necessary to allow the exercise to be performed, typically, the greater the magnitude of stress encountered by the individual (this is influenced by the level of exercise intensity and the degree of training the individual has experienced)
Environmental conditions – heat and cold exposure beyond normal limits can augment the stress hormone responses to exercise
Age – younger individuals tend to have augmented stress hormone responses to exercise than older, mature individuals
Gender – menstrual cycle fluctuations in sex steroid hormones can interact and affect the stress hormonal response during exercise
Nutrition – the timing of a meal and its macronutrient content can alter the magnitude and degree of stress hormone response to exercise
Circadian rhythms – many of the hypothalamic–pituitary–adrenal axis hormones display such rhythms and the exercise response can be influenced by the level of rhythmicity
Genetics – many individuals display some degree of interindividual variation in how they respond to exercise, which has been attributed to genetic variation
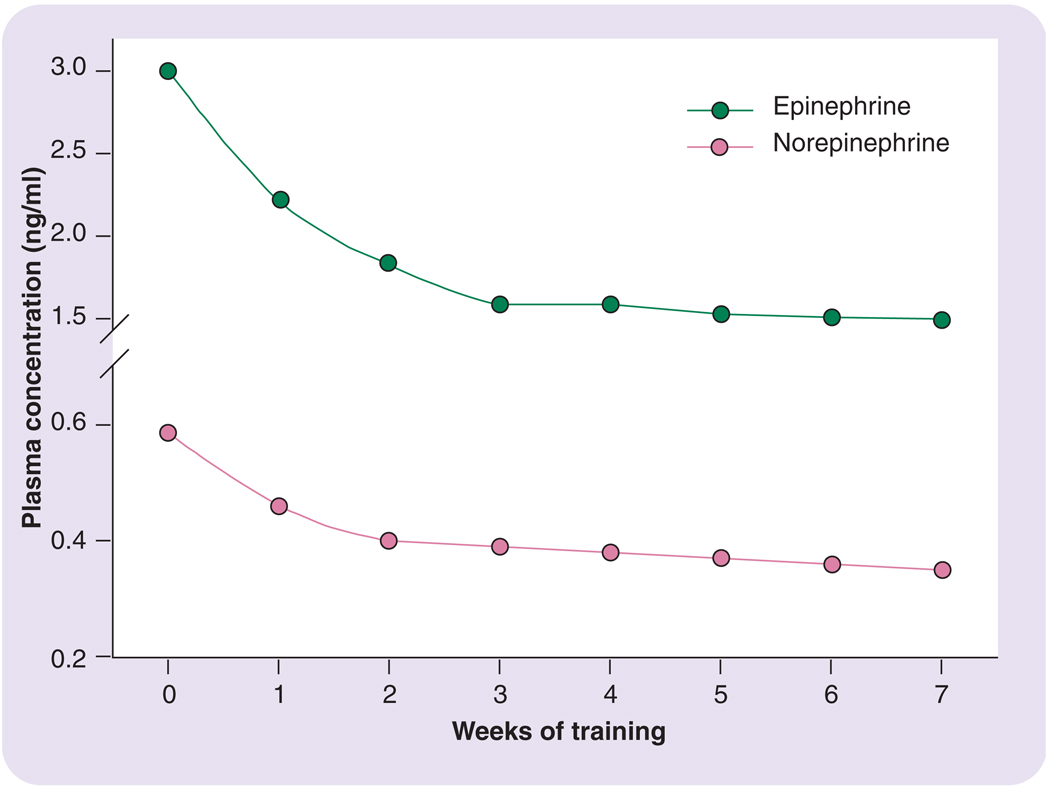
One important factor not mentioned in BOX 3 is exercise training. In fact, the level of chronic exposure to exercise is one of the most potent factors influencing the neuroendocrine stress response to an acute exercise session. That is, it is well established that as a person becomes more regular and chronic in their exercise pattern, the neuroendocrine stress response to exercise becomes attenuated when the exercise session is performed at the same absolute workload. FIGURE 5, which demonstrates findings by Winder, illustrates this adaptation for norepinephrine and epinephrine [32]. Depicted are the catecholamine responses to a repeated series of constant-load submaximal exercise bouts across a several-week training program; as is seen, the hormonal responses to the exercise stimulus become greatly reduced over time. Such findings as this in adults for other stress hormone responses are numerous in the research literature [15–17,19,26,33,34].
Figure 5. Blood (plasma) catecholamine response to exercise of a constant intensity level over a 7-week exercise training period.
Catecholamine responses are substantially reduced by the end of the exercise training period. Redrawn from part of the data presented in [32].
A similar phenomenon of reduced hormonal concentrations is also seen for the resting, basal levels in many situations [19,34,35]. Interestingly, however, an augmented stress response to maximal exercise following exercise training can actually occur. This greater hormonal responsiveness after exercise training appears to be due to both the fact that the absolute workload necessary to elicit a maximal response is much greater and to there being a glandular adaptation resulting in an enhanced hormonal secretory capacity [36–39].
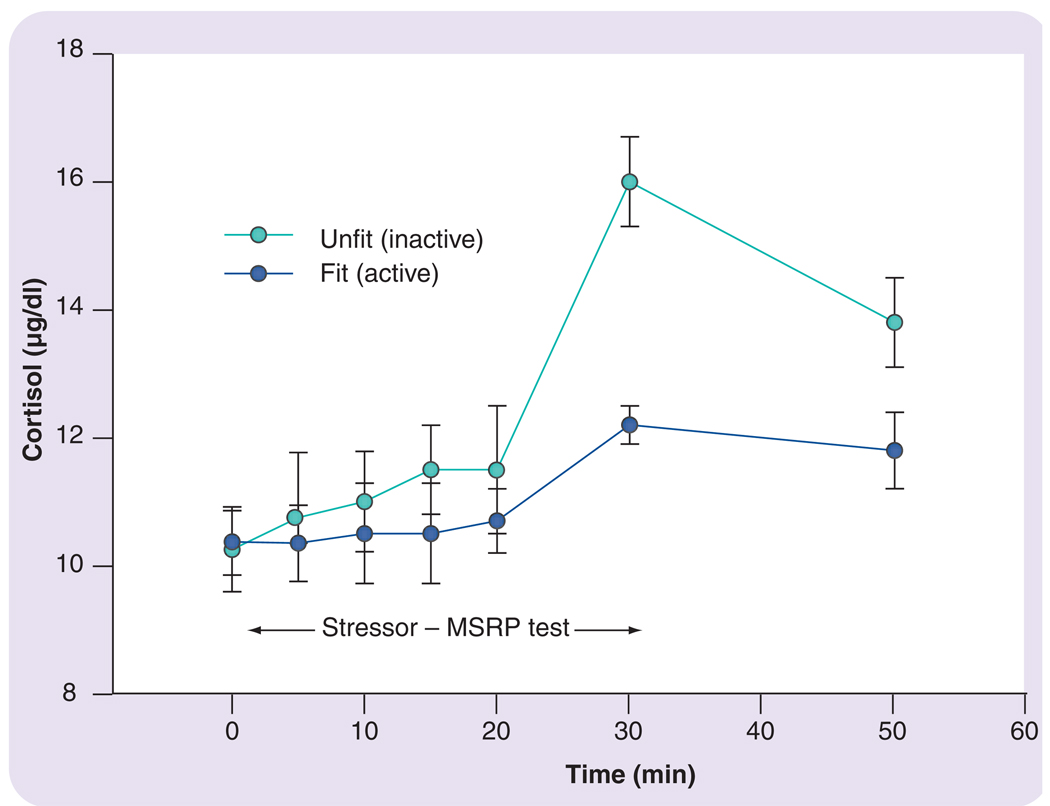
The adaptation of an attenuated neuroendocrine stress response to chronic exercise training has effects on aspects of stress reactions other than just those to acute exercise. Specifically, evidence supports an abatement of the neuroendocrine response to other life stressors by exposure to exercise training. For example, Traustadottir and colleagues examined two groups of adult women who were subjected to a standardized psychosocial stressor. They found that the cortisol response to the stressor was substantially lower in the physically more active women compared with those who were more sedentary (i.e., not very physically active) (FIGURE 6) [40].
Figure 6. Cortisol response of fit and unfit women to a psychosocial stressor.
The cortisol response was measured by the MSRP test [40]. The data in the figure are redrawn from part of the data presented in [40].
MSRP: Matt stress reactivity protocol.
This example only describes an acute stress exposure. By contrast, it is known that chronic exposure to psychosocial, environmental or traumatic stressors can have long-lasting, deleterious effects on a person’s psychological and physiological health, in both adults and children [21,35,40]. The suggestion that exercise may have a carry-over effect on the acute stress response to other stressors suggests physical exercise could, perhaps, be part of an intervention strategy to deal with some chronic stress-related health problems in the young and old alike. Unfortunately, WHO statistics point to a trend in many countries of the world towards a more sedentary lifestyle in both children and adults [41]. Thus, there is a great need in the scientific community to make the public aware of the important role exercise can potentially play as an adjunct to both proper nutrition and medical attention in dealing with health issues related to chronic stress exposure.
The intent of exercise training is to improve health and human performance. These two objectives are not mutually exclusive; but, depending upon the individual, the focus of training can be skewed towards one or the other. In the case of elite athletes, the focus is skewed towards the latter goal. In attempting to enhance human performance and cause positive physiological adaptations, athletes perform a tremendous amount of exercise training. If such exercise training is excessive or inappropriate in intensity, it can, however, be unproductive, leading to inappropriate neuroendocrine stress responses [26,42]. In fact, the stress exercise is placing upon the organism may become distressful and harmful in nature (i.e., excessive allostatic load). Consequently, this may start to induce maladaptations in the athlete and compromise their subsequent ability to perform. In the area of exercise and sports physiology, this process is referred to as over-reaching–overtraining and the potential clinical outcome of the process as the overtraining syndrome [26,43–45].
In appropriate athletic exercise training, the individual is subjected to a gradual increase in training overload (an exercise volume stimulus not previously experienced) followed by time to rest and recover. In turn, as a response to this approach, they begin to adapt to the overload stimulus and promote physiological compensations leading to an improved performance capacity. If the training overload stimulus is too much or adequate rest not allowed, then the athlete may not be able to adapt. That is, they may be over-reaching in their training. If their training is continued to be progressed to a further level of overload (or continued inadequate amounts of rest allowed), they may move to the category of overtraining. If not checked in this progression, the athlete may ultimately develop and display the clinical characteristics of the overtraining syndrome (see BOX 4) [42].
Box 4. The major signs and symptoms of overtraining and the overtraining syndrome
Physiological function
Decreased competitive performance
Decreased muscular strength
Increased muscular soreness
Chronic fatigue
Reduced tolerance to training overload
Sleep–wake cycle abnormalities
Gastrointestinal disturbances
Reduced sexual drive and libido
Altered heart rate responses
Suppressed immunological function
Psychological function
Increased feelings of depression
Lethargy and apathy
Emotional abnormalities
Loss of appetite
Lack of competitive drive
Restlessness
Difficulty in concentrating
The research on over-reaching–overtraining by several investigators suggests that the neuroendocrine stress hormonal response seems to be of two phases: an initial hyperactivity phase, followed by a latter hypoactivity phase [43–46,101]. In the latter phase, elevations in the circulating levels of ACTH, cortisol, prolactin and catecholamines have been reported at rest or in response to acute exercise [45,47–49]; although these hormonal patterns do not seem universal [45,49]. This phase may be reflective of the over-reaching point in the training continuum. Interestingly, in some situations during over-reaching, if the athlete is given short-term rest and recovery, they may actually compensate with greater than normal adaptations and enhancements of performance [49]. This fact explains why some athletes and their coaches push the degree of training so intently. The neuroendocrine hypoactive phase seems to correspond with the overtraining point and/or with the manifestation of the overtraining syndrome. In the latter situations, neuroendocrine function becomes suppressed for certain hypothalamic, pituitary and adrenal cortical hormones (e.g., ACTH, luteinizing hormone, follicle-stimulating hormone catecholamines and cortisol) [49–52]. The development of the hypoactivity phase appears to be the more serious outcome as it may require weeks or months for the athlete to recover and regain normal endocrine function. In some situations, pharmacological intervention (e.g., antidepressant) seems necessary to help the athlete cope and recover from the condition [49].
Currently, it is unclear as to what is the physiological mechanism that induces the overtraining syndrome. The most prevailing theory suggests that clinically overt overtraining syndrome may reflect the exhaustion stage of Selye’s General Adaptation Syndrome [6,42]. This stage is characterized by insufficient glucocorticoid response to increasing demands owing to persistent stressors, a finding already noted in the overtrained athletes (vide supra). Smith has further proposed that with overtraining, the excessive musculoskeletal loading of exercise (associated with inadequate rest and recovery) results in tissue damage, hence, local and system inflammation develops and becomes excessive [53]. Inflammatory cytokines, such as interleukin (IL)-6, tumor necrosis factor (TNF)-α and IL-1β act upon multiple levels of the hypothalamic–pituitary–adrenal (HPA) axis, most notably the hypothalamic paraventricular nucleus (PVN) where corticotropin-releasing hormone production occurs [54,55]. This neuroendocrine peptide influences ACTH secretion (and the whole HPA axis), and can also affect mood, sexual and immune functions [42,44,49]; either directly acting on brain sensitive regions or indirectly via the sympathetic nervous system [42,50,56]. As these systems interact, the negative events of overtraining intensify and the athlete spirals downward from healthy and physically fit to syndrome development and psychophysiological compromise. This proposed mechanism, while not perfect, reconciles and connects many of the major pathogenic and clinical manifestations of the overtraining syndrome.
Regrettably, the degree of exercise training volume or lack of rest–recovery that puts an athlete at risk for moving from overload, to over-reaching, to overtraining in their program is uncertain. It is, fortunately, an issue of intense study and much debate among researchers of this topic. Similarly, the time-line for the transition from normal, to hyper- to hypoactive phases of neuroendocrine responses are also uncertain. This entire line of work is hampered since systematic research on overtraining, for ethical reasons, is very limited and essentially consists of case study-like reports. Nevertheless, careful systematic work on this topic is necessary and warranted, especially in young developing athletes, as the stress response hormones are linked tightly and influence many physiological systems [3,8,57]. For example, abnormal or inappropriate stress responses could potentially impact upon such processes as adrenarche, gonadarche and menarche in the developing youthful athlete.
Summary & conclusion
In summary, there are several key points for the reader to draw from this review:
Acute exercise is a powerful activator of the neuroendocrine system (i.e., a stressor), provided that the exercise is of sufficient intensity and/or duration.
The neuroendocrine stress response to exercise appears to be directly proportional to the volume of exercise exposure. These hormonal results of acute exercise (low-to-moderate intensity) in adults are typically transient in nature and do not last for longer than a few minutes to hours into recovery.
The stress response to exercise in adults can be heightened when the exercise is performed in competitive situations.
Adaptations in the neuroendocrine system with chronic exposure to exercise training results in a reduction in hormonal stress response to submaximal exercise and, in some cases, reduced basal hormone levels in adults.
This abatement of the hormonal stress response associated with exercise training has potential health implications for dealing with chronic stress-related problems, but more research is necessary in this area.
In adults, excessive exercise training can push the neuroendocrine exercise stress response to become inappropriate, resulting in the potential development of the overtraining syndrome.
Research evidence suggests that stress in small amounts with interspersed rest–recovery periods enables the neuroendocrine system to respond and prepare the body for anticipated needs, both in the present and for future challenges. Exercise as a stressor, in this respect, is no different in its acute effects on the neuroendocrine system. However, chronic exposure to exercise training allows adaptation and accommodation within the neuroendocrine system, such that the stress response to subsequent acute exercise is lessened (i.e., the paradoxical effect of exercise on the system). Nevertheless, if the exercise stress presents a challenge beyond the ability of the neuroendocrine system to compensate, there can be more prolonged and profound disruption of homeostasis and a decrement in the function of other bodily systems or the organism as a whole.
Expert commentary
Stress endocrinology is an evolving area of study with many exciting research questions to challenge scientists. It is also a multidisciplinary area in which there is a need for more communication and interaction among fields of study. The dual role of exercise as a means to provoke and abate the neuroendocrine stress response makes it an excellent research tool, as well as a study area, for scientists in the various disciplines who are examining stress.
The most exciting future avenue in need of further pursuit is the impact of exercise on other life stressors (e.g., psychosocial, environmental or traumatic) that can have long-lasting deleterious effects on an individual’s health. The promotion of lifestyle changes to incorporate more regular amounts of daily physical activity (i.e., exercise) holds the promise of being an enhancement in the treatment of stress-related health problems. Such behavioral changes, in concert with proper nutritional practices, appropriate medical supervision and pharmaceutical interventions where needed, can aid tremendously in dealing with these deleterious effects of chronic stress exposure.
Key issues
Physical exercise is a stressor to the human body and serves as a robust activator of the neuroendocrine system, provided that the exercise is of sufficient volume (i.e., intensity and/or duration). The magnitude of the neuroendocrine stress response to exercise seems directly proportional to the volume of exercise exposure. These stress hormone responses are typically very transient in nature and do not last longer than a few minutes to hours into recovery.
Chronic exposure to exercise training results in adaptations in the neuroendocrine system, such that there is a reduction in hormonal stress response to submaximal exercise and, in many cases, reduced circulating basal stress hormone levels. This abatement of the hormonal stress response with exercise training has implications for dealing with many chronic stress-related health problems.
The adaptability and plasticity in the neuroendocrine system to exercise training has limits. Excessive exercise training can push the neuroendocrine exercise stress response to become inappropriate, resulting in the potential development of chronic fatigue and the overtraining syndrome condition.
References
- 1.McEwen BS. Stressed or stressed out: what is the difference? J. Psychiatry Neurosci. 2005;30(5):315–318. [PMC free article] [PubMed] [Google Scholar]
- 2.McEwen BS. Stress, definition and concepts of. In: Fink G, editor. Encyclopedia of Stress, Volume 3. CA, USA: Academic Press; 2000. pp. 508–509. [Google Scholar]
- 3.McEwen BS. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharm. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 5.Cannon WB. The emergency function of the adrenal medulla in pain and in the major emotions. Am. J. Physiol. 1914;33:356–372. [Google Scholar]
- 6.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32–36. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 7.Mason JW. A re-evaluation of the concept of ‘non-specificity’ in stress theory. J. Psychiat. Res. 1971;8:323–328. doi: 10.1016/0022-3956(71)90028-8. [DOI] [PubMed] [Google Scholar]
- 8.Chrousos CP, Gold PW. The concept of stress systems disorders: overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 9.Sterling P, Eyer J. Allostasis: a new paradigm to explain arousal pathology. In: Fisher S, editor. Handbook of Life Stress, Cognition and Health. NY, USA: J Wily & Sons; 1988. pp. 629–649. [Google Scholar]
- 10.Young E, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Front. Neuroendocrinol. 2004;25:69–76. doi: 10.1016/j.yfrne.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Levine S. Developmental determines of sensitivity and resistance to stress. Psychoneuroendocrinol. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DS. Catecholamines and stress. Endocrine Reg. 2003;37:69–80. [PubMed] [Google Scholar]
- 13.Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Ann. Rev. Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 14.Tremblay MS, Chu SY, Mureika R. Methodological and statistical considerations for exercise-related hormone evaluations. Sports Med. 1995;20(2):90–108. doi: 10.2165/00007256-199520020-00004. [DOI] [PubMed] [Google Scholar]
- 15.Galbo H. Hormonal and Metabolic Adaptation to Exercise. Stuttgart, Germany: Georg Thieme Verlag; 1983. pp. 2–117. [Google Scholar]
- 16.Viru A. Hormonal Ensemble in Exercise – Hormones in Muscular Activity, Volume 1. FL, USA: CRC Press; 1985. pp. 34–96. [Google Scholar]
- 17.Viru A. Plasma hormones and physical exercise. Int. J. Sports Med. 1992;13:201–209. doi: 10.1055/s-2007-1021254. [DOI] [PubMed] [Google Scholar]
- 18.Brooks GA, Fahey TD, White TP. Exercise Physiology: Human Bioenergetics and its Application, (2nd Edition) Toronto, Canada: Mayfield Publishing; 1996. Neural-endocrine control of metabolism; pp. 56–196. [Google Scholar]
- 19.McMurray RG, Hackney AC. Endocrine responses to exercise and training. In: Garrett W, editor. Exercise and Sport Science. PA, USA: Lippincott Williams & Wilkins; 2000. pp. 135–162. [Google Scholar]
- 20.Davies CTM, Few JD. Effects of exercise on adrenocortical function. J. Appl. Physiol. 1973;5:887–891. doi: 10.1152/jappl.1973.35.6.887. [DOI] [PubMed] [Google Scholar]
- 21.Borer KT. Exercise Endocrinology. IL, USA: Human Kinetics Publishing; 2003. pp. 77–96. [Google Scholar]
- 22.Lehmann M, Keul J, Huber G, DaPrada M. Plasma catecholamines in trained and untrained volunteers during graded exercise. Int. J. Sports Med. 1981;2(3):143–147. doi: 10.1055/s-2008-1034601. [DOI] [PubMed] [Google Scholar]
- 23.Farrell PA, Garthwaite TL, Gustafson AB. Plasma adrenocorticotropin and cortisol responses to submaximal and exhaustive exercise. J. Appl. Physiol. 1983;55(5):1441–1444. doi: 10.1152/jappl.1983.55.5.1441. [DOI] [PubMed] [Google Scholar]
- 24.Luger A, Deuster PA, Kyle SB, et al. Acute hypothalamic-pituitary-adrenal responses to the stress of treadmill exercise: physiologic adaptations to training. N. Engl. J. Med. 1987;316(21):1309–1315. doi: 10.1056/NEJM198705213162105. [DOI] [PubMed] [Google Scholar]
- 25.Struder HK, Hollmann W, Platen P, Rost R, Weicker H, Weber K. Hypothalamic–pituitary–adrenal and–gonadal axis function after exercise in sedentary and endurance trained elderly males. Eur. J. Appl. Physiol. Occup. Physiol. 1998;77(3):285–288. doi: 10.1007/s004210050334. [DOI] [PubMed] [Google Scholar]
- 26.Hackney AC. Neuroendocrine system, exercise overload and regeneration. In: Lehmann M, editor. Overload, Performance Incompetence, and Regeneration in Sport. Stuttgart, Germany: Kluwer Academic-Plenum; 1999. pp. 131–177. [Google Scholar]
- 27.Hackney AC, Premo MC, McMurray RG. Influence of aerobic versus anaerobic exercise on the relationship between reproductive hormones in men. J. Sports Sci. 1995;13(4):305–311. doi: 10.1080/02640419508732244. [DOI] [PubMed] [Google Scholar]
- 28.Hackney AC, Viru A. Twenty-four hour cortisol response to multiple daily exercise sessions of moderate and high intensity. Clin. Physiol. 1999;19(2):178–182. doi: 10.1046/j.1365-2281.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- 29.Hackney AC, Viru A, Viru M, Karelson K, Janson T, Siim Fischer K. Adrenergic influence on the hormonal response to exercise in endurance trained men; Presented at: 53rd American College of Sports Medicine meeting; Denver, CO, USA: 2006. (Abstract 2572) [Google Scholar]
- 30.Obminski Z, Stupnicki R, Lerczak K, Starczewska-Czapowska J, Olszewska-Lelonkiewicz M, Hackney AC. Cortisol and testosterone responses to training and competition stress in ice-dance skaters – a case study. So. African J. Sports Med. 2002;9:23–25. [Google Scholar]
- 31.Loucks AB, Heath EM. Dietary restriction reduces luteinizing hormone (LH) pulse frequency during waking hours and increases LH pulse amplitude during sleep in young menstruating women. J. Clin. Endocrinol. Metab. 1994;78:910–915. doi: 10.1210/jcem.78.4.8157720. [DOI] [PubMed] [Google Scholar]
- 32.Winder WW, Hagberg JM, Hickson RR, Ehsani AA, McLane JA. Time course of sympathoadrenergic adaptation to endurance exercise training in man. J. Appl. Physiol. 1978;45:370–374. doi: 10.1152/jappl.1978.45.3.370. [DOI] [PubMed] [Google Scholar]
- 33.Bobbert T, Brechtel L, Mai K, et al. Adaptation of the hypothalamic–pituitary hormones during intensive endurance training. Clin. Endocrinol. 2005;63:530–536. doi: 10.1111/j.1365-2265.2005.02377.x. [DOI] [PubMed] [Google Scholar]
- 34.Crews DJ, Landers DM. A meta-analytic review of aerobic fitness and reactivity to psychosocial stressors. Med. Sci. Sports Exerc. 1987;19(5):S114–S120. [PubMed] [Google Scholar]
- 35.Blair SN, Kampert JB, Kohl HW, et al. Influence of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210. [PubMed] [Google Scholar]
- 36.Kjaer M, Mikines KJ, Linstow MV, Nicolaisen T, Galbo H. Effect of 5 wk of detraining on epinephrine response to insulin-induced hypoglycemia in athletes. J. Appl. Physiol. 1992;72(3):1201–1204. doi: 10.1152/jappl.1992.72.3.1201. [DOI] [PubMed] [Google Scholar]
- 37.Deuster PA, Chrousos GP, Luger A, et al. Hormonal and metabolic responses of untrained, moderately trained, and highly trained men to three exercise intensities. Metabolism. 1989;38(2):141–148. doi: 10.1016/0026-0495(89)90253-9. [DOI] [PubMed] [Google Scholar]
- 38.Korkushko OV, Frolkis MV, Shatilo VB. Reaction of pituitary–adrenal and autonomic nervous system to stress in trained and untrained elderly people. J. Auton. Nerv. Syst. 1995;54(1):27–32. doi: 10.1016/0165-1838(95)00015-p. [DOI] [PubMed] [Google Scholar]
- 39.Park E, Chan O, Li Q, et al. Changes in basal hypothalamic–pituitary–adrenal activity during exercise are centrally mediated. Am. J. Physiol. 2005;289:R1360–R1371. doi: 10.1152/ajpregu.00103.2005. [DOI] [PubMed] [Google Scholar]
- 40.Traustadottir T, Bosch PR, Matt KS. The HPA axis response to stress in women: effects of aging and fitness. Pyschoneuroendocrinol. 2005;30:392–402. doi: 10.1016/j.psyneuen.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. Global Strategy on Diet, Physical Activity and Health: Obesity and Overweight Facts Summary. WHO technical report series, No.916. 2003
- 42.Angeli A, Minetto M, Dovio A, Paccotti P. The overtraining syndrome in athletes: a stress-related disorder. J. Endocrinol. Invest. 2004;27(6):603–612. doi: 10.1007/BF03347487. [DOI] [PubMed] [Google Scholar]
- 43.Lehmann M, Foster C, Keul J. Overtraining in endurance athletes: a brief review. Med. Sci. Sports Exerc. 1993;25:854–862. doi: 10.1249/00005768-199307000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Lehmann M, Lormes W, Opitz-Gress A, et al. Training and overtraining: an overview and experimental results in endurance sports. J. Sports Med. Phys. Fit. 1997;37:7–17. [PubMed] [Google Scholar]
- 45.Meeusen R, Duclos M, Gleeson M, Rietjens G, Steinacker J, Urhausen A. Prevention, diagnosis and treatment of the overtraining syndrome: ECSS Position Statement ‘Task Force’. Eur. J. Sport Sci. 2006;6(1):1–14. doi: 10.1249/MSS.0b013e318279a10a. [DOI] [PubMed] [Google Scholar]
- 46.Viru A, Viru M. Cortisol – essential adaptation hormone in exercise. Int. J. Sport Med. 2004;25:461–464. doi: 10.1055/s-2004-821068. [DOI] [PubMed] [Google Scholar]
- 47.Fry AC, Schilling BK, Weiss LW, Chiu L. β adrenergic down-regulation and performance decrements during high intensity resistance exercise overtraining. J. Appl. Physiol. 2006 doi: 10.1152/japplphysiol.01599.2005. (In Press) [DOI] [PubMed] [Google Scholar]
- 48.Hackney AC. Hormonal changes at rest in overtrained endurance athletes. Biol. Sport. 1991;8(2):49–56. [PMC free article] [PubMed] [Google Scholar]
- 49.Fry AC, Steinacker JM, Meeusen R. Endocrinology of overtraining. In: Kraaemer WJ, Rogol AD, editors. The Endocrine System in Sports and Exercise. Oxford, UK: Blackwell Publishing; 2005. pp. 578pp. 584–593. [Google Scholar]
- 50.Tsigo C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 51.Rogero MM, Mendes RR, Tirapegui J. Aspectos neuroendocrinos e nutricionais em atletas com overtraining. Arq. Bras. Endocrinol. Metabol. 2005;49(3):359–368. doi: 10.1590/s0004-27302005000300006. [DOI] [PubMed] [Google Scholar]
- 52.Barron G, Noakes T, Levy W, Smidt C, Millar R. Hypothalamic dysfunction in overtrained athletes. J. Clin. Endocrinol. Metab. 1995;60:803–806. doi: 10.1210/jcem-60-4-803. [DOI] [PubMed] [Google Scholar]
- 53.Smith LL. Tissue trauma: the underlying cause of the overtraining syndrome? J. Strength Cond. Res. 2004;18(30):185–193. doi: 10.1519/1533-4287(2004)018<0185:tttuco>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 54.Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 55.Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc. Sport Sci. Rev. 2005;33(3):114–119. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Mastorakos G, Pavlatou M, Diamanti-Kandaraki E, Chrousos GP. Exercise and the stress system. Hormones (Athens) 2005;4(2):73–89. [PubMed] [Google Scholar]
- 57.Hackney AC. Exercise as a stressor to the human neuroendocrine system. Medicina. 2006;42(10):788–796. [PubMed] [Google Scholar]
Website
- 101.Tsigos C, Kyrou I, Chrousos G. Stress, endocrine physiology and pathophysiology. 2006 www.endotext.com/adrenal/ [Google Scholar]