Abstract
Measurement of iohexol plasma disappearance GFR in children require optimization of duration and sampling times Shortening the plasma disappearance study may overestimate GFR. We examined iohexol plasma disappearance curves in 27 children to determine the degree of overestimation in GFR due to shortening studies from 6 to 5 and to 4 hours. GFR measured after 5 hours was comparable to that after 6 hours, but shortening to 4 hours resulted in a 3% (p< 0.01) overestimation of GFR. Another simplification would be reducing the number of blood samples used to determine GFR. We followed the lead of Brochner-Mortensen and derived the relationship between a single compartment (slowGFR) disappearance curve and that from a double exponential analysis (two compartments sampled at 10, 30, 120, and 300 minutes), using 489 GFR measurements (median= 44 ml/min per1.73m2) in visit 1 of the Chronic Kidney Disease in Children (CKiD) study. Using polynomial regression methods, we developed coefficients to accurately measure GFR from a single compartment: GFR2 = K1*slowGFR + K2*(slowGFR)2. The coefficients (K1=1.0019 ; K2=-0.001258) were then used to generate GFR2 to be compared with 361 four point GFRs in visit 2. There was excellent correlation (r=0.999) and no bias or change in between-individuals’ dispersion. Conclusion: GFR can be accurately measured in children with CKD using the slow component of the iohexol plasma disappearance curve, provided the duration of study is at least 5 hours.
Introduction
Glomerular filtration rate (GFR) can accurately be determined from the plasma disappearance of iohexol after a single bolus injection [1;2]. The mathematical model for the disappearance curve is an open two-compartment system. The GFR marker is injected in the first compartment, equilibrates with the second compartment, and is excreted from the first compartment by glomerular filtration. Initially the concentration falls rapidly but at a progressively diminishing rate, as there is diffusion of the marker in its distribution volume in addition to renal excretion. Thereafter, the slope of the decline of plasma concentration reflects predominately its renal excretion rate. This latter decrease occurs at the same exponential rate in the compartments wherein it is distributed.
In order to perform epidemiological studies in chronic kidney disease (CKD) cohorts, and encourage large scale recruitment, it is imperative that the duration of time spent at the clinic or clinical research center not be excessive and protocols be minimally intrusive. Whereas optimal GFR studies may need to be performed overnight to obtain maximal precision and accuracy of the measurements, such a time commitment would compromise recruitment and retention of subjects. Clearly, a proper balance between accuracy and subject acceptance is necessary for designing feasible kidney disease studies.
To obtain an accurate iohexol plasma disappearance curve, several blood samples are required. Characterizing the curve based only on samples from early time points will result in an overestimation of GFR [3]. The first objective of this report was to compare the GFR values based on time points of up to 4, 5 and 6 hours using data on 27 children with sampling at 10, 20, 30, 60, 120, 180, 240, 300 and 360 minutes.
The NIH-funded North American cohort study of children with chronic kidney disease (CKiD) has used the plasma disappearance of iohexol to generate a four point GFR (iGFR or GFR4, with sampling at 10, 30, 120, and 300 minutes) [4]. In order to further simplify the iohexol protocol for subjects and study personnel, we have considered condensing the measurement to two sampling points at 120 and 300 minutes. As described by Brochner-Mortensen [5], this is facilitated by the tight relationship between the GFR obtained using multi-compartment 51Cr-EDTA plasma disappearance curves (i.e., area under the full disappearance curve) and the GFR obtained based only on a one-compartment (renal curve) model corresponding to the slow rate of decline (i.e., area under the slow curve) to which hereafter we referred as the slowGFR, A similar relationship has not been generated in children using iohexol. In our pilot study [6] the relationship between the 9 point iohexol plasma disappearance GFR and the one-compartment GFR followed a similar quadratic equation but there were too few subjects to generate a relationship to three significant figures. However, the CKiD study has collected enough data with four time points for us to examine the relationship between iohexol disappearance GFR based on four time points and the GFR based on two time points. Accordingly, the second aim of this report was to evaluate whether a Brochner-Mortensen like equation calibrated to the CKiD study accurately measures GFR and obviates early blood sampling.
Results
Table 1 provides the descriptive statistics of the pilot study (n= 27) as well as those of the CKiD study with complete data for the analysis at baseline (N= 489 of the 570 enrolled prior to February 2009) and for the subcohort which has completed the first follow up visit (N= 362). The profile of the subset of 489 in this analysis from the 570 enrolled (see methods) were very similar.
Table 1.
Descriptive Statistics with [median (interquartile range) or percentage] of Study Populations.
| CKiD Study |
|||
|---|---|---|---|
| Variable |
Pilot Study |
Baseline |
Follow-up |
| N | 27 | 489 | 362 |
| Age (years) | 14.0 (12.5, 18.0) | 11.0 (7.4, 14.4) | 12.1 (8.5, 15.6) |
| Male | 56% | 64% | 65% |
| Height (m) | 1.55 (1.45, 1.66) | 1.39 (1.19, 1.57) | 1.45 (1.23, 1.62) |
| Weight (kg) | 51.6 (42.7, 74.4) | 35.8 (23.4, 53.6) | 40.0 (26.1, 56.6) |
| BSA (m2) | 1.50 (1.30, 1.85) | 1.18 (0.88, 1.54) | 1.26 (0.95, 1.60) |
| Serum Creatinine (mg/dl) | 1.5 (1.1, 2.3) | 1.3 (1.0, 1.8) | 1.4 (1.0, 2.0) |
| Iohexol-based GFR (ml/min|1.73m2) | 51.3 (32.4, 70.0) | 43.3 (32.8, 56.0) | 42.1 (31.4, 54.3) |
Duration of GFR study
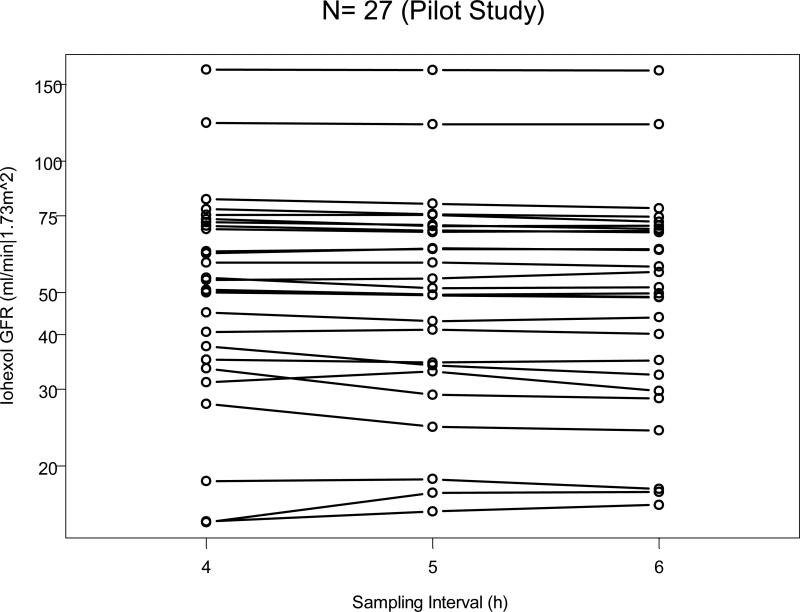
Figure 1 shows the three GFR estimates for each of the 27 children in the original pilot study [4] joined by a line and according to the sampling interval. Shortening the sampling interval of the slow (renal) curve from 6 to 5 hours did not significantly alter the measurement of GFR in the 27 subjects. However, shortening to 4 hours slightly but significantly increased the measured GFR compared to 6 hours. Indeed the median GFR (Table 2) at 6 hours was 51.3 ml/min per 1.73 m2, and the median ratio of GFRs based on 5 hour sampling compared to that based on 6 hours was 1.010 with an interquartile range (IQR) of 0.995 to 1.021. At 4 hours: the median ratio was 1.030 with IQR of 1.005 to 1.043 and the p value for testing whether the ratio was different from one was less than 0.01. That is, there was a significant 3% overestimation if sampling was truncated at 4 hours after iohexol injection.
1.
Iohexol-based GFR as a function of time of sampling for the slow curve. GFR for each of the 27 studies of the pilot study was calculated after 4, 5, and 6 hours of sampling.
Table 2.
Median (IQR) of Plasma Disappearance Parameters for the 6 Hour Study and the ratios of the 5 hour and the 4 hour to the 6 hour studies (N= 27)
| Ratios |
|||
|---|---|---|---|
| 6 hr | 5 / 6 hr | 4 / 6 hr | |
| |
|||
| Slow Curve | |||
| Intercept: exp(A) [mg/ml] | 0.253 (0.169, 0.318) | 1.016 (0.993, 1.035) | 1.057** (1.000, 1.092) |
| half life: ln(2)/α [min] | 172 (148, 297) | 0.975 (0.949, 1.009) | 0.940** (0.880, 0.996) |
| area: exp(A)/ α [mg min/ml] | 68.1 (41.5, 99.2) | 0.999 (0.981, 1.009) | 0.991 (0.973, 1.025) |
| Fast Curve | |||
| Intercept: exp(B) [mg/ml] | 0.172 (0.143, 0.225) | 0.983* (0.924, 1.001) | 0.983* (0.922, 1.007) |
| half life: ln(2)/β [min] | 19 (16, 25) | 0.970 (0.928, 1.045) | 0.926* (0.730, 1.004) |
| area: exp(B)/β [mg min/ml] | 5.1 (3.5, 6.3) | 0.952 (0.882, 1.031) | 0.910* (0.695, 1.003) |
| Total area: slow + fast | 74.4 (47.1, 104.3) | 0.990 (0.979, 1.005) | 0.971** (0.956, 0.995) |
| iGFR [ml/min|1.73m^2] | 51.3 (32.4, 70.0) | 1.010 (0.995, 1.021) | 1.030** (1.005, 1.043) |
0.01< p < 0.05
p < 0.01 by the sign test relative to the 6 hour study.
Examination of the plasma disappearance parameters in Table 2 shows that except for the area under the slow curve, all other parameters of the 4 hour study were significantly (p< 0.05) different from those of the 6 hour study. In contrast, the only parameter of the 5 hour study which was significantly different (0.01 < p < 0.05) from those of the 6 hour study was the intercept of the fast curve, but it was only 1.7% lower. It should be noted that the departures from 1 of the ratios of the descriptors of the fast component are entirely due to the variability of the coefficients of the slow component, the data from which are subtracted from the observed concentrations at 10 and 30 minutes to determine the parameters of the fast component.
Determining GFR from slow component of disappearance curve
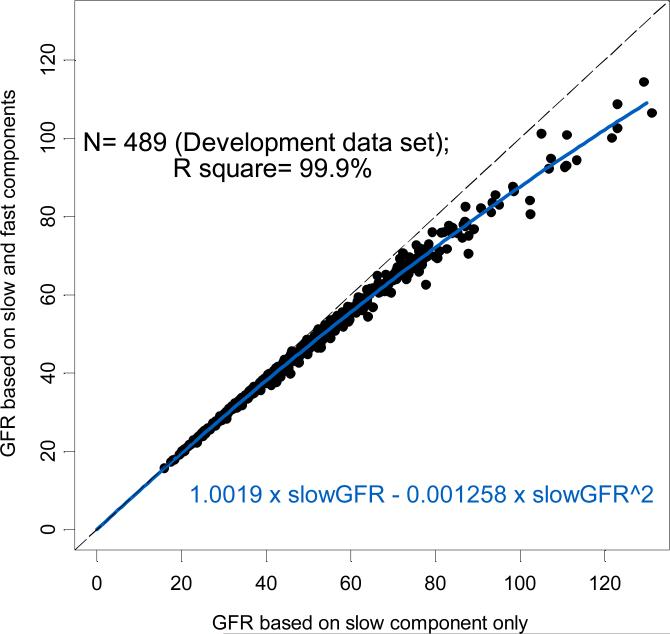
In order to derive a Brochner-Mortensen-like equation for subsequent use by the CKiD study, we examined the GFR calculated from the slow component of the double exponential curve against that determined from the slow and fast (double exponential) components. As expected, in 489 successful four-point GFR studies (Figure 2), the GFR calculated from the slow component alone (slowGFR) consistently overestimated that from the double exponential GFR, which was calculated from the fast and slow components. Using least squares methods for a polynomial regression with no intercept, we found that GFR could be determined from the slow component (slowGFR) according to the equation:
2.
Relationship between GFRs based on the slow component only (x axis) and the four point GFR (double exponential, y axis) in 489 subjects at Visit 1 of the CKiD study. The regression equation for GFR is given near the bottom of the graph.
These coefficients compare favorably with those published by Brochner-Mortensen for adults [5] (k1= 0.99078, k2= -0.001218) and for children [7] (k1= 1.01, k2= -0.0017) using 51Cr-EDTA plasma disappearance clearances.
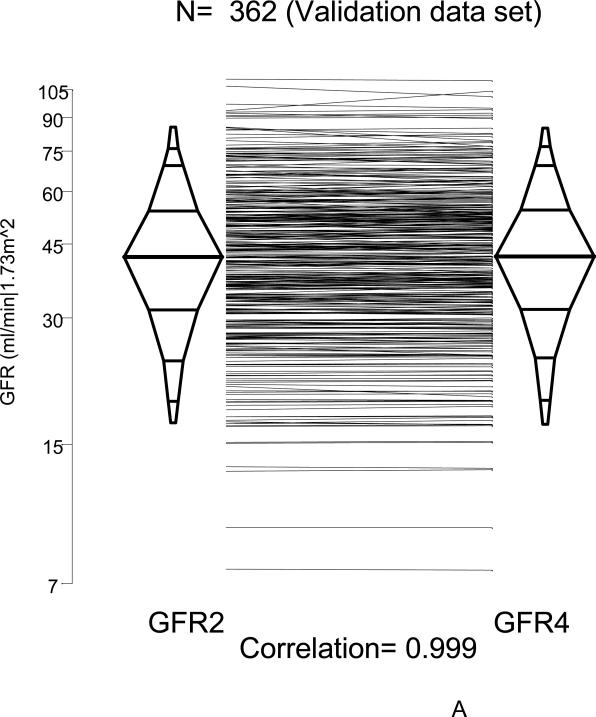
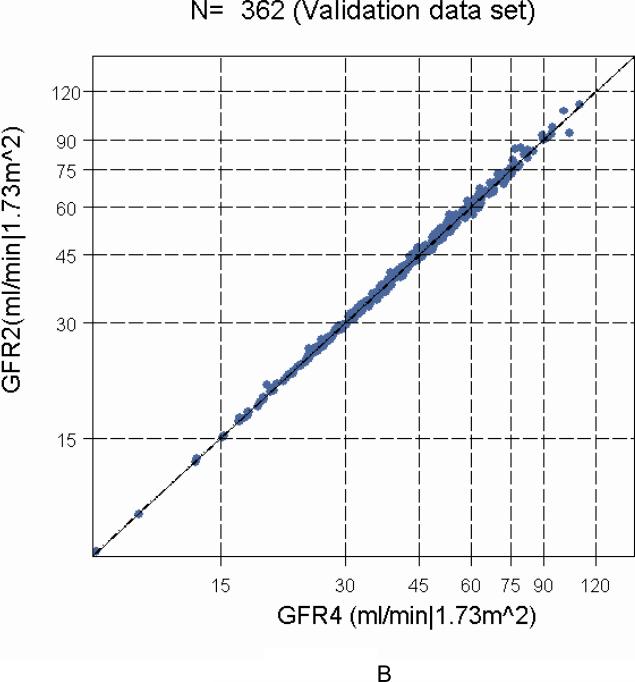
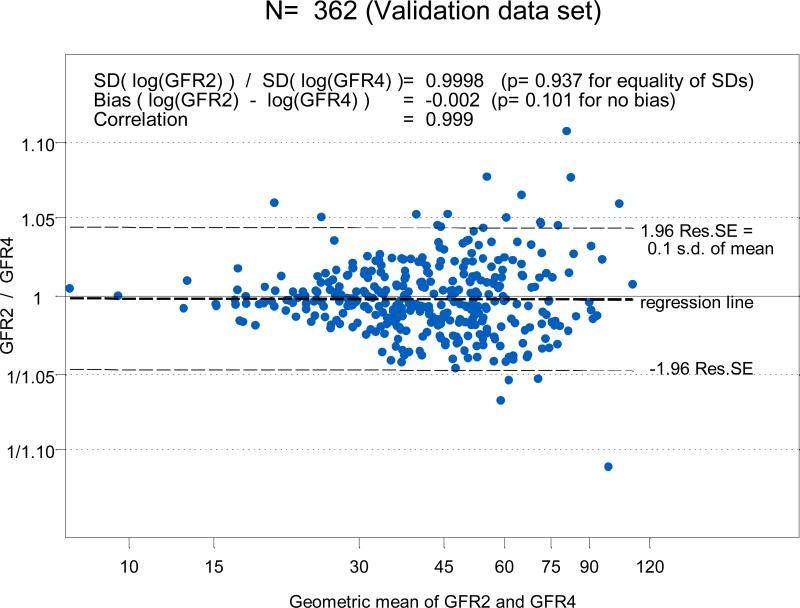
To determine the agreement between GFR4 based on four points (two for the slow curve at 120 and 300 minutes and two for the fast curve at 10 and 30 minutes) with the GFR2 obtained from just the two points in the slow curve (120 and 300 minutes) and the derived Brochner-Mortensen-like equation, we used the 362 successful four-point GFR studies performed in CKiD subjects one year later at Visit 2. GFR2 was well correlated (r = 0.999) and essentially identical to the four-point GFR4 (Figures 3A, histograms, and B, regression line). Since the regression line of the difference on the mean of the log transformed GFR2 and GFR4 had intercept and slope equal to zero, the Bland-Altman graph in Figure 4 showed that there was no bias when comparing GFR based on the single exponential clearance (GFR2) with four-point GFR4, and that the standard deviation of the GFR2 was essentially identical to that observed with the four-point GFR4. Furthermore, by the fact that 95% of differences in Figure 4 were within 5% and corresponded to only 0.1 of the standard deviation of the mean, this is congruent with a very high correlation (r= 0.999).
3.
Comparison of GFR2 (based on the slow component using constants determined from Visit 1) to four-point GFR4 studies in Visit 2 (N= 362) of the CKiD study. Panel A, Percentile (2.5th, 5th, 10th, 25th, 50th, 75th, 90th, 95th and 97.5th) boxplots showing a high correlation (r= 0.999). Panel B, linear regression of GFR2 on GFR4 showing a very close agreement and a very high correlation.
4.
Bland-Altman analysis of GFR2 versus four-point GFR in the 362 subjects at Visit 2 of the CKiD study showing an insignificant bias of -0.002. The ratio of the SD of the GFR2 and the four-point GFR was essentially equal to 1.
When GFR2 based on these coefficients for iohexol were compared with GFR2 using Brochner-Mortensen's coefficients for 51Cr-EDTA [7], the agreement along the line of identity was apparent with a correlation of >0.999 and, although statistically significant, the GFR values based on the original formula were downward biased by only 1.4%.
Repeating the development using only two-thirds of the data from Visit one (N=326) with a one-third random sample (N=163) reserved for validation resulted in identical inferences. Resampling of the development and validation sets yielded results from the Bland-Altman analysis of an average bias of -0.02% and an average ratio of the standard deviations of 1.0008 after resampling 5 times.
Discussion
Choice of iohexol plasma disappearance
In developing clinical and epidemiological studies using GFR as the primary measure of disease progression, a method that is reproducible but acceptable to subjects is critical. Retention is of primary importance in longitudinal studies and can be affected by challenging or time-consuming protocols. In preparation for CKiD, we planned to perform formal GFR measurements in alternate years after the first two study years, and based on the epidemiology of CKD in children (with vesico-ureteral reflux, bladder dysfunction, etc.), conventional renal clearance studies requiring the collection of urine would be notoriously inaccurate. In view of the fact that nearly 80% of our recruited subjects had some form of genitourinary disease, plasma disappearance technology would be a preferred method to determine GFR. Obviously, we chose a non-radioactive marker, because repeated measurements should not add additional radioactive risk to the subjects. The choice of iohexol over non-radioactive iothalamate was based primarily on the finding that iothalamate is actively secreted by renal proximal tubular cells [8] and its clearance exceeds that of inulin or 51Cr-EDTA [8;9]; either agent can be readily measured by high performance liquid chromatography [10-12]. There is abundant data to suggest that iohexol clearance is quite comparable to 51Cr-EDTA [13;14] and inulin [11;15-17]. Indeed, iohexol has been heralded as the new gold standard measure of GFR [15;18].
Duration of plasma disappearance study
A very important component for the proper determination of the iohexol plasma disappearance curve is the duration of examination. Empirical studies during renal insufficiency indicate that a later sampling time is necessary to obtain a correct determination of GFR [3;19-21]; the lower the GFR, the longer the interval after injection required for plasma sampling. Indeed, for single plasma samples and estimated GFR of 30 ml/min, the optimal sampling time was recommended to be 8-9 hours [19]. Stake et al [21] examined plasma clearances in 11 children with chronic kidney failure (mean GFR 12.5 ml/min per 1.73 m2) and the effect of the timing of sampling. When plasma was sampled several times within 3.5 h GFR was overestimated by more than 50%, compared to the reference method which obtained samples over 3 to 24 hours. They recommended that plasma sampling at 3 and 24 hours provided the best interval when clearance was less than 20 ml/min per 1.73 m2. Similarly, in adult patients with advanced CKD the most reliable assessment of GFR can be obtained from two blood samples drawn 5 and 24 hours after an intravenous single injection [22;23]. Thus, late plasma sampling is critical for the accurate determination of GFR by plasma disappearance methods in patients with renal failure. There are no data on the timing of sampling for children with mild to moderate CKD, but for adults with estimated GFR of 15 to 40 ml/min per 1.73 m2 it is recommended that two samples be obtained at 6 and 8 hours after injection; for GFR above 40 ml/min per 1.73 m2, the timing should be at 3 and 4 hours [24]. Using single injection iothalamate plasma clearances in men with CKD stages 3 and 4, Agarwal et al [25] found that short plasma sampling intervals substantially overestimated GFR and reduced precision of the measurement especially in larger-sized adult patients. Presumably, the area under the disappearance curve was underestimated in shorter studies and in bigger adults. In the group with estimated GFR above 30 ml/min per 1.73 m2 the two compartment GFR sampled for a duration of 5 hours overestimated GFR by 17% and sampling for 2 hours by 54%, compared with a 10 hour reference. In the group with GFR below 30 ml/min per 1.73 m2 the magnitude of overestimation was larger: 5 hour sampling was 36% higher and 2 hour sampling was 126% higher than the 10 hour GFR. The overestimation in calculated GFR between 4 and 6 hours was 9% for estimated GFR above 30 ml/min per 1.73 m2 and 21% for estimated GFR below 30 ml/min per 1.73 m2. These data need to be contrasted for the 3% overestimation we found in the children (see Figure 1). The mechanism for the overestimation observed by Agarwal et al [25] is underestimation of the area under the curve particularly with the slow phase of the disappearance curve. Based on the slope of the slow (renal) curve, Agarwal et al [25] indicate that 5 hour sampling would be adequate for adults with estimated GFRs above 50 ml/min per 1.73 m2. But for those with an estimated GFR of 30 ml/min per 1.73 m2 9 hours of sampling would be required.
Whereas the Agarwal study [25] attests that the overestimation of GFR is greater in the larger adults, one could interpret this to mean that the overestimation might be less in children, who are smaller than adults. Perhaps the smaller size of the children in this study permitted more rapid equilibration of the disappearance curves. Indeed, we found in our 6 hour pilot study in children, with median GFR of 51.3 ml/min per 1.73 m2 that a 5 hour study yielded GFR values practically identical to those at 6 hours. The 4 hour study overestimated GFR by 3%, a significant difference but much smaller than the differences observed by Agarwal et al [25] in adult subjects with a median GFR of 37.7 ml/min per 1.73 m2. Moreover, when those children with lower GFR values were examined (see Figure 1), the differences between 4 and 6 hour studies did not appear to be much larger than in those with higher GFR. Whereas this overestimation of GFR at 4 hours is statistically significant, in fact it is probably not clinically important. Therefore termination of plasma disappearance studies by 4 hours in children with moderate CKD but without edema or obesity should not markedly alter the GFR measurement. The fact that the mean BMI of the patients in the Agarwal study [25] was high at 32.8 kg/m2 compared to the value of 24.0 kg/m2 of our pilot study [4], suggests the possibility that the magnitude of body size or obesity may adversely influence the attainment of steady state conditions and the determination of the area under the curve.
Another possibility to consider in examining the duration of plasma disappearance is that electro-neutral iohexol may equilibrate faster than anionic iothalamate, thereby not requiring as much observation time for calculating GFR. While this may only be speculation, other explanations for why the overestimation of GFR is smaller in children given iohexol than adults with higher absolute and scaled GFR given iothalamate are not currently available.
Number of blood sample collections
Another important issue is the timing and the number of the samples required for an accurate measure of iohexol-based GFR measurement. In considering a longitudinal study with growth and development, the use of a single sampling point, with assumptions about the volume of distribution of the iohexol, [15;26;27] seemed too unreliable for regular use, particular in growing children of various sizes and muscle mass. In our previous pilot study [4] we initially obtained 9 sampling points from 10 to 360 minutes. But we found that sampling at four points (10, 30, 120, and 300 minutes) gave nearly identical results with very high correlation (r=0.999) and no bias. Reduction to two points would be even more advantageous, an intravenous line would only need to be indwelling for 3 hours. Moreover, less blood would be taken from each subject, resulting in less stress and work for the subjects, nurses, and study coordinators, important considerations in epidemiological studies. Finally, we hope to utilize iohexol disappearance from two fingerprick samples [28], and the coefficients generated in this manuscript to accurately calculate GFR, thereby providing a significant epidemiological advantage for future studies.
In order to further limit the study to two points along the slow component of the disappearance curve, we needed to develop a Brochner-Mortensen equation based on the iohexol studies with four points. The coefficients obtained in the present study were k1= 1.0019 and k2= -0.001258, compared with those generated by Brochner-Mortensen in 30 children by 51Cr-EDTA plasma disappearance [7], which were 1.01 and -0.0017. The agreement with Brochner-Mortensen's studies, which utilized 51Cr-EDTA plasma disappearance, and ours, which utilized iohexol, indicates that iohexol is most likely a reliable marker for GFR that is handled at least comparably to 51Cr-EDTA. In addition, the similarities of the coefficients suggest that the distribution and renal excretion of iohexol are similar to that of 51Cr-EDTA.
It is clear that the one compartment clearance (slowGFR) always exceeds true GFR because the area under the curve is smaller when determined from the one compartment than from the two or multi-compartment clearance. For low clearances the differences between one compartment clearance and GFR are very small, but for higher clearances the one compartment model exceeds GFR by 25-30% [5;27]. These observations suggest that a single correction factor would not be adequate to correlate the one compartment clearance to the GFR [27]. Brochner-Mortensen [5] showed the original quadratic relationship by employing the method of least squares. We have used a comparable analysis to generate coefficients in a similar relationship between the one compartment slowGFR and the two compartment (four point) GFR based on iohexol plasma disappearance.
We have shown here that the combination of the slow (>= 120 minutes) GFR and a Brochner-Mortensen like equation yielded estimates of GFR very close to those obtained from measurements in both the slow and fast (<= 60 minutes) components. This does not imply that the fast area is useless. Indeed, the slow GFR and the estimate of the two component GFR via Brochner-Mortensen type equations provide the basis to estimate the fast area to be linked to variables of interest for specific scientific aims. However, initiatives which require not only the fast area but the intercept and slope of the fast curve would require the collection and analysis of samples prior to 60 minutes from the infusion of iohexol, as it was done in the first two visits of the CKiD study.
It is important to note that the single compartment approximation of GFR may not be correctly determined in patients with large collections of fluids such as ascites, severe peripheral edema, or pleural effusions, or in severe obesity, [29;30]; examination of the fast disappearance curves may be important in these situations. There were no edematous participants in the pilot study and only eight of the participants in the validation sample of CKiD were noted to have edema at the time of iohexol measurement. In this situation the full plasma disappearance curve may need to be measured, using samples at typically 10, 15, 30, 45, 60, 90, 120, 180, 240, and 300 minutes post injection [4;27]. It may be necessary to sample beyond 300 minutes particularly if the GFR is reduced. Similarly, it is possible that other characteristics could contribute to a poor approximation of the two compartment GFR using the methods detailed here. However, the distributions of factors such as BSA, BMI and age were equivalent between those with good agreement (within the 95% LOA) and those with poor agreement (outside the 95% LOA).
In conclusion, GFR can be accurately measured in children with chronic kidney disease using only the slow component of the iohexol plasma disappearance curve in combination with accurate formulas to estimate the two component based GFR from the slow GFR, provided the duration of study is at least 5 hours. Based on our analysis of the demographics of the CKiD population, a single set of coefficients can be utilized to accurately compute GFR from the slow (renal) iohexol plasma disappearance curve.
Methods
Study Participants
The pilot study included 27 children [6]. There were 12 with obstructive uropathy-dysplasia, four with kidney transplants, four with chronic glomerulonephritis, and seven with other diseases (including hereditary and cystic kidney disease).
For the CKiD study, eligible subjects were 1 to 16 years of age with mild to moderate CKD, based on GFR estimates by the original Schwartz formula [31-33] in the 30 to 90 ml/min per 1.73 m2 range. Subjects from 46 local sites were recruited. At the study visit, demographics, height, weight, and vital signs were determined. Body surface area (BSA) was determined using the formula of Haycock et al [34]. A total of 570 children were enrolled in the study by February 2009 of whom 62% were male, 70% Caucasian, 21% African-American, and 15% were of Hispanic ethnicity; 21% had glomerular disease. At enrollment, fifty percent of the cohort had age below 11 years, duration of CKD above 6.5 years, enzymatic serum creatinine above 1.3 mg/dL, cystatin C above 1.75 mg/L, urine protein:creatinine ratio above 0.5 and iohexol-based GFR below 44 ml/min per m2.
Both the pilot study and the CKiD study were approved by Research Review Boards at all participating sites in the United States and Canada.
Effect of time span of Iohexol Plasma Disappearance Studies
An intravenous line or butterfly needle was used to administer 5 ml of iohexol and was removed following the injection. A second intravenous line was saline locked and used for obtaining blood samples. Blood samples (1 ml) for the pilot study were obtained at 10, 20, 30, 60, 120, 180, 240, 300, and 360 minutes after the iohexol injection [4].
The plasma disappearance curve can be partitioned into two exponential decay curves and parameters for the two curves obtained by the technique of curve stripping. Specifically, since the contribution of the fast component is negligible after 120 minutes, fitting the line log(iohexol concentration)= A - α × time for times past 120 minutes yields the slow curve with expected concentration at time zero (=exp(A)) and the slope (=α). When the values along the slow curve (= exp(A - α × time)) are subtracted from the concentrations observed within the first 2 hours, a second linear function (fast curve) by fitting log(concentration - exp(A - α × time)) = B - β × time is obtained. The area under the sum of the two (slow and fast) curves is given by exp(A)/α + exp(B)/β. The plasma clearance of the substance, taken as GFR calibrated to a body surface area of 1.73 m2, can be calculated as [1]:
where Dose is the administered amount of iohexol in mg (mean= 3200 mg). To determine the possible bias due to shortening the time span of iohexol plasma disappearance studies, we calculated the medians of ratios between studies of 5 and 6 hours and between studies of 4 and 6 hours. Statistical significance for whether the median ratio was 1 was determined by the signed rank test.
Agreement between one compartment and two compartment GFR measures
At the baseline and first annual follow up visits of the CKiD study, the protocol for the determination of GFR from disappearance curves of iohexol specified the collection of sera at 10, 30, 120, and 300 minutes after infusion of iohexol. Since the GFR is based on 4 time points we refer to it hereafter as GFR4. Of the 570 CKiD children with an initial study visit by February 2009, 489 (86%) had a successful GFR4. Sera in serum separator tubes (SSTs) were shipped at room temperature to the Central Biochemistry Laboratory based in Rochester, NY. Iohexol is stable at room temperature for more than one week (G.J. Schwartz, B. Erway, and T. Kwong, unpublished observations). Iohexol concentration in mcg/ml was determined in deproteinated sera by HPLC as previously described [4]. The methods to determine the intercepts and slopes of the slow (A and α) and fast (B and β) components were the same as those indicated above for the nine point protocol of the pilot study [4].
The data from the Visit 1 iohexol studies which rendered GFR based on the four time points were used to derive a Brochner-Mortensen-like equation [5] relating the GFR based on the two compartment disappearance curves (slow and fast) to the GFR using simply the slow component, slowGFR, defined as slowGFR = {Dose/[exp (A)/α]} × {1.73/BSA}. Specifically, we used standard polynomial regression methods to fit the equation k1 × slowGFR + k2 × (slowGFR)2 to the measured GFR4. Using the estimates of the coefficients k1 and k2 obtained from the 489 data points from the baseline visit, the definitive comparison between the GFR determined from four points (GFR4) and that based on two points with the CKiD-based Brochner-Mortensen equation (GFR2) was accomplished by examining the test set of 362 children who completed a successful Visit 2 with GFR measured using 4 time points. The agreement was assessed by extending the methods proposed by Bland and Altman [35]. Results from the validation using Visit 2 data were compared with those from a development and validation done solely in Visit 1 using a 2/3 – 1/3 data split. Resampling of the development and validation data sets was done five times.
Acknowledgments
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children's Mercy Hospital and the University of Missouri – Kansas City (Bradley Warady, MD) and Johns Hopkins School of Medicine (Susan Furth, MD, Ph.D.), data coordinating center at the Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz, Ph.D.), and the Central Biochemistry Laboratory at the University of Rochester (George J. Schwartz, MD). The CKiD is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Institute of Neurological Disorders and Stroke, the National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01 DK82194, U01-DK-66143, U01-DK-66174, and U01-DK-66116). The CKID website is located at http://www.statepi.jhsph.edu/ckid. We are grateful to GE Healthcare, Amersham Division, for providing the CKiD study with iohexol (Omnipaque™) for the GFR measurements. We are indebted to Paula Maier for coordinating the central laboratory's preparation of GFR kits and tracking the blood samples, and to Brian Erway and Dr. Tai Kwong for skillfully developing and maintaining the iohexol HPLC assay at the University of Rochester Medical Center
References
- 1.Sapirstein LA, Vidt DG, Mandel MJ, Hanusek G. Volumes of distribution and clearances of intravenously injected creatinine in the dog. Am J Physiol. 1955;181:330–336. doi: 10.1152/ajplegacy.1955.181.2.330. [DOI] [PubMed] [Google Scholar]
- 2.Koch-Weser J. Bioavailability of drugs (first of two parts). N Engl J Med. 1974;291:233–237. doi: 10.1056/NEJM197408012910505. [DOI] [PubMed] [Google Scholar]
- 3.Sterner G, Frennby B, Hultberg B, Almen T. Iohexol clearance for GFR-determination in renal failure--single or multiple plasma sampling? Nephrol Dial Transplant. 1996;11:521–525. [PubMed] [Google Scholar]
- 4.Schwartz GJ, Furth S, Cole S, et al. Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 5.Brochner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest. 1972;30:271–274. doi: 10.3109/00365517209084290. [DOI] [PubMed] [Google Scholar]
- 6.Fujikawa-Adachi K, Nishimori I, Taguchi T, et al. cDNA sequence, mRNA expression, and chromosomal localization of human carbonic anhydrase-related protein, CA-RP XI. Biochim Biophys Acta. 1999;1431:518–524. doi: 10.1016/s0167-4838(99)00067-9. [DOI] [PubMed] [Google Scholar]
- 7.Brochner-Mortensen J, Haahr J, Christoffersen J. A simple method for accurate assessment of the glomerular filtration rate in children. Scand J Clin Lab Invest. 1974;33:139–143. [PubMed] [Google Scholar]
- 8.Odlind B, Hällgren R, Sohtell M, Lindström B. Is 125I iothalamate an ideal marker for glomerular filtration? Kidney Int. 1985;27:9–16. doi: 10.1038/ki.1985.3. [DOI] [PubMed] [Google Scholar]
- 9.Perrone RD, Steinman TI, Beck GJ, et al. Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I-iothalamate, 169Yb-DTPA, 99mTc-DTPA, and inulin. Am J Kid Dis. 1990;XVI:224–235. doi: 10.1016/s0272-6386(12)81022-5. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz GJ, Kwong T, Erway B, et al. Validation of creatinine assays utilizing HPLC and IDMS traceable standards in sera of children. Pediatr Nephrol. 2009;24:113–119. doi: 10.1007/s00467-008-0957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaspari F, Perico N, Ruggenenti P, et al. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol. 1995;6:257–263. doi: 10.1681/ASN.V62257. [DOI] [PubMed] [Google Scholar]
- 12.Isaka Y, Fujiwara Y, Yamamoto S, et al. Modified plasma clearance technique using nonradioactive iothalamate for measuring GFR. Kidney Int. 1992;42:1006–1011. doi: 10.1038/ki.1992.380. [DOI] [PubMed] [Google Scholar]
- 13.Brändström E, Grzegorczyk A, Jacobsson L, et al. GFR measurement with iohexol and 51Cr-EDTA. A comparison of the two favoured GFR markers in Europe. Nephrol Dial Transplant. 1998;13:1176–1182. doi: 10.1093/ndt/13.5.1176. [DOI] [PubMed] [Google Scholar]
- 14.Krutzen E, Back SE, Nilsson-Ehle I, Nilsson-Ehle P. Plasma clearance of a new contrast agent, iohexol: a method for the assessment of glomerular filtration rate. J Lab Clin Med. 1984;104:955–961. [PubMed] [Google Scholar]
- 15.Brown SCW, O'Reilly PH. Iohexol clearance for the determination of glomerular filtration rate in clinical practice: evidence for a new gold standard. J Urol. 1991;146:675–679. doi: 10.1016/s0022-5347(17)37891-6. [DOI] [PubMed] [Google Scholar]
- 16.Erley CM, Bader BD, Berger ED, et al. Plasma clearance of iodine contrast media as a measure of glomerular filtration rate in critically ill patients. Crit Care Med. 2001;29:1544–1550. doi: 10.1097/00003246-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Lindblad HG, Berg UB. Comparative evaluation of iohexol and inulin clearance for glomerular filtration rate determinations. Acta Paediatr. 1994;83:418–422. doi: 10.1111/j.1651-2227.1994.tb18133.x. [DOI] [PubMed] [Google Scholar]
- 18.Rahn KH, Heidenreich S, Bruckner D. How to assess glomerular function and damage in humans. J Hypertens. 1999;17:309–317. doi: 10.1097/00004872-199917030-00002. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsson L. A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol. 1983;3:297–305. doi: 10.1111/j.1475-097x.1983.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 20.Brochner-Mortensen J. Current status on assessment and measurement of glomerular filtration rate. Clin Physiol. 1985;5:1–17. doi: 10.1111/j.1475-097x.1985.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 21.Stake G, Monn E, Rootwelt K, Monclair T. The clearance of iohexol as a measure of the glomerular filtration rate in children with chronic renal failure. Scand J Clin Lab Invest. 1991;51:729–734. doi: 10.3109/00365519109104587. [DOI] [PubMed] [Google Scholar]
- 22.Brochner-Mortensen J, Freund LG. Reliability of routine clearance methods for assessment of glomerular filtration rate in advanced renal insufficiency. Scand J Clin Lab Invest. 1981;41:91–97. doi: 10.3109/00365518109092020. [DOI] [PubMed] [Google Scholar]
- 23.Groth S, Aasted M. Determination of 51Cr-EDTA clearance between 15 and 40 ml/min/1.73 m2 by a single plasma sample. Scand J Clin Lab Invest. 1989;49:711–717. doi: 10.3109/00365518909091549. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson-Ehle P, Grubb A. New markers for the determination of GFR: Iohexol clearance and cystatin C serum concentration. Kidney Int. 1994;46:S-17–S-19. [PubMed] [Google Scholar]
- 25.Agarwal R, Bills JE, Yigazu PM, et al. Assessment of iothalamate plasma clearance: duration of study affects quality of GFR. Clin J Am Soc Nephrol. 2009;4:77–85. doi: 10.2215/CJN.03720708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaspari F, Guerini E, Perico N, et al. Glomerular filtration rate determined from a single plasma sample after intravenous iohexol injection: Is it reliable? J Am Soc Nephrol. 1996;7:2689–2693. doi: 10.1681/ASN.V7122689. [DOI] [PubMed] [Google Scholar]
- 27.Fleming JS, Zivanovic MA, Blake GM, et al. Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nuclear Medicine Communications. 2004;25:759–769. doi: 10.1097/01.mnm.0000136715.71820.4a. [DOI] [PubMed] [Google Scholar]
- 28.Niculescu-Duvaz I, D'Mello L, Maan Z, et al. Development of an outpatient finger-prick glomerular filtration rate procedure suitable for epidemiological studies. Kidney Int. 2006;69:1272–1275. doi: 10.1038/sj.ki.5000240. [DOI] [PubMed] [Google Scholar]
- 29.Chantler C, Barratt TM. Estimation of glomerular filtration rate from plasma clearance of 51-Chromium edetic acid. Arch Dis Childh. 1972;47:613–617. doi: 10.1136/adc.47.254.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chantler C, Garnett ES, Parsons V, Veall N. Glomerular filtration rate measurement in man by the single injection method using 51Cr-EDTA. Clin Sci. 1969;37:169–180. [PubMed] [Google Scholar]
- 31.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr. 1985;106:522–526. doi: 10.1016/s0022-3476(85)80697-1. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571–590. doi: 10.1016/s0031-3955(16)36251-4. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 34.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 35.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]