Abstract
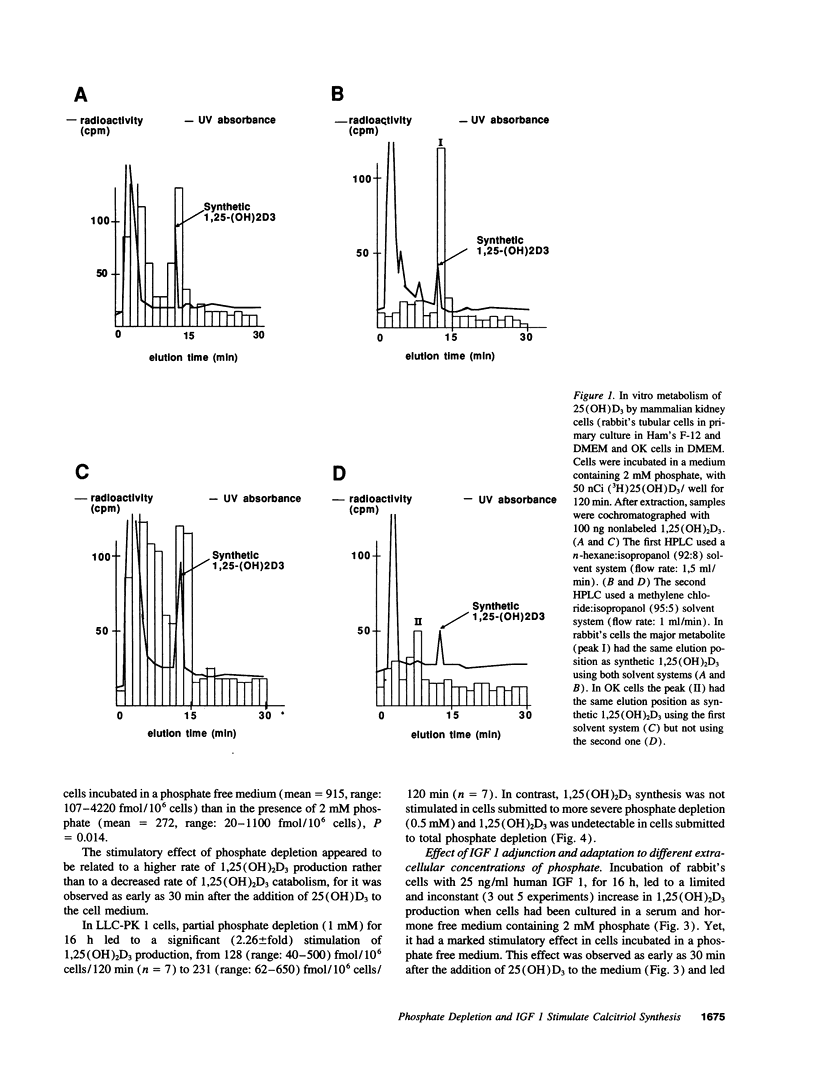
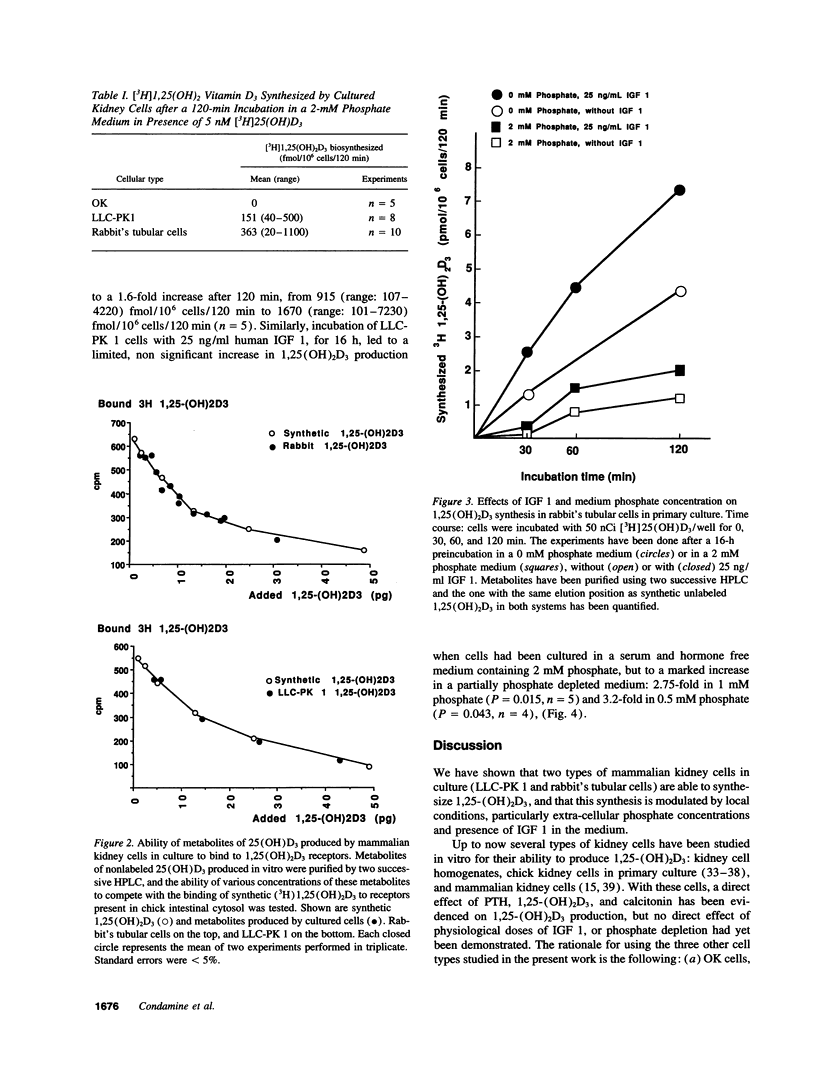
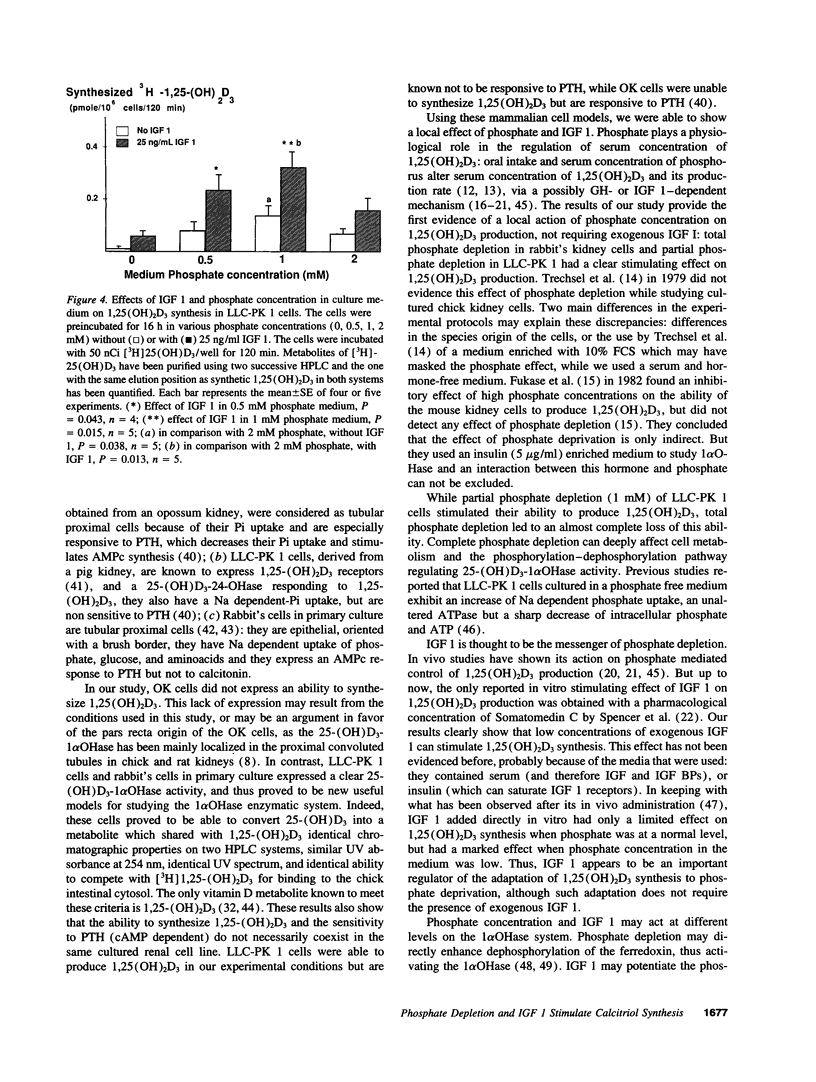
The hormonal form of vitamin D, 1,25(OH)2D, is synthesized mostly in proximal renal tubular cells. Experimental and clinical studies suggest that the growth hormone may be involved in growth-related fluctuations of plasma 1,25(OH)2D and in the increase of 1,25(OH)2D induced by in vivo phosphate deprivation, an action possibly mediated by insulin-like growth factor 1 (IGF 1). We tested the effects of phosphate depletion and IGF 1 addition on 1,25(OH)2D3 production in cultured kidney cells: opossum kidney (OK) cells, LLC-PK 1, and rabbit's proximal tubular cells. Confluent cell monolayers were preincubated in various phosphate concentrations, in the presence and absence of IGF 1. Then, 5 nM of [3H]25 (OH)D3 or 2 microM of 25 (OH)D3 were added to the medium and the cells were incubated for a further 120 min. The amount of biosynthesized 1,25(OH)2D3 in lipid extracts was determined after two different straight phase high performance liquid chromatographies. The experiment showed the following: (a) LLC-PK 1 and rabbit's cells expressed a detectable ability to synthesize 1,25(OH)2D3, while OK cells did not. (b) Partial or total phosphate deprivation increased the amount of 1,25(OH)2D3 produced, respectively in LLC-PK 1 and in rabbit's cells. (c) IGF 1 (25 ng/ml) increased 1,25(OH)2D3 production in rabbit's cells, particularly in phosphate-free medium (1.6-fold), and in LLC-PK 1 cells, in partial phosphate depletion (2.75-fold in 1 mM phosphate, P = 0.015, n = 5, and 3.2-fold in 0.5 mM phosphate, P = 0.043, n = 4). Our findings demonstrate a local action of phosphate depletion and of IGF 1 on 1,25-dihydroxyvitamin D3 production.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksnes L., Aarskog D. Plasma concentrations of vitamin D metabolites in puberty: effect of sexual maturation and implications for growth. J Clin Endocrinol Metab. 1982 Jul;55(1):94–101. doi: 10.1210/jcem-55-1-94. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Breslau N. A. Normal and abnormal regulation of 1,25-(OH)2D synthesis. Am J Med Sci. 1988 Dec;296(6):417–425. doi: 10.1097/00000441-198812000-00009. [DOI] [PubMed] [Google Scholar]
- Caverzasio J., Bonjour J. P. Insulin-like growth factor I stimulates Na-dependent Pi transport in cultured kidney cells. Am J Physiol. 1989 Nov;257(5 Pt 2):F712–F717. doi: 10.1152/ajprenal.1989.257.5.F712. [DOI] [PubMed] [Google Scholar]
- Caverzasio J., Montessuit C., Bonjour J. P. Stimulatory effect of insulin-like growth factor-1 on renal Pi transport and plasma 1,25-dihydroxyvitamin D3. Endocrinology. 1990 Jul;127(1):453–459. doi: 10.1210/endo-127-1-453. [DOI] [PubMed] [Google Scholar]
- Chesney R. W., Rosen J. F., Hamstra A. J., DeLuca H. F. Serum 1,25-dihydroxyvitamin D levels in normal children and in vitamin D disorders. Am J Dis Child. 1980 Feb;134(2):135–139. doi: 10.1001/archpedi.1980.02130140009004. [DOI] [PubMed] [Google Scholar]
- Chuman L., Fine L. G., Cohen A. H., Saier M. H., Jr Continuous growth of proximal tubular kidney epithelial cells in hormone-supplemented serum-free medium. J Cell Biol. 1982 Sep;94(3):506–510. doi: 10.1083/jcb.94.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. D., Alavi N., Livingston D., Hiller S., Taub M. Characterization of primary rabbit kidney cultures that express proximal tubule functions in a hormonally defined medium. J Cell Biol. 1982 Oct;95(1):118–126. doi: 10.1083/jcb.95.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colston K., Feldman D. 1,25-Dihydroxyvitamin D3 receptors and functions in cultured pig kidney cells (LLC PK1). Regulation of 24,25-dihydroxyvitamin D3 production. J Biol Chem. 1982 Mar 10;257(5):2504–2508. [PubMed] [Google Scholar]
- DeLuca H. F. Vitamin D metabolism and function. Arch Intern Med. 1978 May 15;138(Spec No):836–847. [PubMed] [Google Scholar]
- Escoubet B., Djabali K., Amiel C. Adaptation to Pi deprivation of cell Na-dependent Pi uptake: a widespread process. Am J Physiol. 1989 Feb;256(2 Pt 1):C322–C328. doi: 10.1152/ajpcell.1989.256.2.C322. [DOI] [PubMed] [Google Scholar]
- Fine L. G., Sakhrani L. M. Proximal tubular cells in primary culture. Miner Electrolyte Metab. 1986;12(1):51–57. [PubMed] [Google Scholar]
- Fraser D. R. Regulation of the metabolism of vitamin D. Physiol Rev. 1980 Apr;60(2):551–613. doi: 10.1152/physrev.1980.60.2.551. [DOI] [PubMed] [Google Scholar]
- Friedlander G., Amiel C. Protein kinase C activation has dissimilar effects on sodium-coupled uptakes in renal proximal tubular cells in primary culture. J Biol Chem. 1989 Mar 5;264(7):3935–3941. [PubMed] [Google Scholar]
- Friedlander G., Couette S., Coureau C., Amiel C. Mechanisms whereby extracellular adenosine 3',5'-monophosphate inhibits phosphate transport in cultured opossum kidney cells and in rat kidney. Physiological implication. J Clin Invest. 1992 Sep;90(3):848–858. doi: 10.1172/JCI115960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander G., Shahedi M., Le Grimellec C., Amiel C. Increase in membrane fluidity and opening of tight junctions have similar effects on sodium-coupled uptakes in renal epithelial cells. J Biol Chem. 1988 Aug 15;263(23):11183–11188. [PubMed] [Google Scholar]
- Fukase M., Birge S. J., Jr, Rifas L., Avioli L. V., Chase L. R. Regulation of 25 hydroxyvitamin D3 1-hydroxylase in serum-free monolayer culture of mouse kidney. Endocrinology. 1982 Mar;110(3):1073–1075. doi: 10.1210/endo-110-3-1073. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazarian J. G. The renal mitochondrial hydroxylases of the vitamin D3 endocrine complex: how are they regulated at the molecular level? J Bone Miner Res. 1990 Sep;5(9):897–903. doi: 10.1002/jbmr.5650050902. [DOI] [PubMed] [Google Scholar]
- Gray R. W. Evidence that somatomedins mediate the effect of hypophosphatemia to increase serum 1,25-dihydroxyvitamin D3 levels in rats. Endocrinology. 1987 Aug;121(2):504–512. doi: 10.1210/endo-121-2-504. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Garthwaite T. L. Activation of renal 1,25-dihydroxyvitamin D3 synthesis by phosphate deprivation: evidence for a role for growth hormone. Endocrinology. 1985 Jan;116(1):189–193. doi: 10.1210/endo-116-1-189. [DOI] [PubMed] [Google Scholar]
- Gray R. W., Garthwaite T. L., Phillips L. S. Growth hormone and triiodothyronine permit an increase in plasma 1,25(OH)2D concentrations in response to dietary phosphate deprivation in hypophysectomized rats. Calcif Tissue Int. 1983;35(1):100–106. doi: 10.1007/BF02405013. [DOI] [PubMed] [Google Scholar]
- Halloran B. P., Spencer E. M. Dietary phosphorus and 1,25-dihydroxyvitamin D metabolism: influence of insulin-like growth factor I. Endocrinology. 1988 Sep;123(3):1225–1229. doi: 10.1210/endo-123-3-1225. [DOI] [PubMed] [Google Scholar]
- Handler J. S. Studies of kidney cells in culture. Kidney Int. 1986 Aug;30(2):208–215. doi: 10.1038/ki.1986.173. [DOI] [PubMed] [Google Scholar]
- Harbison M. D., Gertner J. M. Permissive action of growth hormone on the renal response to dietary phosphorus deprivation. J Clin Endocrinol Metab. 1990 Apr;70(4):1035–1040. doi: 10.1210/jcem-70-4-1035. [DOI] [PubMed] [Google Scholar]
- Henry H. L. Insulin permits parathyroid hormone stimulation of 1,25-dihydroxyvitamin D3 production in cultured kidney cells. Endocrinology. 1981 Feb;108(2):733–735. doi: 10.1210/endo-108-2-733. [DOI] [PubMed] [Google Scholar]
- Henry H. L. Metabolism of 25-hydroxy-vitamin D3 by primary cultures of chick kidney cells. Biochem Biophys Res Commun. 1977 Jan 24;74(2):768–774. doi: 10.1016/0006-291x(77)90368-0. [DOI] [PubMed] [Google Scholar]
- Juan D., DeLUCA H. F. The regulation of 24,25-dihydroxyvitamin D3 production in cultures of monkey kidney cells. Endocrinology. 1977 Oct;101(4):1184–1193. doi: 10.1210/endo-101-4-1184. [DOI] [PubMed] [Google Scholar]
- Kawashima H., Kurokawa K. Metabolism and sites of action of vitamin D in the kidney. Kidney Int. 1986 Jan;29(1):98–107. doi: 10.1038/ki.1986.12. [DOI] [PubMed] [Google Scholar]
- Koyama H., Goodpasture C., Miller M. M., Teplitz R. L., Riggs A. D. Establishment and characterization of a cell line from the American opossum (Didelphys virginiana). In Vitro. 1978 Mar;14(3):239–246. doi: 10.1007/BF02616032. [DOI] [PubMed] [Google Scholar]
- Kumar R. The metabolism and mechanism of action of 1,25-dihydroxyvitamin D3. Kidney Int. 1986 Dec;30(6):793–803. doi: 10.1038/ki.1986.258. [DOI] [PubMed] [Google Scholar]
- Lund B., Clausen N., Lund B., Andersen E., Sørensen O. H. Age-dependent variations in serum 1,25-dihydroxyvitamin D in childhood. Acta Endocrinol (Copenh) 1980 Jul;94(3):426–429. doi: 10.1530/acta.0.0940426. [DOI] [PubMed] [Google Scholar]
- Mayer E., Bishop J. E., Ohnuma N., Norman A. W. Biological activity assessment of the vitamin D metabolites 1,25-dihydroxy-24-oxo-vitamin D3 and 1,23,25-trihydroxy-24-oxo-vitamin D3. Arch Biochem Biophys. 1983 Jul 15;224(2):671–676. doi: 10.1016/0003-9861(83)90254-0. [DOI] [PubMed] [Google Scholar]
- Nesbitt T., Drezner M. K. Insulin-like growth factor-I regulation of renal 25-hydroxyvitamin D-1-hydroxylase activity. Endocrinology. 1993 Jan;132(1):133–138. doi: 10.1210/endo.132.1.8419119. [DOI] [PubMed] [Google Scholar]
- Portale A. A., Halloran B. P., Morris R. C., Jr Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J Clin Invest. 1989 May;83(5):1494–1499. doi: 10.1172/JCI114043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portale A. A., Halloran B. P., Murphy M. M., Morris R. C., Jr Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans. J Clin Invest. 1986 Jan;77(1):7–12. doi: 10.1172/JCI112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley R., Baum M. Effects of growth hormone and insulin-like growth factor I on rabbit proximal convoluted tubule transport. J Clin Invest. 1991 Aug;88(2):368–374. doi: 10.1172/JCI115312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel H., Koeffler H. P., Norman A. W. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989 Apr 13;320(15):980–991. doi: 10.1056/NEJM198904133201506. [DOI] [PubMed] [Google Scholar]
- Rosen J. F., Chesney R. W. Circulating calcitriol concentrations in health and disease. J Pediatr. 1983 Jul;103(1):1–17. doi: 10.1016/s0022-3476(83)80767-7. [DOI] [PubMed] [Google Scholar]
- Shepard R. M., Horst R. L., Hamstra A. J., DeLuca H. F. Determination of vitamin D and its metabolites in plasma from normal and anephric man. Biochem J. 1979 Jul 15;182(1):55–69. doi: 10.1042/bj1820055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos E., Barrett D. I., Chong K. T., MacIntyre I. Effect of oestrogen and 1,25-dihydroxycholecalciferol on 25-hydroxycholecalciferol metabolism in primary chick kidney-cell cultures. Biochem J. 1978 Jul 15;174(1):231–236. doi: 10.1042/bj1740231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R. E., Preston A. S., Johnson J. P., Handler J. S. Porous-bottom dishes for culture of polarized cells. Am J Physiol. 1986 Jul;251(1 Pt 1):C136–C139. doi: 10.1152/ajpcell.1986.251.1.C136. [DOI] [PubMed] [Google Scholar]
- Trechsel U., Bonjour J. P., Fleisch H. Regulation of the metabolism of 25-hydroxyvitamin D3 in primary cultures of chick kidney cells. J Clin Invest. 1979 Jul;64(1):206–217. doi: 10.1172/JCI109441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay P., Gougoux A., Lemieux G. Isolation of a pure suspension of rat proximal tubules. Am J Physiol. 1981 Oct;241(4):F403–F411. doi: 10.1152/ajprenal.1981.241.4.F403. [DOI] [PubMed] [Google Scholar]