Abstract
Background
Health care practitioners, including sports physical therapists, commonly prescribe and recommend aerobic exercise for those patients seeking to improve their cardiovascular fitness across all ages. Current literature demonstrates that weight bearing activities such as walking or running may lead to foot and ankle edema.
Objectives
The purpose of this study is to determine if a significant difference exists between foot volumes (edema) in pre versus post-exercise measurements during a loaded activity (treadmill walking) or an unloaded activity (upright exercise bike) in 31 healthy subjects 50 years of age and older.
Methods
After a rest period, a pre-exercise volumetric measurement of the right leg was obtained by the use of a foot volumeter. The first condition (walking or cycling) was randomly chosen. Each subject completed two 10-minute exercise sessions. Immediately following both exercise sessions, a post-exercise volumetric measurement was completed.
Results
A statistically significant difference in foot volume was found between pre (mean = 742.39ml, 95% CI: 685.23 – 799.55) and post (mean = 753.03ml, 95% CI: 697.51ml – 808.55ml) measurements for the treadmill (weight bearing) protocol. When considering each sex separately, males produced significant increases in foot volume following tread-mill walking (pre mean = 871.00ml, 95% CI: 793.95ml – 948.05ml; post mean = 886.20ml, 95% CI: 811.28ml – 961.13ml), while females displayed no significant changes.
Discussion and Conclusion
This study demonstrated a 1.4% increase in foot volume after 10 minutes of treadmill walking. Based on these results, it may be advisable to prescribe non-weight bearing exercise to active older individuals with pre-existing conditions for edema.
Keywords: edema, volumetrics, unloaded and loaded activities
INTRODUCTION
Health care practitioners (including sports physical therapists) commonly prescribe and recommend aerobic exercise for those individuals of all ages seeking to improve their cardiovascular fitness. These aerobic activities often include walking, running, and cycling programs. Current literature demonstrates, however, that weight-bearing activities, such as walking or running, may lead to foot and ankle swelling.1–4 This acute swelling, which is a common complication resulting from weight bearing activities, can lead to more serious conditions such as fibrosis, joint stiffness, pain, and dysfunction.1,5–13
One of the problems that results from aerobic exercise is edema formation in the lower extremities which can lead to peripheral vascular disease or other circulatory insufficiencies.1,3,13 Other research has shown an opposite effect, with aerobic activity resulting in a decrease in lower extremity volume.1,2,14 This decrease in lower extremity volume may be the result of the muscle-pumping effect of the gastroc-soleus complex.1,2,15,16 In addition to aerobic activities resulting in an increase in swelling, static activities have also been shown to produce edema in the lower leg.14,15,17,18
Other research has examined the discrepancies involving the effects of loaded dynamic activity on lower extremity volume. Cloughley and Mawdsley3 found a greater increase in foot volume during running as compared to walking. These findings are supported by McWhorter et al13 who found significant increases in foot swelling during walking and running. Other researchers found increases in foot volume during running, but a decrease during walking.1 Evidence also exists demonstrating increases in interstitial and intracellular volume during and after exercise which are directly related to exercise workload.19
During quiet static standing, subjects have been shown to increase lower extremity edema formation as a result of pooling of fluid in the lower extremity.17 Specifically, foot volume during standing has been shown to increase compared to a supine position, possibly the result of a decrease in perfusion of the veins that return to normal when the supine position is resumed.18
Stationary bicycle ergometry is an example of an unloaded dynamic activity. Research by Stick et al14 found that stationary bicycling demonstrated a gradual decrease in foot volume. They also discovered a more significant decrease in foot volume occurred when subjects pedaled against a stronger resistance.
Other studies have examined the effects of differing postures on changes in foot volumes. Seated positioning has been shown to cause an increase in foot volume.14 Another posture, supine lying with or without leg elevation, has demonstrated a significant decrease in foot volume.20,21 All of these studies demonstrate that foot volume can be affected by its gravitational position.
In the young or recreational athlete, the small changes that occur as a result of running or walking may not be a problem. However, in older or geriatric adults, these small increases in foot volume when coupled with chronic resting edema could prove to be harmful.22,23 Peripheral edema is a common finding in the elderly, however, actual figures for prevalence are not known. A survey conducted on peripheral edema in elderly patients admitted to a geriatric ward found that 48% of patients had an overall presence of edema during their admission.24 Compounding the effects of existing edema with co-morbidities will make exercise prescription increasingly more difficult.
The increasing incidence of morbidities involving resting edema, such as coronary heart disease (CHD) and diabetes, showcases the need for further research in this area.8,10,24–26 Arnold et al25 reported the incidence of CHD to be 39.6 per 1,000 persons in men and 22.3 per 1,000 persons in women. Moreover, the incidence of CHD increases 9% with each year of age past 65. Bertoni et al26 reported a high incidence of mortality among older adults with diabetes and heart failure; 32.7 per 100 persons-years compared with 3.7 per 100 person-years among those with diabetes who did not suffer from congestive heart failure (CHF).
The purpose of this study is to determine if a significant difference exists between foot volumes (edema) in pre versus post-exercise measurements during a loaded activity (treadmill walking) or an unloaded activity (upright exercise bike) in the active healthy older population. Based on the findings of McWhorter et al,13 the hypothesis tested was that loaded activities will result in greater edema in the foot and ankle than unloaded activities. Additionally, it is hypothesized that the unloaded position of the bike coupled with the active and passive movement of the ankle may result in a reduction in foot volume secondary to a muscle pumping action moving fluid out of the area.26 Results of this study may help health care practitioners prescribe a more appropriate exercise mode when addressing the cardiovascular health of the active geriatric individuals.
METHODS
Subjects
The subjects consisted of 21 female and 10 male volunteers between the ages of 50 to 67 years without a history of musculoskeletal injuries, health problems, or surgery to the lower extremities, and who had no difficulty or discomfort during walking on a treadmill of riding a bicycle ergometer. The mean age of all 31 participants was 56.26 (SD = 4.89) years. For the 21 females, the mean age was 56.1 (SD = 3.97) years and for the males was 56.6 (SD = 6.33) years. All subjects were recruited by using emails sent throughout the university. All participants completed the Physical Activity Readiness Questionnaire (PAR-Q)27 and signed an informed consent to participate. This research study was approved by the Institutional Review Board at the University of Nevada, Las Vegas. Participants were excluded if they met any of the following conditions: injury to the right lower extremity within the past year, abnormal swelling in the ankles or feet, history of bone or joint disorders that is aggravated by exercise, or any other physical reason provided by a physician that they should not exercise.
Instrumentation
All lower extremity measurements were obtained using a Lucite (Foot Volumeter, P.O. Box 146, Idyllwild, CA, 92349) foot volumeter set (Figure 1) which included the volumeter container, an obturator which was used to calibrate the water levels prior to each measurement, a receiver to catch the water overflow, and a 1000-ml gradated cylinder with 10-ml gradations. All measurements were taken following the manufacturer's guidelines. Several studies have been performed establishing the reliability and validity of obtaining foot/ankle volume measurements using this equipment.1–3,5,6,13,28
Figure 1:
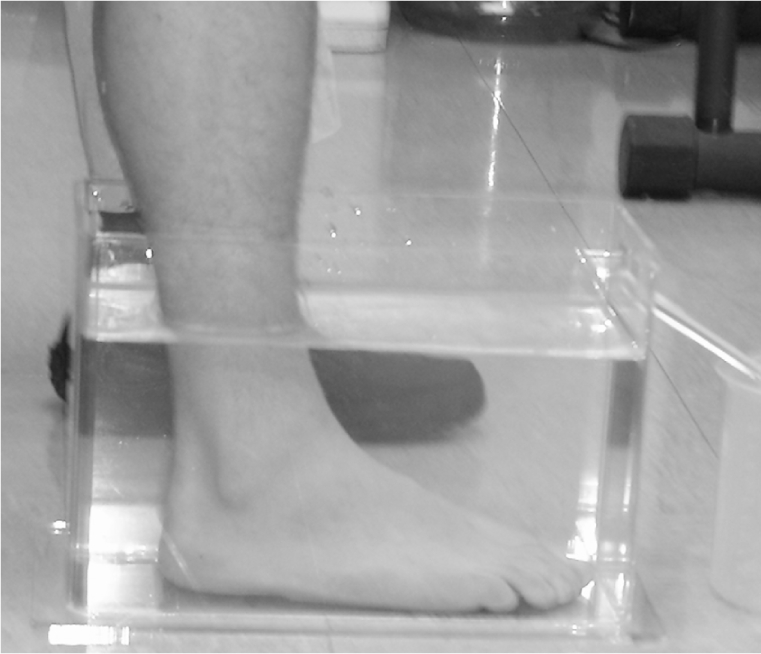
Lucite foot volumeter.
The displaced water was captured in a plastic container and subsequently measured in a gradated cylinder. All data was immediately recorded on a personalized data sheet. As water has a tendency to creep up the sides of the plastic cylinder, measurements were taken from the lowest level at the water line. Each volume measurement was taken by the same observer to ensure proper consistency.
A pilot test was performed previously in order to allow the testers to practice taking foot volumetric measurements. At this time, the researchers performed the volumetric measurements on 10 volunteers. The three researchers involved in data collection were asked to take the measurements from the participants. The data were recorded by an independent observer. Two days later, the researchers, who were blinded to the previous results, took the measurements from the same participants. All measurements were compared for reliability and demonstrated a reliability intra-class correlation coefficient of 0.99 (95% limits of agreement +/− 7.54 ml).29
Procedure
The subjects were given an individual instructional session at which time all aspects of the research study were explained and possible complications as a result of participation were discussed. All subjects were tested at the same time of day. They were informed to not exercise or consume alcoholic beverages on the days prior to being tested and to maintain their present eating habits. Each subject was required to sit in a straight back chair and slowly lower their right leg into the foot volumeter until the foot rested flat against the bottom. They remained in this position until all of the displaced water was collected. All subjects were tested during walking and cycling, thus serving as their own controls.
Prior to each exercise session, all subjects were instructed to bring their athletic footwear. All subjects were required to rest in a supine position for at least 10 minutes prior to testing. The subjects were seated in a chair immediately following their 10-minute rest period. At this time, a pre-exercise volumetric measurement of the right leg was obtained by having the subject slowly lower the right foot into the volumeter.
The activity for the first condition (walking or cycling) was randomly chosen by a flip of a coin. The tread-mill (Star Trac Unisen, Inc., 4500 Treadmill, Star Trac, 14410 Myford Rd., Irvine, CA. 92606) speed and cycling (Fitron Cycle, First Fitness Equipment International, 4750 Bryant Irvine Rd., Ste 808, PMB 229, Ft. Worth, TX 76132-3631) cadence were set at a self-selected comfortable pace for each individual participant. The speed of the treadmill and cadence of the cycle ergometer were recorded on the data sheet. These values were then used for the post-test measure. Due to the fact that walking and cycling speeds vary greatly among individuals, each individual was allowed to determine their own comfortable speed to more closely mimic a real life scenario.30,31
As the exercise session began, each subject performed a 2-minute warm-up session on the treadmill or cycle ergometer at which time they gradually increased their speed until they felt comfortable for the remaining 8 minutes. Subjects were not allowed to grip the handrails on the treadmill during the exercise sessions. The treadmill was kept at a level (zero degrees) elevation for all exercise sessions. During cycling, the seat was adjusted for each participant individually and used for all subsequent sessions. In addition, all cycling sessions used toe straps to minimize foot extraneous movements. The cycle tension was chosen by the participant and used for all subsequent sessions. At least 48 hours of rest was allowed between the two exercise sessions. All procedures were performed in a consistent manner for both sessions.
Data Analysis
Means and standard errors for the fluid volume data were calculated. Given the repeated measures in each exercise protocol, a 2 (exercise) × 2 (time) within factorial ANOVA was utilized to determine if statistical interaction existed in the data. The alpha level was set at 0.05. If statistical interaction was found, simple main effects were calculated using paired t-tests to determine if statistical significance was present.
RESULTS
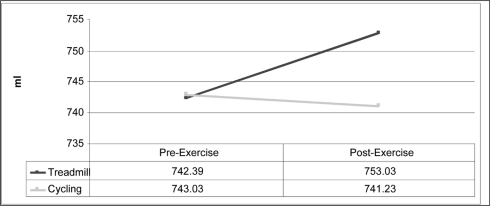
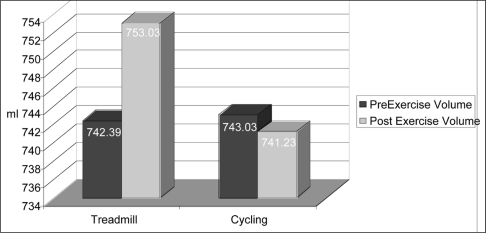
Factorial results of the 2 × 2 ANOVA revealed a statistical interaction (Figure 2). Because statistical interaction was observed {F(1,30)=5.705, p=.023}, simple main effects were analyzed. Two paired samples t-tests using a Bonferroni correction (p =.025) were used to determine the effect of weight-bearing vs. non-weight bearing exercise on foot volume (Table 1). A statistically significant difference in foot volume was found between pre (mean=742.39, 95% Confidence Interval: 685.229 – 799.546) and post (mean=753.03, 95% Confidence Interval: 697.511 – 808.553) measurements for the tread-mill protocol (weight-bearing), t=−2.952, p=.006 (Figure 3). There was no statistically significant difference between the pre (mean = 743.03, SD = 162.68) and post (mean = 741.23, SD = 155.83), cycle ergometry measurements (non-weight bearing), t = .376, p = .710.
Figure 2:
2 × 2 within factorial ANOVA
Table 1:
Comparison of Fluid Volume Changes During Treadmill Walking (Weight Bearing) and Cycle Ergometry (Non-weight Bearing)
| Mode of Exercise N=31 | Mean Volume (ml) | Standard Error | t-values | p-values |
|---|---|---|---|---|
| Pre-walk | 742.39 | 27.99 | −2.952 | .006* |
| Post-walk | 753.03 | 27.19 | ||
| Pre-bike | 743.03 | 29.22 | 0.376 | .710 |
| Post-bike | 741.23 | 28.30 |
Significance at α = .025
Figure 3:
Pre and Post Foot Volume Measurements
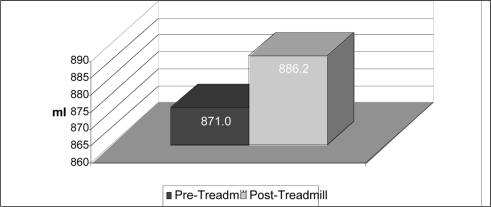
A 2 (exercise) by 2 (time) within factorial ANOVA was also utilized to determine if statistical interaction was present in data sorted by sex. Because statistical interaction was observed for males (F(1,9)=10.545, p=.010), simple main effects were analyzed. Paired samples t-testing using a Bonferroni correction (p=.0125) found a statistically significant difference in male foot volumes (Figure 4) between pre (mean=871.00, SD=107.706) and post (mean=886.20, SD=104.739) measurements for the treadmill protocol, t=−5.429, p<.0005. Statistical interaction was not observed for females (F(1,20)=.990, p=.332) and no further statistical analysis was performed.
Figure 4:
Pre and Post Foot Volume Measurements for Males
DISCUSSION
Numerous methods have been used to measure extremity volume. Previous research has demonstrated the water displacement method of volumetry to be valid and reliable, and thus is known to be the gold standard.32–34 Many variables need to be considered when taking volumetric measurements. These include time of day, water temperature, positioning of subject, the temperature of the room, and recent musculoskeletal injuries. However, Moholkar and Fenelon28 showed that time of day does not significantly effect volume measurements of the extremities. Tepid water temperatures between 20 and 35° C did not significantly increase or decrease volume measurements, however, extreme hot or cold temperatures have been shown to affect volume.21,35,36 The temperature of the room was not shown to have an effect on the measurements of foot volumes following quiet standing.16 It also has been shown that individuals with a recent history of musculoskeletal injuries in the lower extremity will have an increase in edema formation.37 Therefore, it can be safely assumed that the performance of an activity rather than the previously mentioned variables affects foot volumes, except for the conditions of extreme water or room temperatures and recent injuries.
The data from this study showed that, in healthy older subjects, treadmill walking resulted in significant increases in foot and ankle fluid volumes compared to resting measurements. This finding was in agreement with the original hypothesis that weight-bearing exercise will increase foot volume. Additionally, when considering each sex separately, males produced significant increases in foot volume following treadmill walking, while females displayed no significant changes. However, when comparing pre and post-test measurements for biking, no significant changes were observed in the present sample. This lack of change also held true when considering each sex separately. The results did not support the hypothesis that the biking protocol would cause a significant decrease in foot volume.
The edema in the foot and ankle during walking can be attributed to the increase in blood flow to the exercising muscles. The increase in edema following weight-bearing activity has been shown to increase foot volume by as much as 8%.26
Stegall38 has demonstrated an 80 mmHg drop in venous pressure at the saphenous vein in the ankle during running as compared to quiet standing. As a result, the author suggests that there is an inability of the lower extremities to maintain a steady rate of venous return following vigorous weight-bearing activities.
Prior to this study, the hypothesis was made that the bike protocol would cause a significant decrease in foot volume secondary to a muscle pumping action. However, as previously mentioned, no significant difference was found. After analyzing the data, one possible explanation for these results was that riding an upright exercise bike does not require enough active foot and ankle muscular activity to cause a muscle pumping effect away from the distal lower extremities. Although movement does occur in the foot and ankle during this activity, much of the movement may be passive, thus not adequately activating the muscle pumping mechanism.
Sochart et al39 examined the relationship between passive and active movement of the foot and ankle related to venous return in the lower extremity. They found that an active “combined” movement (plantarflexion/dorsiflexion and inversion/eversion) produced a significantly larger increase in mean blood velocity than any of the three passive movement patterns. The results found by Sochart et al39 could help explain why a significant decrease in foot volume for the bike protocol was not observed in this present study.
A difference in fluid volumes after treadmill walking was also found to be significant between sex. In the present study, the participants demonstrated a mean increase in foot volume after a 10 minute walk on the treadmill, but significance between genders was only found in males. The females' 1.2% increase in foot volume failed to produce significance. A review of the literature failed to identify any studies of post-exercise foot and ankle volume changes based on sex. Chalk et al26 demonstrated a slight non-significant decrease in foot volume in female inter-collegiate volleyball players after a 2-hour rigorous exercise session. In addition, the benefits of reducing foot volume in females during weight-bearing activities may be offset by the greater benefit of osteoporosis prevention.40–42 It should be noted that the sample size for this study is not large enough to draw firm conclusions with regard to sex and foot swelling.
The results of this study provide important clinical implications for physical therapists. This study demonstrated a 1.4% increase in foot volume after 10 minutes of treadmill walking. It is important to note that this statistically significant increase in foot volume occurred only after 10 minutes. Ambulating patients in the acute or rehab settings often takes longer than 10 minutes secondary to their age, medical condition, and other physical limitations. This information is also important for the older recreational athlete who may walk for an extended period of time. Keeping these patients on their feet for longer periods of time could cause further increases in foot volume, resulting in potential further constriction of venous return with possible negative consequences in those with already compromised circulation.3,43 Even in cases with mild edema, instability may result due to poorly fitting foot wear as well as the increased weight of the edematous limb.24 Patients may experience a decline in walking confidence and become immobile, further aggravating the problem. It may be safer and more effective to prescribe non-weight bearing exercise as a warm-up or treatment alternative to patients with pre-existing peripheral edema or conditions that place them at risk for impaired venous return. Based on the small sample size, no concrete clinical suggestions can be made with regard to sex at this time.
This study had several limitations. For example, the statistical results would be more convincing if the sample had more subjects, particularly when considering statistics sorted by sex. The total sample of 31 provides a fairly strong field to draw conclusions, but when considering each sex separately, the sample size was reduced significantly. Another limitation in this study is the inability to control each subject's activity level prior to testing. A subject that was active versus sedentary could have a different circulation status upon arrival for exercise testing which could affect foot volume pre and post exercise testing. The non-weight bearing exercise protocol in this study utilized an upright exercise bike. The upright exercise bike caused a considerable flexion angle in the hip that could potentially impair venous return to the heart. A better alternative would be to use a recumbent bike, thus reducing the hip flexion angle.
Future studies should focus on patients with real pre-existing co-morbidities that cause foot edema to determine if these results hold true in such populations. Studies utilizing more subjects with these conditions could help draw firm conclusions regarding sex and foot edema. Although an upright stationary bike was utilized in this study, other forms of non-weight bearing exercise equipment are available. Future studies involving these different exercise modes could help determine the optimal piece of exercise equipment to help minimize foot swelling.
CONCLUSION
The results from this study suggest that treadmill walking (loaded exercise) results in an increase in foot edema when compared to riding an upright stationary exercise bike (unloaded exercise). Although it is difficult to conclude that riding an exercise bike is an effective way to decrease foot edema, these results suggest that stationary biking is a safer mode of exercise than treadmill walking in controlling foot edema, especially in older men. Therefore, unloaded activities may be the most appropriate exercise to prescribe when an increase in foot volume is unwanted. Further investigations into the injured (ankle sprains) and chronically ill (congestive heart failure, peripheral vascular disease, diabetes) patients are necessary to ascertain whether similar findings would result. This knowledge would be very applicable for clinical use of the cycle ergometer as a modality to positively influence venous stasis and decrease the problems associated with ankle and foot edema.
REFERENCES
- 1.Stick C, Jaeger H, Witzleb E. Measurements of volume changes and venous pressure in the human lower leg during walking and running. J Appl Physiol. 1992;72:2063–2068 [DOI] [PubMed] [Google Scholar]
- 2.Chalk PJ, McPoil T, Cornwall MW. Variations in foot volume before and after exercise. J Am Podiatr Med Assoc. 1995;85:470–472 [DOI] [PubMed] [Google Scholar]
- 3.Cloughley WB, Mawdsley RH. Effect of running on volume of the foot and ankle. J Orthop Sports Phys Ther. 1995;22:151–154 [DOI] [PubMed] [Google Scholar]
- 4.McPoil TG, Hunt GC. Evaluation and management of foot and ankle disorders: present problems and future directions. J Orthop Sports Phys Ther. 1995;21:381–388 [DOI] [PubMed] [Google Scholar]
- 5.Lundvall J, Mellander S, Westling H, et al. Fluid transfer between blood and tissues during exercise. Acta Physiol Scand. 1972;85:258–269 [DOI] [PubMed] [Google Scholar]
- 6.Baker CH, Davis DL. Isolated skeletal muscle blood flow and volume changes during contractile activity. Blood Vessels. 1974;11:32–44 [DOI] [PubMed] [Google Scholar]
- 7.Schnizer W, Hinneberg H, Moser H, et al. Intra- and extravascular volume changes in the human forearm after static hand grip exercise. Eur J Appl Physiol Occup Physiol. 181979;41:131–140 [DOI] [PubMed] [Google Scholar]
- 8.Evanski P. The Geriatric Foot. Disorders of the Foot. In: M J. ed. Phildelphia: WB Saundersn Co; 1982:964–978 [Google Scholar]
- 9.Stick C, Stofen P, Witzleb E. On physiological edema in man's lower extremity. Eur J Appl Physiol Occup Physiol. 1985;54:442–449 [DOI] [PubMed] [Google Scholar]
- 10.Shereff MJ. Geriatric foot disorders: How to avoid undertreating them. Geriatrics. 1987;42:69–72, 75-80 [PubMed] [Google Scholar]
- 11.McGough C, Zurwasky ML. Effect of exercise on volumetric and sensory status of the asymptomatic hand. J Hand Ther. 1991;4:177–180 [Google Scholar]
- 12.Gordon GM, Cuttic MM. Exercise and the aging foot. South Med J. 1994;87:S36–41 [PubMed] [Google Scholar]
- 13.McWhorter JW. The effects of walking, running, and shoe size on foot volumetrics. Physical Therapy in Sport. 2003;4:87–92 [Google Scholar]
- 14.Stick C, Grau H, Witzleb E. On the edema-preventing effect of the calf muscle pump. Eur J Appl Physiol Occup Physiol. 1989;59:39–47 [DOI] [PubMed] [Google Scholar]
- 15.Man LG, Morrissey MC, Cywinski JK. Effect of neuromuscular electrical stimulation on foot/ankle volume during standing. Med Sci Sports Exerc. 2003;35:630–634 [DOI] [PubMed] [Google Scholar]
- 16.Stick C, Hiedl U, Witzleb E. Volume changes in the lower leg during quiet standing and cycling exercise at different ambient temperatures. Eur J Appl Physiol Occup Physiol. 1993;66:427–433 [DOI] [PubMed] [Google Scholar]
- 17.Gardner AM, Fox RH. The venous pump of the human foot–preliminary report. Bristol Med Chir J. 1983;98:109–112 [PMC free article] [PubMed] [Google Scholar]
- 18.Mayrovitz HN, Larsen PB. Effects of compression bandaging on leg pulsatile blood flow. Clin Physiol. 1997;17:105–117 [DOI] [PubMed] [Google Scholar]
- 19.Jacobsson S, Kjellmer I. Accumulation of fluid in exercising skeletal muscle. Acta Physiol Scand. 1964;60:286–292 [DOI] [PubMed] [Google Scholar]
- 20.Ciocon JO, Galindo-Ciocon D, Galindo DJ. Raised leg exercises for leg edema in the elderly. Angiology. 1995;46:19–25 [DOI] [PubMed] [Google Scholar]
- 21.Sims D. Effects of positioning on ankle edema. JOSPT. 1986;8. [DOI] [PubMed] [Google Scholar]
- 22.Guyton AC, Hall JE. Textbook of Medical Physiology. 10th ed Philadelphia: Saunders; 2000 [Google Scholar]
- 23.Sorenson MK. The edematous hand. Phys Ther. Dec 1989;69:1059–1064 [DOI] [PubMed] [Google Scholar]
- 24.Smith E. A survey of peripheral oedema in elderly patients admitted to a geriatric ward. Br J Clin Pract. 1996;50:20–21 [PubMed] [Google Scholar]
- 25.Arnold AM, Psaty BM, Kuller LH, et al. Incidence of cardiovascular disease in older Americans: the cardiovascular health study. J Am Geriatr Soc. 2005;53:211–218 [DOI] [PubMed] [Google Scholar]
- 26.Bertoni AG, Hundley WG, Massing MW, et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703 [DOI] [PubMed] [Google Scholar]
- 27.ACSM's Guidelines For Exercise Testing And Prescription. 7 ed Philadelphia: Lippincott Williams & Wilkins; 2006 [Google Scholar]
- 28.Moholkar K, Fenelon G. Diurnal variations in volume of the foot and ankle. J Foot Ankle Surg. 2001;40:302–304 [DOI] [PubMed] [Google Scholar]
- 29.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310 [PubMed] [Google Scholar]
- 30.Rodgers MM. Dynamic biomechanics of the normal foot and ankle during walking and running. Phys Ther. 1988;68:1822–1830 [DOI] [PubMed] [Google Scholar]
- 31.Winter D. Kinematic and kinetic patterns in human gait: Variability and compensating effects. Human Movement Science. 1984;3:51–76 [Google Scholar]
- 32.Bednarczyk JH, Hershler C, Cooper DG. Development and clinical evaluation of a computerized limb volume measurement system (CLEMS). Arch Phys Med Rehabil. 1992;73:60–63 [PubMed] [Google Scholar]
- 33.Karges JR, Mark BE, Stikeleather SJ, et al. Concurrent validity of upper-extremity volume estimates: comparison of calculated volume derived from girth measurements and water displacement volume. Phys Ther. 2003;83:134–145 [PubMed] [Google Scholar]
- 34.Waylett-Rendall J. Sensibility evaluation and rehabilitation. Orthop Clin North Am. 1988;19:43–56 [PubMed] [Google Scholar]
- 35.King TI. The effect of water temperature on hand volume during volumetric measurement using the water displacement method. J Hand Ther. 1993;6:202–204 [DOI] [PubMed] [Google Scholar]
- 36.McCulloch J BV. The effects of whirlpool and the dependant position of lower extremity volume. J Orthop Sports Phys Ther. 1992;16:169–173 [DOI] [PubMed] [Google Scholar]
- 37.Powell AA, Armstrong MA. Peripheral edema. Am Fam Physician. 1997;55:1721–1726 [PubMed] [Google Scholar]
- 38.Stegall H. Muscle pumping in the dependent leg. Circ Res. 1966;19:180–190 [Google Scholar]
- 39.Sochart DH. The relationship of foot and ankle movements to venous return in the lower limb. J Bone Joint Surg. 1999;81:700–704 [DOI] [PubMed] [Google Scholar]
- 40.Keen R. Osteoporosis: Strategies for prevention and management. Best Pract Res Clin Rheumatol. 2007;21:109–122 [DOI] [PubMed] [Google Scholar]
- 41.Lewiecki EM, Silverman SL. Redefining osteoporosis treatment: Who to treat and how long to treat. Arq Bras Endocrinol Metabol. 2006;50:694–704 [DOI] [PubMed] [Google Scholar]
- 42.Schmiege SJ, Aiken LS, Sander JL, et al. Osteoporosis prevention among young women: Psychosocial models of calcium consumption and weight-bearing exercise. Health Psychol. 2007;26:577–587 [DOI] [PubMed] [Google Scholar]
- 43.Sheriff D. The muscle pump raises muscle blood flow during locomotion. J Appl Physiol. 2005;99:371–375 [DOI] [PubMed] [Google Scholar]