Abstract
Background
Neuromuscular electrical stimulation initiated by a surface electromyographic biofeedback threshold (sEMG-triggered NMES) has been studied in populations of patients with neurological problems, but has not been applied to orthopedic populations.
Objectives
The purpose of this single-blinded, randomized clinical trial was to investigate sEMG-triggered NMES on knee extension active range of motion (AROM), function, and torque in patients with post-operative arthroscopic knee surgery.
Methods
Twenty-five participants were randomly assigned to either: (1) sEMG-triggered NMES with exercise group, or (2) exercise-only comparison group. Participants received outpatient physical therapy treatment 2 to 3 times a week for 12 visits. Knee AROM and function determined by the lower extremity functional scale (LEFS) were collected at the first, sixth, and twelfth visits. Peak isometric extensor torque was assessed using an electromechanical dynamometer at 3 months post surgery. Two analysis of variance tests with repeated measures were used to analyze knee AROM and LEFS data. An independent samples t-test was used to analyze the peak torque index (%) of the involved extremity compared to the uninvolved.
Results
A significant difference in AROM was found between groups. No significant difference was found between groups in the LEFS, nor in the peak isometric extensor torque. A 72.5% strength deficit was found compared to the uninvolved extremity.
Conclusion
Using sEMG-triggered NMES intervention improved extension AROM but did not improve function or torque.
Keywords: electrical stimulation, biofeedback, knee, rehabilitation
INTRODUCTION
Surgical interventions due to knee pathologies are becoming more common in society today due to the aging population, increasing sport participation, and occupational hazards. Surgical interventions often result in physiological impairments including decreased strength, range of motion (ROM), and function. Inability to voluntarily activate the quadriceps femoris muscle is frequently reported among patients following knee surgery and has been attributed to post-surgical pain and swelling.1–7 Providing intervention in the earlier stages of rehabilitation is believed to limit inhibition of the quadriceps femoris muscle, thus improving prognosis and function.4,8
Surface electromyography (sEMG) biofeedback and neuromuscular electrical stimulation (NMES) applications to the knee extensors are commonly used clinically to facilitate quadriceps femoris muscle recruitment. Improved knee extensor muscle recruitment aids in activities of daily living such as transfers and gait. Previous studies on the effectiveness of sEMG biofeedback and NMES, or a combination of the two, have shown conflicting results.9–25 Biofeedback from sEMG has been demonstrated to be effective for improving maximal isometric strength and muscle recruitment in healthy individuals.9,10 Biofeedback from sEMG has also been shown to enhance patellar alignment and muscle recruitment in individuals with knee pathology, such as patellofemoral syndrome.11,12 In post-surgical patients, the application of sEMG biofeedback showed improvement in maximal isometric strength, muscle recruitment, and ROM.13,14 However, other investigators have not observed improvements in strength or ROM with sEMG biofeedback intervention following knee surgery.15,16
Like sEMG, effectiveness of neuromuscular electrical stimulation (NMES) remains debated. Neuromuscular electrical stimulation has been demonstrated to be effective for improving maximal isometric strength in healthy individuals.17,18 Improvements in maximal isometric strength and gait with NMES were also shown in individuals following knee surgery.19–22 In contrast, other authors have found no benefit for the use of NMES as an intervention in the healthy and post-operative population.23–25
Technological advances have enabled the combined application of sEMG and NMES. In the neurological population, sEMG combined with NMES has been shown to improve functional recovery of stroke patients.26–31 However, the effectiveness of sEMG and NMES in combination has not been studied in an orthopedic population. The effectiveness of independent sEMG and NMES application has been shown and the combination may provide an additional benefit in the rehabilitation of orthopedic pathologies. This combination utilizes the activation of asynchronous motor units firing during volitional sEMG contraction and the activation of synchronous motor units firing during the electrically induced contraction by NMES. The muscle is also required to recruit and maintain a volitional contraction at a threshold prior to the initiation of the electrically elicited contraction by the NMES. The addition of sEMG to NMES has the potential to augment the physiological feed-forward system of motor control. An anticipatory motor response is initiated by the use of the sEMG, providing a more normal neuromuscular control feedback loop than by the NMES alone.
The purpose of this study was to investigate the effectiveness of sEMG-triggered NMES combined with exercise for improving knee active range of motion (AROM), functional status, and strength in patients after arthroscopic surgery. The research hypotheses were that sEMG-triggered NMES combined with exercise would be more effective than exercise alone for improving knee AROM and function over 12 physical therapy visits and for improving strength at three months post-surgery.
METHODS
Participants
Participants were recruited consecutively from patients referred to the outpatient physical therapy clinic where the principal investigator practiced. Patients who were 14 years of age or older and had undergone knee arthroscopic surgery within the last three weeks met the inclusion criteria. Patients were excluded if they had post-operative weight bearing or ROM restrictions, systemic diseases, uncontrolled hypertension, multiple joint surgery, total knee arthroplasty, pathology of the uninvolved extremity, pregnancy, or inability to complete the required time frame of the study. Patients who qualified for the study and wished to participate were asked to read and sign an informed consent form in accordance with the institutional review board guidelines. Participant demographic information including age, time post-surgery, and surgical procedure were obtained. Participants were then randomly assigned to either the sEMG-triggered NMES with exercise group or the exercise-only group, but they were unaware of whether their assigned treatment was considered the experimental or comparison group.
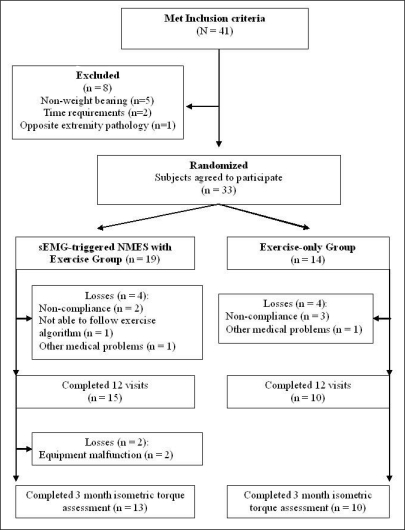
A consort diagram (Figure 1) presents patient enrollment and randomization. Forty-one patients met the inclusion criteria and were recruited for the study. Of the 41 subjects, eight participants had one or more exclusion criteria: weight bearing restriction (n = 5), inability to fulfill time requirement (n =2), and bilateral involvement (n =1).
Figure 1.
Consort diagram for patient enrollment and randomization
Consequently, 33 patients were eligible to participate in the study and randomly assigned to one of the two treatment groups; 19 in the sEMG-triggered NMES with exercise group and 14 in the exercise-only group (Table 1).
Table 1.
Description of Participants by Group
| Characteristic | Treatment | Comparison | All |
|---|---|---|---|
| Age (years) | 29.2 ± 12.2 | 39.4 ± 13 | 33.3 ± 13.3 |
| Body Weight (Kg) | 77.5 ± 21.7 | 89.8 ± 21.1 | 82.4 ± 21.9 |
| Height (cm) | 172.4 ± 12.7 | 171.5 ± 8.5 | 172 ± 11 |
| Time Post-surgery (days) | 10.3 ± 4.4 | 7.5 ± 3.7 | 8.6 ± 4.2 |
| Visual Analog Scale for pain | |||
| Visit 1 | 4.2 ± 2.8 | 4.6 ± 2.6 | 4.3 ± 2.7 |
| Visit 6 | 2.2 ± 1.8 | 2.3 ± 1.5 | 2.2 ± 1.7 |
| Visit 12 | 1.3 ± 1.1 | 1.5 ± 1.5 | 1.4 ± 1.2 |
| Gender | |||
| Male | 7 | 4 | 11 |
| Female | 8 | 6 | 14 |
| Surgical Procedure | |||
| Patellar tendon graft ACL reconstruction | 5 | 5 | 10 |
| Hamstring-graft ACL reconstruction | 5 | 0 | 5 |
| Menisectomy | 4 | 4 | 8 |
| Chondroplasty | 1 | 1 | 2 |
Note. All (n=25); Treatment Group (n=15); Comparison group (n=10), Treatment =sEMG-triggered NMES with exercise; Comparison = Exercise-only.
Outcome Measures
Height, weight, and pain assessment using a visual analog scale (VAS) were first obtained from all participants for descriptive purposes. Three outcome measures were collected: knee ROM, functional status, and muscle strength. Knee extension AROM and function were collected over the course of physical therapy treatment at visit 1, visit 6, and visit 12. Peak isometric knee extensor torque was compared with that of the non-operative knee at a 3-month post-operative follow-up.
Knee extension AROM was measured manually in supine with support under the ankle, using a universal goniometer and recorded in degrees. The average of two trials was used for data analysis. Intratester reliability of goniometric measurements of knee extension taken in a similar manner as this study has been shown to be excellent in the clinical setting.32,33 A concurrent intratester reliability sub-study was performed for the measurement of knee extension AROM. The investigator demonstrated excellent reliability (ICC3,2 = 0.99).
The Lower Extremity Functional Scale (LEFS) was used to assess functional status. The LEFS is a self-administered questionnaire that asks the patient to rate 20 individual items on a scale of 0 to 4 based on difficulty in performing the task. A score of 0 represents no difficulty while a score of 4 signifies extreme difficulty or inability to perform. The scores from the 20 individual items are summed and divided by the maximal possible score of 80 to obtain a percentage score. The LEFS has been shown to have construct validity and excellent test-retest reliability (ICC2,1 =.94).34
At three months after the surgery date, knee extensor muscle torque was assessed using the Biodex dynamometer (Biodex Medical Systems Inc., New York, NY). The three-month time lapse from surgery date to the torque assessment was the earliest that surgeons would allow tensile force to be applied to the graft harvest sites in the participants who had anterior cruciate ligament reconstruction. Peak knee extensor isometric torque of the operated (involved) knee was compared with that of the non-operated (uninvolved) knee creating a peak torque index. The participant's knee was placed in 60 degrees of knee flexion and correction procedures for the effects of gravity were performed. The participant performed a maximal isometric contraction for 5 seconds with a 30-second relaxation period. One practice trial and two test trials were completed for the uninvolved extremity followed by the involved extremity. The average of the two test trials was used for data analysis. A concurrent intratester reliability assessment was also performed for the muscle strength measurement. The investigator demonstrated excellent reliability (ICC3,2 = 0.99) for both involved and uninvolved extremities.
Interventions
Each participant received treatment 2 to 3 times a week in the clinic. Cryotherapy was applied for 15 minutes after each treatment session for post-operative inflammation and exercise-induced muscle soreness. All participants were also given a home exercise program that was modified at each clinical visit according to their progression.
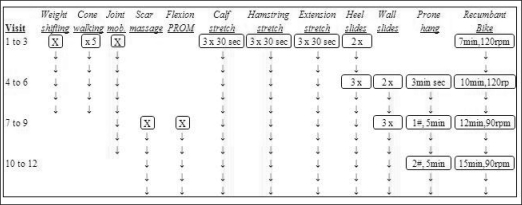
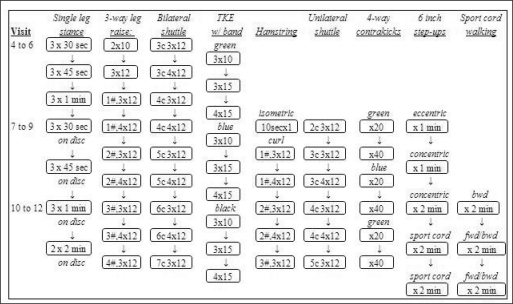
Participants in the sEMG-triggered NMES with exercise g r o u p r e c e i v e d application of the sEMG-triggered NMES to the quadriceps muscle while performing standard exercises. A standard exercise program included pain-free ROM exercises for flexion and extension of the knee, functional activities, gait training, and aerobic exercises (Figure 2). Advanced strengthening exercises of the quadriceps femoris musculature were added as the participant improved according to a specific progression (Figure 3). The exercise program was monitored for adverse responses.
Figure 2.
Algorithm for aerobic, gait, flexibility and manual exercise progression
Figure 3.
Algorithm for strength exercise progression
The Care EMG-Triggered Stimulation (Care Rehab and Orthopedic Products Inc., McClean, VA) was used for the sEMG-triggered NMES intervention. The following parameters of the NMES settings were used: pulse rate 60 Hz, pulse width 250 μS, ramp 0.6 seconds, and auto setting “on.” In this auto mode, the EMG threshold to initiate the NMES automatically increased or decreased the EMG threshold by 12.5% for the next trial. The participant assumed knee extension in the long sitting position, without support under the ankle, to help isolate the ability of the quadriceps femoris muscle to a c t i v e l y extend the knee through the available range. For both the sEMG and NMES application, three circular 2.75 inch self-adhesive electrodes (EMPI, St. Paul, MN) were u s e d . Participants were prepped with new electrodes and a new battery was issued for every sEMGt r i g g e r e d NMES intervention visit. The negative electrode was placed on the vastus medialis oblique muscle (VMO), positive electrode on the mid rectus femoris muscle, and reference electrode on the head of the fibula (Figure 4). For electrode placement, both the VMO and rectus femoris muscles have been shown to have better reliability than that of the vastus lateralis muscle for sEMG applications to the quadriceps femoris muscle.35,36 The electrodes and the outline of the patella were traced onto a transparent film to ensure consistent placement of electrodes during subsequent applications.
Figure 4.
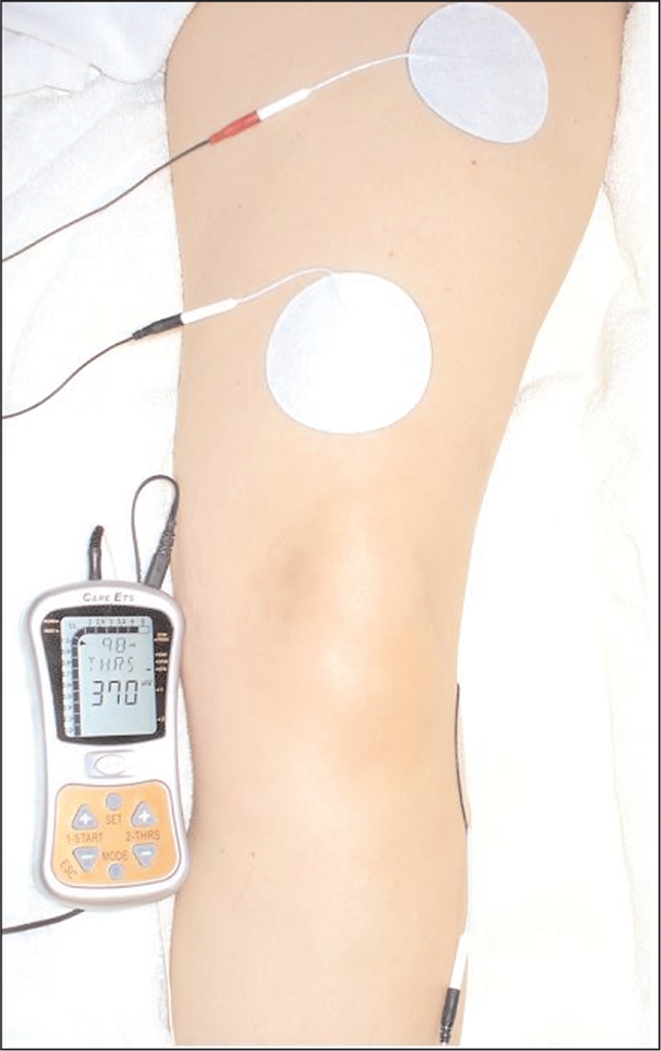
sEMG-triggered NMES unit and electrode placement
For normalization of the sEMG application, a maximal volitional isometric contraction (MVIC) was performed and the EMG amplitude was recorded at each visit to determine the baseline VMO and rectus femoris muscle recruitment for that day. Two MVIC trials were performed with the contraction held for 10 seconds with a 20-second rest in between trials. The average of the MVIC trials was calculated and the target sEMG biofeedback threshold was set at 75% of MVIC value for sub-maximal quadriceps m u s c l e strengthening. The participants were then asked to contract their quadriceps muscle and the NMES was gradually increased to maximal tolerance. During the quadriceps m u s c l e strength training, sEMG-triggered NMES was set at the previously determined target sEMG biofeedback threshold value. Once this sEMG biofeedback threshold value was exceeded, the sEMG-triggered NMES unit automatically turned on the NMES to electrically elicit an even stronger quadriceps muscle contraction. The sEMG-triggered NMES intervention duty cycle was set at 1:2 with an on-time of 10 seconds for muscle contraction and an off-time of 20 seconds for a total of 22 repetitions. The exercise-only group performed the exact same exercise without the sEMG-triggered NMES intervention.
Data Analysis
The SPSS 16.0 statistical software program (SPSS Inc., Chicago, IL) was used to analyze the collected data. A mixed design 2x3 (group by visit) analysis of variance (ANOVA) with repeated measures on the visit factor was used to compare knee AROM and LEFS data between and within groups. The peak torque index was calculated using the average involved lower extremity peak torque value divided by the peak torque value for the uninvolved extremity, multiplied by 100. An independent samples t-test was used to compare the differences in peak torque index between the sEMG-triggered NMES with exercise and exercise-only groups. The alpha level was set at 0.05 for all statistical tests.37
RESULTS
Twenty-five participants completed the 12 intervention visits in the 6-8 week post-operative program and returned for post-test measurements. Fifteen of these participants were in the treatment (sEMG-triggered NMES with exercise) group and ten in the comparison (exercise-only) group with equal attrition for both groups. Four participants dropped out of each group. Table 1 shows the descriptive statistics and characteristics of the participants who completed the study. All data were found to be normally distributed by analyzing the degree of skewness. No baseline differences were found between groups for any characteristics or measures.
Knee Extension Active Range of Motion
Descriptive statistics for knee extension AROM assessed at visits 1, 6, and 12 are reported in Table 2. Both the treatment and comparison groups showed a decrease in degrees of flexion, suggesting knee extension AROM improved from visit 1 to visit 6 and continued to improve at visit 12. The ANOVA results for AROM revealed statistically significant interaction for knee extension AROM (F = 3.99, p = 0.049). The significant interaction signifies that groups responded differently over time, so tests of simple effects were conducted to further investigate differences.
Table 2.
Descriptive statistics (mean ± SD, SEM) and Minimal Detectable Change (MDC) for knee extension active range of motion (AROM) and the Lower Extremity Functional Scale (LEFS) score by group for visits #1, 6,and 12
| Treatment | Comparison | All | MDC | |
|---|---|---|---|---|
| Knee Extension AROM (°) | ||||
| Visit 1 | 8.3 ± 5.2, 1.3 | 5.3 ± 3.2, 1.0 | 7.1 ± 4.7, 0.9 | 2 |
| Visit 6 | 2.4 ± 2.6, 0.7 | 2.0 ± 1.8, 0.6 | 2.2 ± 2.2, 0.4 | 0.6 |
| Visit 12 | 1.1 ± 1.8, 0.5 | 1.2 ± 1.4, 0.4 | 1.1 ± 1.6, 0.3 | 0.3 |
| LEFS Score (%) | ||||
| Visit 1 | 28.9 ± 15.0, 3.9 | 26.9 ± 12.1, 3.8 | 28.1 ± 13., 2.7 | 11.3 (9 pts) |
| Visit 6 | 49.2 ± 17.8, 4.6 | 48.4 ± 15.0, 4.8 | 48.9 ± 16.4, 3.3 | 11.3 (9 pts) |
| Visit 12 | 65.1 ± 16.4, 4.2 | 61.7 ± 13.7, 4.3 | 63.7 ± 15.2, 3.0 | 11.3 (9 pts) |
Note. All (n=25); Treatment Group (n=15); Comparison group (n=10), Treatment = sEMG-triggered NMES with exercise; Comparison = Exercise-only.
Two one-way ANOVAs were performed for each group to follow up the significant interaction effect and both ANOVAs were found to be significant. Next, three t- tests were conducted to examine pair-wise differences within each group. Bonferroni's correction was used to control the family-wise error because three post-hoc comparison tests were performed. After correction, the alpha level was set at 0.0167 for each t-test. Differences in knee extension AROM were significant between visits 1 and 6 for the sEMG-triggered NMES with exercise group (t = 5.72, p < 0.01) and exercise only group (t = 5.07, p = 0.001). Differences in knee extension AROM were also significant between visits 1 and 12 for the sEMG-triggered NMES with exercise group (t = 6.57, p < 0.01) and exercise only group (t = 5.84, p < 0.01). Differences in knee extension AROM were significant between visits 6 and 12 for the sEMG-triggered NMES with exercise group (t = 4.34, p = 0.001) but not for the exercise-only group (t = 1.70, p = 0.124).
Lower Extremity Functional Scale
Descriptive statistics for functional status using the LEFS assessed at visits 1, 6, and 12 are reported in Table 2. Both the treatment and comparison groups showed an increase in LEFS score suggesting that function improved from visit 1 to visit 6 and continued to improve at visit 12. The ANOVA results for LEFS scores revealed no statistically significant interaction for function (F = 0.12, p = 0.887) and no significant differences for the main effect of group (F = 0.14, p = 0.709). A significant difference was found for the visit factor (F = 90.11, p < 0.01). Three t-tests were conducted to follow up the significant visit main effect. Bonferroni's correction was used to control for family-wise error rate across the three post-hoc comparison tests by adjusting the alpha level to 0.0167 for each test. Differences in the LEFS scores were significant between visits 1 and 6 (t = −8.96, p < 0.01), between visits 1 and 12 (t = −12.83, p < 0.01), and between visits 6 and 12 (t = −5.82, p < 0.01). Significance was found across the visit factor for both groups combined, indicating that there was improved function over time regardless of group.
Peak Knee Extensor Torque
Table 3 illustrates the descriptive statistics for the peak isometric knee extensor torque (Nm) assessed at three months after surgery. Both the treatment and comparison groups showed a lower peak torque in the involved extremity compared to the uninvolved extremity. Descriptive statistics for the peak torque index (%) assessed at three months after surgery are shown in Table 4. The independent t-test results for the torque index revealed a non-significant difference for torque index between the sEMG-triggered NMES with exercise and exercise only groups (t = 0.245, p = 0.809). The torque index was 73.5% ± 18.3% for the sEMG-triggered NMES with exercise group, and 71.2% ± 26.9% for the exercise-only group.
Table 3.
Descriptive Statistics for Bilateral Peak Isometric Knee Extensor Torque (Nm) by Group
| Extremity | M | SD | SEM |
|---|---|---|---|
| Involved | |||
| All | 87.3 | 44.5 | 9.3 |
| Treatment Group | 83.7 | 37.3 | 10.3 |
| Comparison Group | 91.9 | 54.3 | 17.2 |
| Uninvolved | |||
| All | 117.7 | 46.5 | 9.7 |
| Treatment Group | 111.7 | 37.4 | 10.4 |
| Comparison Group | 125.6 | 57.4 | 18.2 |
Note. All (n=23); Treatment Group (n=13); Comparison group (n=10), Treatment = sEMG-triggered NMES with exercise; Comparison = Exercise-only.
Table 4.
Descriptive Statistics for Mean Isometric Knee Extensor Torque Index (%) by Group
| M | SD | SEM | |
|---|---|---|---|
| All | 72.5 | 21.9 | 4.6 |
| Treatment Group | 73.5 | 18.3 | 5.1 |
| Comparison Group | 71.2 | 26.9 | 8.5 |
Note. All (n=23); Treatment Group (n=13); Comparison group (n=10), Treatment = sEMG-triggered NMES with exercise; Comparison = Exercise-only.
DISCUSSION
Knee Extension Active Range of Motion
The knee extension AROM results showed a significant improvement over time and this improvement was different for the two groups. Both groups made significant AROM improvement in knee extension in the first six visits but only the treatment group continued to improve significantly in the next six visits. The comparison group did not improve significantly from visit 6 to visit 12. As shown in Table 2, the treatment group had an average of a 5.9 degree increase from visit 1 to visit 6, while the comparison group had an average of a 3.3 degree increase. From visit 6 to visit 12, the treatment group had an average of a 1.2 degree increase, while the comparison group only had a 0.9 degree increase. It should be noted that no significant differences were found between the two groups for subjective pain perception on each data collection between visits 1 and 6 (p =0.779), between visits 1 and 12 (p =0.880), and between visits 6 and 12 (p =0.871). Therefore, pain was not likely to have been a factor accounting for differences between the two groups on measures of AROM.
One may argue the clinical importance of a 1.2 degree improvement in knee extension. This study assessed terminal knee extension AROM, which is only a small percentage of full spectrum extension to flexion ROM of the knee. A common clinical observation is that lack of the last few degrees of knee extension may affect normal gait and function and these last few degrees of knee extension are most difficult to attain. Therefore, the minimal detectable change (MDC) for knee extension should be examined. The MDC for knee flexion AROM has been reported to be between 3 degrees and 10 degrees, but it is not feasible to use the established MDC of full knee ROM for this data.38,39 The MDC was calculated for knee extension AROM from the reliability data. At the 95% confidence level, the MDC was 0.96 degrees. Although a 1.2 degree increase of AROM from visit 6 to 12 in the treatment group is statistically and clinically significant based on the MDC, interpreting such small improvements should be done cautiously.
The AROM results are similar to findings in the Draper and Ballard study,16 in which they compared sEMG with exercise to NMES with exercise in patients following ACL reconstruction. Draper and Ballard found no difference in knee extension AROM between the two groups at postoperative weeks 1, 2, 4, and 6 as both showed a significant improvement over time. Additionally, Levitt et al13 found no changes from extension to flexion AROM between exercise-only and sEMG with exercise groups in patients after minor knee surgeries, but the authors did not examine terminal knee extension AROM.
Functional Status
The LEFS for functional status results did not show a statistical difference between the sEMG-triggered NMES intervention with exercise group and exercise-only group. The LEFS mean scores (%) for all participants, regardless of group, progressively improved over the 12-visit treatment course with a greater improvement from visit 1 to visit 6 (28.1 – 48.9) compared to visit 6 to visit 12 (48.9 – 63.7). A 9-point (11.25%) change has been shown to be the MDC for function using the LEFS outcome measure.34 For both groups, the change in LEFS scores from visit 1 to 6 and visit 6 to 12 were greater that the established MDC. Therefore, we may attribute the increase to true improvement in function rather than to measurement error. The LEFS changes were clinically significant and may indicate the importance of functional restoration throughout the stages of knee rehabilitation.
No other studies have been conducted to investigate the functional effects of sEMG-triggered NMES on post-operative knee rehabilitation. However, Snyder-Mackler et al20 found that the application of NMES with exercise significantly increased flexion-extension excursion during the gait cycle when compared to exercise only. The application of NMES with exercises by Snyder-Mackler et al20 also lead to significant improvement in cadence, walking velocity, stance time, and flexion-extension excursion during the gait cycle compared to exercises alone.
The present study revealed a significant improvement in AROM and LEFS across the visit factor regardless of group. Post hoc comparisons showed that the significant differences were between visit 1 and visit 6, visit 6 and visit 12, and visit 1 and 12. This finding would imply that both sEMG-triggered NMES with exercise and exercise only are effective treatments for improving AROM and function following knee surgery. However, without a true control group, the possibility that the participants improved because of time alone cannot be ruled out.
Peak Knee Extensor Torque
The peak torque index results did not show a statistical difference between the two groups at 3 months post-surgery. Knee strength in the involved extremity was only 72.5% of the uninvolved extremity in both groups combined. The residual strength impairment was not different between the two intervention groups with a 73.5% impairment in the sEMG-triggered NMES group and 71.2% impairment in the exercise-only group. The peak torque index at 3 months post surgery showed that participants, regardless of group, still exhibited almost 25% in strength deficits. Further knee rehabilitation focusing on strength gains is clearly warranted.
No other studies have been conducted to investigate the torque effects of sEMG-triggered NMES on post-operative knee rehabilitation. However, other studies have reported the effects of NMES without sEMG triggered stimulation. Delitto et al19 for example, reported a 78.8% torque index using NMES with exercise versus 51.7% with exercise alone six weeks after ACL reconstruction. Although the torque index for NMES reported by Delitto et al19 is similar to the torque index with sEMG triggered NMES in the present study, torque index for the Delitto et al study19 exercise alone group was considerably lower. Additionally, the present study did not solely investigate patients with ACL reconstruction. In contrast to the Delitto et al19 study, Draper and Ballard16 revealed lower torque indices of 46.4% with sEMG alone and 37.9% with NMES alone six weeks after surgery.
Draper14 conducted another study to examine torque index at 45, 60, and 90 degrees of flexion at 12 weeks postoperatively. She found a significant difference in strength following sEMG biofeedback with exercise verses exercise alone. Based on the findings of Draper et al16 of greater strength gain with sEMG biofeedback, it has been accepted practice to use sEMG in knee rehabilitation. However, adding NMES to sEMG in the present study seemed to negate the sEMG benefit. The addition of sEMG may overly complicate the treatment resulting in reduced compliance. The results of the present study are most consistent with Pasternostro-Sluga et al.24 The 78% torque index with NMES and exercise and 76.3% with exercise alone in patients with ACL reconstruction who were 12 weeks postoperative are very similar to the current findings. Both Pasternostro-Sluga et al24 and the current study concluded that the addition of the sEMG and NMES to exercises did not provide additional benefit for strength gains.
The specific type of surgery could have been a confounding factor in the results. Surgical procedure subgroups were unable to be analyzed individually due to insufficient sample size. In addition, other potential confounding factors such as sex, age, time from surgery, and initiation of physical therapy to specific outcome measures assessment did not influence the results.
The application of sEMG-triggered NMES with exercise in outpatient physical therapy can improve AROM, although a 3 month rehabilitation period is not long enough to regain full strength. Recommendations for future study include a long-term follow-up and administering the sEMG-triggered NMES at 3 months postoperatively or later to determine its efficacy in the advanced rehabilitation phase. Randomized controlled trials with a true control group are recommended to examine the effectiveness of sEMG-triggered NMES in larger specific patient populations with greater surgical procedure homogeneity. In addition, the effectiveness in different patient populations and additional outcome measures such as balance and gait should be examined.
CONCLUSION
This study has shown that adding the sEMG-triggered NMES to knee rehabilitative exercises may be beneficial for knee extension AROM improvement in patients with knee surgery especially from week 6 to week 12. Both treatment groups improved from week 1 to week 6 but only the sEMG-triggered NMES group showed statistical improvement after the next six visits. The use of sEMG-triggered NMES did not provide additional improvement in function or knee extensor muscle strength in post-arthroscopic knee rehabilitation. Without a true control group, however, whether the improvements were the result of the treatments or time alone cannot be ascertained. A considerable strength deficit in the quadriceps femoris muscle of the surgical knee remained at 3 months post-surgery in this patient population, indicating that the continuation of knee strength rehabilitation beyond 3 months should be strongly recommended.
ACKNOWLEDGEMENTS
The authors would like to thank the Texas Physical Therapy Foundation and the Texas Society of Allied Health Professionals for the grant funding to allow completion of this clinical study.
REFERENCES
- 1.Krebs DE, Staples WH, Cuttita D, et al. Knee joint angle: Its relationship to quadriceps femoris activity in normal and post arthrotomy limbs. Arch Phys Med Rehabil. 1983;64:441–447 [PubMed] [Google Scholar]
- 2.Fahrer H, Rentsch HU, Gerber NJ, et al. Knee effusion and reflex inhibition of the quadriceps. A bar to effective retraining. J Bone Joint Surg Br. 1988;70:635–638 [DOI] [PubMed] [Google Scholar]
- 3.McNair PJ, Marshall RN, Maguire K. Swelling of the knee joint: Effects of exercise on quadriceps muscle strength. Arch Phys Med Rehabil. 1996;77:896–899 [DOI] [PubMed] [Google Scholar]
- 4.Mizner RL, Stevens JE, Snyder-Mackler J. Voluntary activation and decreased force production of the quadriceps femoris muscle after total knee arthroplasty. Phys Ther. 2003;83:359–365 [PubMed] [Google Scholar]
- 5.Berth A, Urbach D, Awiszus F. Improvement of voluntary quadriceps muscle activation after total knee arthroplasty. Arch Phys Med Rehabil. 2002;83:1432–1436 [DOI] [PubMed] [Google Scholar]
- 6.Suter E, Herzog W, Bray RC. Quadriceps inhibition following arthroscopy in patients with anterior knee pain. Clin Biomech. 1998;13:214–319 [DOI] [PubMed] [Google Scholar]
- 7.Stam HJ, Binkhorst RA, Kuhlmann P, et al. Clinical progress and quadriceps torque ratios during training of menisectomy patients. Int J Sports Med. 1992;13:183–188 [DOI] [PubMed] [Google Scholar]
- 8.Snyder-Mackler L, De Luca PF, Williams PR, et al. Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1994;76:555–560 [DOI] [PubMed] [Google Scholar]
- 9.Lucca JA, Recchiuti SJ. Effect of electromyographic biofeedback on an isometric strengthening program. Phys Ther. 1983;63:200–203 [DOI] [PubMed] [Google Scholar]
- 10.Croce RV. The effects of biofeedback on strength acquisition. Biofeedback Self Regul. 1986;11:299–310 [DOI] [PubMed] [Google Scholar]
- 11.Wise HH, Fiebert IM, Kates JL. EMG biofeedback as treatment for patellofemoral pain syndrome. J Orthop Sports Phys Ther. 1984;6:95–103 [DOI] [PubMed] [Google Scholar]
- 12.Ingersoll CD, Knight KL. Patellar location changes following EMG biofeedback or progressive resistive exercises. Med Sci Sports Exerc. 1991;23:1122–1127 [PubMed] [Google Scholar]
- 13.Levitt R, Deisinger JA, Remondet Wall J, et al. EMG feedback-assisted postoperative rehabilitation of minor arthroscopic knee surgeries. Sports Med Phys Fitness. 1995;35:218–223 [PubMed] [Google Scholar]
- 14.Draper V. Electromyographic biofeedback and recovery of quadriceps femoris muscle function following anterior cruciate ligament reconstruction. Phys Ther. 1990;70:11–17 [DOI] [PubMed] [Google Scholar]
- 15.Dursun N, Dursun E, Kilic Z. Electromyographic biofeedback-controlled exercise versus conservative care for patellofemoral pain syndrome. Arch Phys Med Rehabil. 2001;82:1692–1695 [DOI] [PubMed] [Google Scholar]
- 16.Draper V, Ballard L. Electrical stimulation versus electromyographic biofeedback in the recovery of quadriceps femoris muscle function following anterior cruciate ligament surgery. Phys Ther. 1991;71:455–461 [DOI] [PubMed] [Google Scholar]
- 17.Soo C, Currier DP, Threlkeld AJ. Augmenting voluntary torque of healthy muscle by optimization of electrical stimulation. Phys Ther. 1988;68:333–337 [DOI] [PubMed] [Google Scholar]
- 18.Selkowitz D. Improvement in isometric strength of the quadriceps femoris muscle after training with electrical stimulation. Phys Ther. 1985;65:186–196 [DOI] [PubMed] [Google Scholar]
- 19.Delitto A, Rose SJ, McKowen JM, et al. Electrical stimulation versus voluntary exercise in strengthening thigh musculature after anterior cruciate surgery. Phys Ther. 1988;68:1145–1150 [DOI] [PubMed] [Google Scholar]
- 20.Snyder-Mackler L, Ladin Z, Schepsis AA, et al. Electrical stimulation of the thigh muscles after reconstruction of the anterior cruciate ligament. Effects of electrically elicited contraction of the quadriceps femoris and hamstring muscles on gait and strength of the thigh muscles. J Bone Joint Surg Am. 1991;73:1025–1036 [PubMed] [Google Scholar]
- 21.Lewek M, Stevens J, Snyder-Mackler L. The use of electrical stimulation to increase quadriceps femoris muscle force in an elderly patient following a total knee arthroplasty. Phys Ther. 2001;81:1565–1571 [DOI] [PubMed] [Google Scholar]
- 22.Snyder-Mackler L, Delitto A, Bailey SL, et al. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg Am. 1995;77:1166–1173 [DOI] [PubMed] [Google Scholar]
- 23.Currier DP, Mann R. Muscular strength development by electrical stimulation in healthy individuals. Phys Ther. 1983;63:915–921 [DOI] [PubMed] [Google Scholar]
- 24.Paternostro-Sluga T, Fialka C, Alacamliogliu Y, et al. Neuromuscular electrical stimulation after anterior cruciate ligament surgery. Clin Orthop. 1999:166–175 [PubMed] [Google Scholar]
- 25.Sisk TD, Stralka SW, Deering MB, et al. Effect of electrical stimulation on quadriceps strength after reconstructive surgery of the anterior cruciate ligament. Am J Sports Med. 1987;15:215–220 [DOI] [PubMed] [Google Scholar]
- 26.Bolton DA, Cauraugh JH, Hausenblas HA. Electromyogram-triggered neuromuscular stimulation and stroke recovery of arm/hand function: A meta-analysis. J Neurol Sci. 2004;223:121–127 [DOI] [PubMed] [Google Scholar]
- 27.Cauraugh JH, Kim S. Two coupled motor recovery protocols are better than one: Electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke. 2002;33:1589–1594 [DOI] [PubMed] [Google Scholar]
- 28.Kraft GH, Fitts SS, Hammond MC. Techniques to improve function of the arm and hand in chronic hemiplegia. Arch Phys Med Rehabil. 1992;73:220–227 [PubMed] [Google Scholar]
- 29.Fields RW. Electromyographically triggered stimulation for chronic hemiplegia. Arch Phys Med Rehabil. 1987;68:407–414 [PubMed] [Google Scholar]
- 30.Rakos M, Freudenshu B, Girsch W, et al. Electromyogram-controlled electrical stimulation for treatment of the paralyzed upper extremity. Artificial Organs. 1999;23:466–469 [DOI] [PubMed] [Google Scholar]
- 31.Francisco G, Chae J, Chawla H, et al. Electromyogram-triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: A randomized study. Arch Phys Med Rehabil. 1998;79:570–575 [DOI] [PubMed] [Google Scholar]
- 32.Watkins MA, Riddle DL, Lamb RL, et al. Reliability of goniometric measurements and visual estimates of knee range of motion obtained in the clinical setting. Phys Ther. 1991;71:90–96 [DOI] [PubMed] [Google Scholar]
- 33.Rothstein JM, Miller PJ, Roettger RF. Goniometric reliability in a clinical setting. Phys Ther. 1983;63:1611–1615 [DOI] [PubMed] [Google Scholar]
- 34.Binkley JM, Stratford PW, Lott SA, et al. The lower extremity functional scale (LEFS): Scale development, measurement, properties, and clinical application. Phys Ther. 1999;79:371–383 [PubMed] [Google Scholar]
- 35.Larsson B, Karlsson S, Eriksson M, et al. Test-retest reliability of EMG and peak torque during repetitive maximum concentric knee extensions. J Electromyogr Kinesiol. 2003;13:281–287 [DOI] [PubMed] [Google Scholar]
- 36.Kollmitzer J, Ebenbichler GR, Kopf A. Reliability of surface electromyographic measurements. Clin Neurophysiol. 1999;110:725–734 [DOI] [PubMed] [Google Scholar]
- 37.Portney LG, Watkins MP. Clinical Foundations of Clinical Research: Application to Practice. 2nd ed.Upper Saddle River, NJ: Prentice Hall Heath; 2000 [Google Scholar]
- 38.Boone DC, Azen SP, Lin CM, et al. Reliability of goniometric measurements. Phys Ther. 1978;58:1355–1390 [DOI] [PubMed] [Google Scholar]
- 39.Bovens AM, van Baak MA, Vrencken JG, et al. Variability and reliability of joint measurements. Am J Sports Med. 1990;18:58–63 [DOI] [PubMed] [Google Scholar]