Abstract
Available data concerning the non-hepatic source of plasma factor VIII are conflicting. In the present study, dogs with factor VIII deficiency hemophilia were transplanted with spleen or vascularized lymph node grafts obtained from normal donor dogs. Postoperative immunosuppression was done with azathioprine and heterologous antilymphocyte globulin. Four spleen transplants were successful and the recipients had positive technetium sulfide spleen scans along with adequate plasma factor VIII levels for three to eight weeks. Cessation of graft isotope uptake was accompanied by prompt disappearance of plasma factor in all four dogs. Histologically, the grafts were rejected at this time. In one dog, a second spleen graft immediately restored therapeutic factor VIII levels. Four lymph node transplants were successful and plasma factor VIII was detected for one, one, two, and ten weeks. Three dogs rejected their lymph node grafts with disappearance of factor VIII, while one had a viable transplant removed with loss of factor VIII within two days. The data suggest that factor VIII is produced in the lymphatic tissue of the dog.
Data provided by recent experiments with organ transplantation10, 11, 19 has indicated a multiorgan site of synthesis of plasma antihemophilic factor (factor VIII). Thus, a major role of hepatic tissue in the process was established by the finding that transplantation of a normal liver into a hemophilic dog raised plasma factor VIII to an essentially normal level. But in the same studies, evidence of production of the antihemophilic factor in other organs was adduced by the observation that replacement of the liver in a normal dog with a hemophilic organ led to only a temporary decrease in plasma factor VIII. Available data concerning the non-hepatic source of the factor are conflicting. One group of investigators reported correction of hemophilia following splenic transplantation in dogs,14, 17 but this finding could not be reproduced by others. 10–12, 19 In other canine experiments, transplantation of the kidney and the bone marrow were found not to cause detectable changes in the antihemophilic factor.10, 19
In the present study, canine hemophilic recipients of splenic or vascularized lymph node grafts were both found to maintain significant levels of plasma factor VIII. This result, in conjunction with the previous findings, indicates that canine antihemophilic factor is produced in tissues common to the liver, spleen, and lymph node.
METHODS
Eleven Labrador dogs with factor VIII deficiency hemophilia (four females and seven males, age 15 to 20 months, weighing 12 to 22 kilograms) were used.* All but two had had bleeding complications which ranged from simple surface hematomas to hemarthroses that had resulted in crippling arthritis. With blood drawing, the venipuncture site required prolonged compression. During observation in our laboratory for three to six months, plasma factor VIII was less than one percent when assayed on numerous occasions. Healthy, normal Labrador dogs served as graft donors; the animals were heparinized just prior to graft removal. The anesthetic agent was sodium pentobarbital supplemented with phencyclidine hydrochloride.
Whole organ splenic transplantation was achieved with vascular anastomoses to the recipient iliac vessels as described previously.9 Ischemia time for the grafts was 25 to 30 minutes. The recipient spleen was removed. Postoperatively, splenic graft function was assessed twice weekly by 99M-technetium sulfide radioisotope scans.
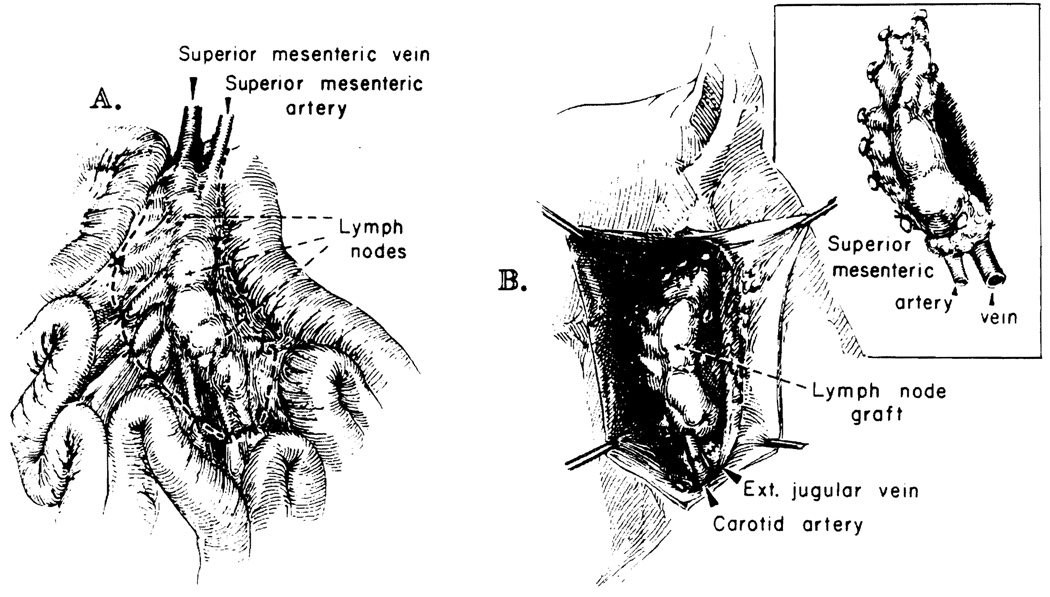
Lymph node grafts were prepared from the root of the mesentery of the small bowel, preserving the superior mesenteric artery and vein (Fig. 1). In such a preparation, there are regularly two clusters of nodes, each having a size of approximately 1 by 1 by 5 cm. Revascularization in the recipient was done with end-to-end anastomosis of the mesenteric vessels to the carotid artery and external jugular vein. The graft was placed in a subcutaneous pocket in the neck (Fig. 1). Ischemia time was 21 to 33 minutes.
Fig. 1.
Technique of vascularized lymph node transplantation. A, The root of the mesentery is dissected free from the small bowel in the donor, preserving the mesenteric vessels. B, The graft has been revascularized in the neck of the recipient. Vascular anastomoses are to the carotid artery and external jugular vein. The graft is placed in a subcutaneous pocket.
Prior to operation, each recipient dog was transfused with 25 ml. per kilogram of fresh, normal dog plasma. To prevent or reduce allergy-like transfusion reactions, intravenous hydrocortisone, 250 mg. was given simultaneously. Postoperative immunosuppression was achieved with a two-drug regimen. Azathioprine, 1 to 8 mg. per kilogram per day, was given orally; the daily dose was adjusted according to the peripheral white blood cell count. Horse antilymphocyte globulin (ALG) (leukoagglutinin titer 1:4000, cytotoxic titer 1:256), 10 mg. per kilogram per dose, was given subcutaneously daily for the first two postoperative weeks. The ALG was produced as described for the human material at our institution,5 except that canine thymic lymphocytes were used for the immunization.
Factor VIII in citrated plasma samples was measured by the thromboplastin generation time assay of Pool and Robinson15; the normal reference plasma (100 percent activity) was pooled normal dog plasma. Unlike one-stage assays for factor VIII (partial thromboplastin time assays), this method is relatively insensitive to activated coagulation factors or traces of tissue thromboplastin or thrombin.13 Assays for fibrinogen and factors II, V, and VII + X were done as previously described6 with the use of pooled normal dog plasma as the normal standard. The whole blood clotting time, prothrombin time, thrombin time, euglobulin lysis time, platelet count, hematocrit, and total white cell count were performed by standard methods.
Biopsy and autopsy specimens from the grafts were stained with hematoxylin and eosin, methyl green pyronin, silver for reticulin, Weigert’s for elastic, and periodic acid–Schiff reagent, and then they were examined by light microscopy.
RESULTS
Effect of plasma infusion and immunosuppression only
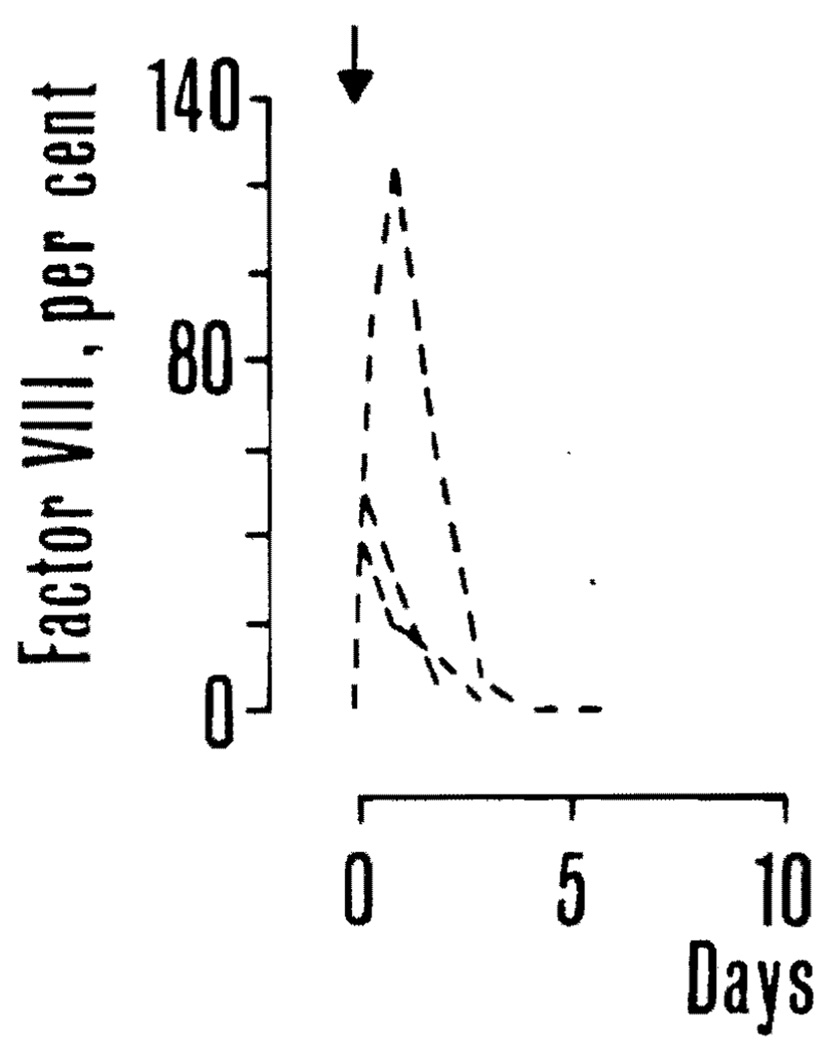
Infusion of fresh canine plasma, 25 ml. per kilogram, to three hemophilic dogs not undergoing concomitant transplantation caused a prompt rise in factor VIII. Maximum values recorded after transfusion were 40, 50, and 125 percent in the three dogs, respectively. Three days later the factor had returned to near zero in all instances (Fig. 2). One of the dogs was given the immunosuppressive regimen used in the graft recipients, starting at the day of transfusion. There was no apparent effect on the plasma factor VIII. The two other dogs were used as graft recipients six to eight weeks later.
Fig. 2.
Effect of plasma infusion on plasma factor VIII in three hemophilic dogs. In all instances, factor VIII returned to near zero in three days. One of the dogs received the immunosuppressive regimen used in the graft recipients but the effect of plasma infusion was not thereby prolonged.
Splenic transplantation
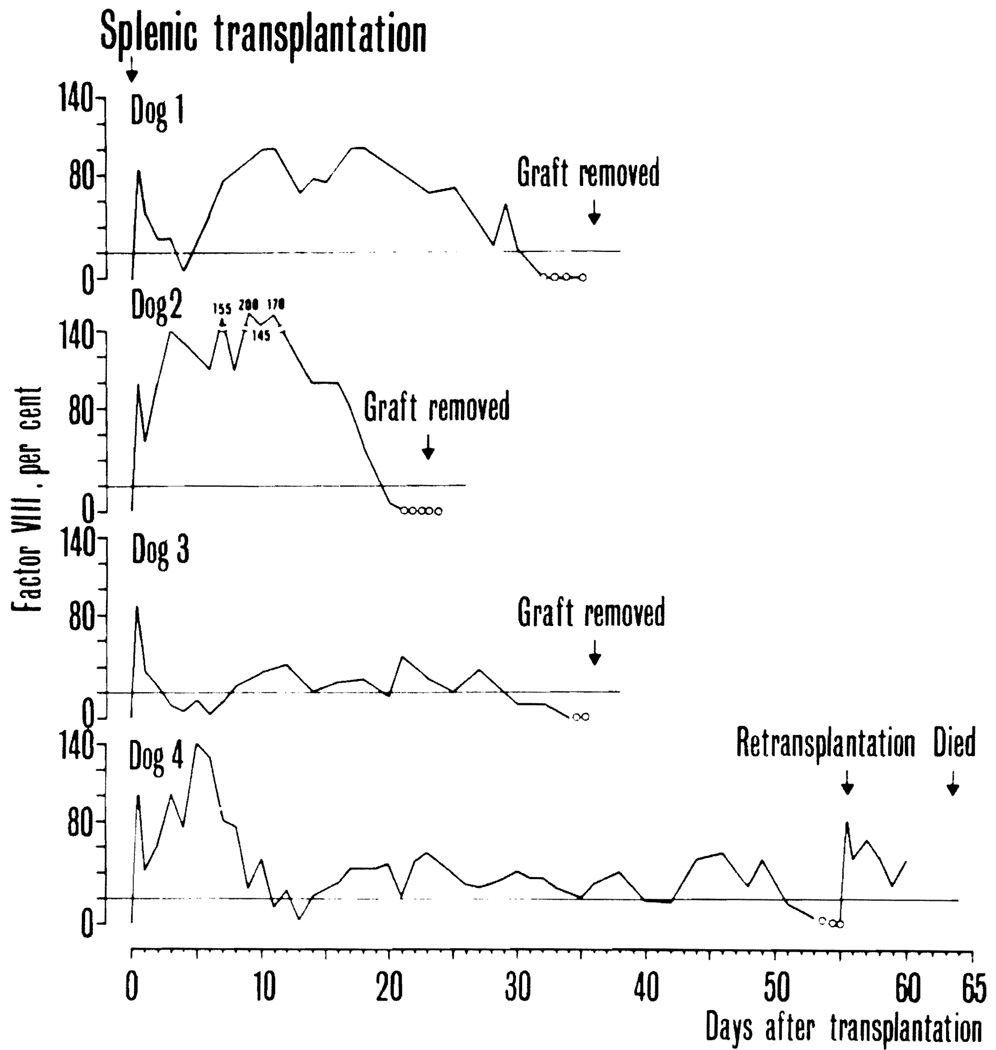
There were four hemophilic recipients of splenic transplants; one of the dogs received two consecutive grafts. Radioisotope scanning showed uptake in the grafts from the day of transplantation to day 28, 19, 32, and 50 after operation, respectively. During the last several days before disappearance of uptake, there was a gradual decline in the density of the radiological picture (Fig. 3). The dog (No. 4) in which the graft picture disappeared after 50 days, underwent retransplantation six days later. The second graft showed excellent isotope uptake, but the dog died nine days postoperatively with pneumonia.
Fig. 3.
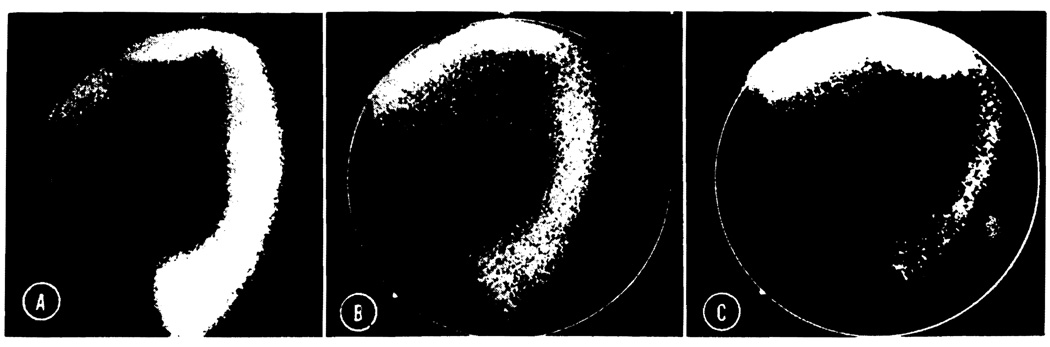
Postoperative technetium disulfide scan in a splenic recipient (Dog No. 2). A, Eight days after transplantation. The graft which is visualized in the left lower abdomen shows an excellent uptake. The lower edge of the liver is also seen. The animal’s own spleen was removed. B and C, Seventeen and 19 days after transplantation the uptake is decreasing rapidly. On day 21 the graft could no longer be visualized radiographically.
On the day of transplantation, plasma factor VIII in the four donor dogs was 100, 40, 100, and 105 percent, respectively. In the recipients, preoperative antihemophilic factor was less than one percent in all instances. Postoperatively, an initial peak, caused by plasma infusion, was followed by a few days of wide fluctuations (Fig. 4). Following this, the plasma factor VIII was essentially normal during three to four weeks in two dogs (Nos. 1 and 2). In the other two (Nos. 3 and 4) the level stabilized around 20 to 40 percent for four and eight weeks, respectively. There were no clinical clotting problems during or after surgery except that one dog (No. 4) had a brief period of wound bleeding 12 days after transplantation at a time when the factor was near zero. At the cessation of graft isotope uptake the plasma antihemophilic factor disappeared in all four dogs (Fig. 4). In the retransplanted dog, the provision of a second graft was again followed by a significant plasma level of factor VIII (Fig. 4).
Fig. 4.
Plasma factor VIII in four hemophilic dogs that received whole organ splenic grafts. The solid line denotes that there was isotope uptake on the splenic scan. The dotted line indicates that isotope uptake was no longer recorded. Horizontal lines mark the 20 percent level.
On assay one week after transplantation, the previously prolonged clotting time was shortened to near normal. Several other clotting parameters studied were unaffected. The hematocrit and white blood cell count (WBC) had dropped moderately (Table I).
Table I.
Hematological findings before and one week after splenic or lymph node transplantation (mean ± S.E. in the groups)*
| Splenic transplantation | Lymph node transplantation | Normal range | |||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Whole blood clotting time (min.) | 38 ± 10 | 13 ± 8 | 77 ± 25 | 24 ± 4 | 5–10 |
| Prothrombin time (sec.) | 7.5 ± 0.3 | 8.1 ± 0.3 | 7.8 ± 0.2 | 8.2 ± 0.3 | 6–7 |
| Thrombin time (sec.) | 9.1 ± 0.1 | 9.2 ± 0.3 | 9.0 ± 0.4 | 9.6 ± 0.5 | 7–9 |
| Fibrinogen (mg./100 ml.) | 571 ± 50 | 524 ± 23 | 604 ± 31 | 554 ± 46 | 400–600 |
| Factor II (%) | 95 ± 18 | 93 ± 5 | 93 ± 11 | 87 ± 7 | 70–100 |
| Factor V (%) | 125 ± 15 | 97 ± 3 | 115 ± 5 | 118 ± 2 | 70–100 |
| Factor VII (%) | 120 ± 19 | 111 ± 11 | 88 ± 15 | 85 ± 13 | 70–150 |
| Platelets (1000/mm.3) | 248 ± 75 | 198 ± 10 | 218 ± 44 | 194 ± 80 | 200–400 |
| Euglobulin lysis time (min.) | 122 ± 15 | 58 ± 13 | 123 ± 17 | 104 ± 33 | 90–300 |
| Hematocrit (%) | 41 ± 2 | 29 ± 3 | 42 ± 3 | 33 ± 2 | — |
| White blood cell count (100/mm.3) | 21.8 ± 3.0 | 14.0 ± 3.7 | 22.4 ± 3.1 | 14.1 ± 3.4 | — |
The four original splenic grafts were re-moved three to seven days after the cessation of their isotope uptake. Histologically, they showed end-stage rejection. There was necrosis of most of the lymphocytes in the white pulp; only a few large lymphoid cells with pyroninophilic cytoplasm survived immediately adjacent to the penicillary arterioles. The red pulp was necrotic and hemorrhagic, and there was fibrinoid necrosis of the walls of most of the arteries and arterioles.
At recipient death, nine days after retransplantation, the second graft of one dog (No. 4) showed a normal white pulp with abundant germinal centers, and a red pulp that was congested and filled with mononuclear cells possessing pyroninophilic cytoplasm. The arteries and arterioles were normal.
Lymph node transplantation
Six hemophilic dogs received vascularized lymph node grafts. In one dog, a severely congested graft was removed four days after transplantation ; there was thrombosis at the venous anastomosis. Another recipient that had operations on the first and second postoperative day because of bleeding from the graft site did not recover from anesthesia after the latter procedure. The other four dogs had an uneventful early postoperative course. Only these latter dogs will be discussed further.
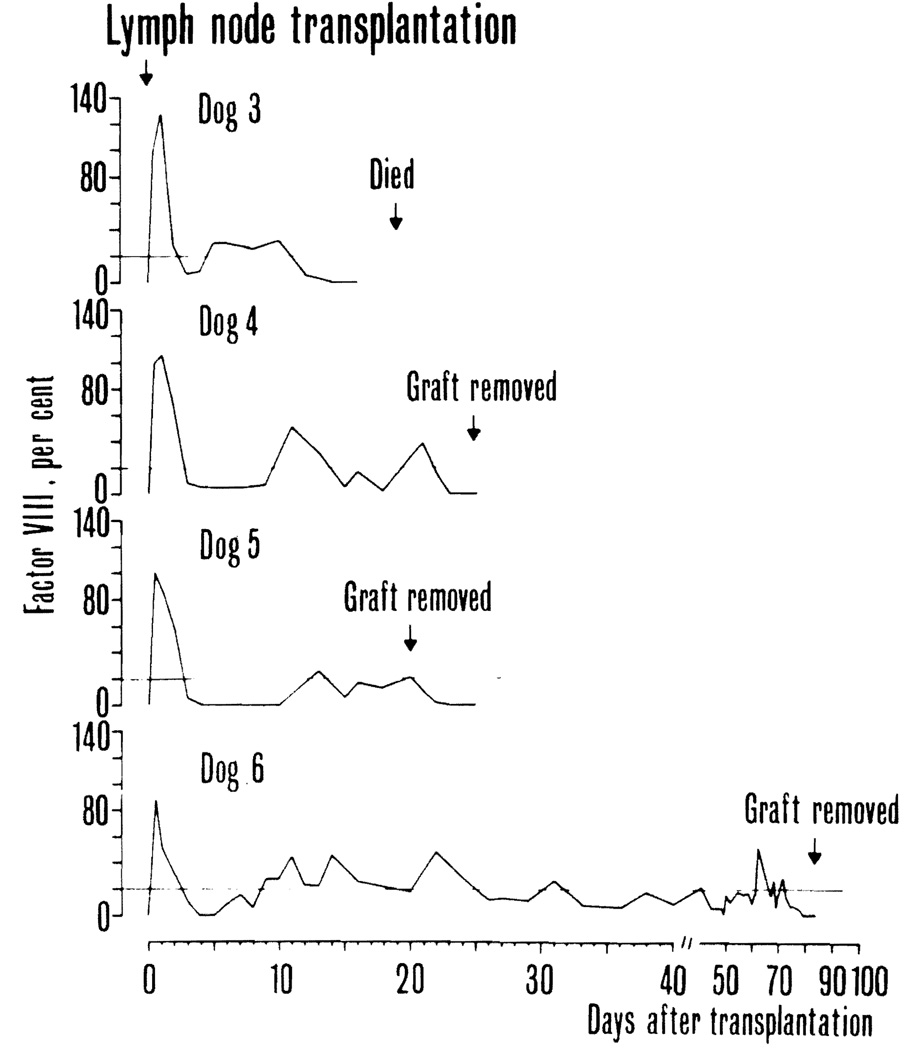
On the day of transplantation, plasma factor VIII in the donor dogs used in the four technically successful ·experiments was 62, 100, 110, and 102 percent, respectively. Prior to transplantation, the antihemophilic factor was less than one percent in all recipient dogs. After operation, an initial peak caused the plasma infusion was followed by lecline to levels around five percent except in one dog (No. 5) in which the level dropped to zero for several days (Fig. 5). At this time, there was a transient bleeding diathesis in this dog. Subsequently, factor VIII levels rose in every animal and then fluctuated between 10 and 40 percent. In one dog (No.5), the graft was surgically removed 21 days after transplantation; in the next few days, the factor declined to less than one percent. In the other three dogs, the factor level was maintained between 10 and 40 percent for one, two, and ten weeks, respectively (Fig. 5).
Fig. 5.
Plasma factor VIII in four hemophilic dogs that recovered after receipt of a vascularized lymph node graft. Horizontal lines mark the 20 percent level.
There was a significant shortening in the previously prolonged clotting time on assay one week after transplantation. The findings in other clotting parameters and in hematocrit and WBC were similar to those found in the spleen transplant group (Table I).
Graft tissue was obtained on two different occasions when the recipient had circulating factor VIII; it was obtained in one dog (No. 6) by an open biopsy performed ten days after transplantation and in another (No. 5) when the whole graft was deliberately removed on the twenty-first postoperative day. Histologically, rejection was not apparent in the 10 day graft, which showed a normal cortex with many germinal centers and a normal paracortical zone and medulla. In the 21 day graft, rejection was occurring. The cortex contained fewer lymphocytes than normal and only occasional germinal centers, and there were a few focal areas of hemorrhagic necrosis. The paracortical zone was expanded and filled with a mass of lymphoid cells, many of them large and with pyroninophilic cytoplasm. Mitoses were frequent in this zone. The medulla contained many plasma cells.
Three lymph node transplants were examined three to seven days after plasma antihemophilic factor had returned to less than one percent. In one instance, the tissue became available at animal death 19 days after transplantation; in the two others the grafts were surgically removed. All showed marked rejection. In the two surgical specimens, the cortex was depleted of all lymphocytes, there were a few groups of large lymphoid cells with pyroninophilic cytoplasm in the paracortical zone, and the medulla contained some plasma cells. In the autopsy specimen, rejection was less advanced and small areas remained in which there were some cortical lymphoid cells, a prominent paracortical zone of pyroninophilic lymphoid cells, and abundant plasma cells in the medulla. In the two surgical specimens, the reticular cells survived and were the main cells in the cortex.
DISCUSSION
In 1967, Webster and colleagues18 first reported on transplantation of the spleen in the hemophilic dog. Their animals were bred at the University of North Carolina, Chapel Hill, N. C. In this pioneer study, two recipient animals were found to have improved hemostasis and clotting tests for 52 and 72 hours after transplantation. More recently, the same investigators reported no sustained beneficial effect when studying similarly transplanted animals for weeks and months after operation.19 They concluded that the role of the spleen is limited to the release of stored factor VIII as opposed to synthesis. Similar results were obtained by McKee and associates12 and Marchioro and associates10, 11; both groups worked with dogs from the hemophilic beagle colony at Oklahoma State University, Stillwater, Okla. In McKee’s series,12 two spleen transplant recipients survived for seven and eight months, respectively; they displayed no postoperative rise in plasma factor VIII despite continuous and excellent isotope uptake by their grafts. Marchioro’s group11 reported on one dog in which the splenic graft remained viable as confirmed by biopsy for 93 days but with plasma antihemophilic factor levels never exceeding six percent.
In contrast to the findings of these investigators. Norman and associates14 and Sise and co-workers17 recorded significant levels of factor VIII in hemophilic canine recipients of splenic transplants which were also from the Oklahoma colony. This favorable finding was recorded in each of seven dogs and for a maximum length of 47 days after operation. Unfortunately, the use of normal human plasma as a reference in the factor VIII assay precluded a truly quantitative assessment of the degree of correction achieved in these experiments.
In the present study, therapeutic levels (exceeding 20 percent) of antihemophilic factor developed for at least three weeks in all recipients of splenic grafts, with the longest period being eight weeks. A close correlation between graft function and plasma factor VIII was indicated by the concomitant disappearance of radioisotope uptake in the graft and of the antihemophilic factor.
The reason for the discrepant findings with splenic transplantation in the hemophilic dog reported by different groups is obscure. The dogs used in the present study were from a different colony than those of the other investigators, but the sex-linked recessive pattern of our Guelph animals was the same. In our series, immunosuppression consisted of azathioprine in combination with antilymphocyte globulin while all earlier investigators used azathioprine and prednisone, but it seems unlikely that this difference affected the hematologic results.
The finding in the present study that the provision of a vascularized lymph node graft, as well as a splenic graft, was accompanied by a sustained increase in plasma factor VIII is evidence that lymphoreticuloendothelial tissue produces the factor. The initial lack of plasma antihemophilic factor in the lymph node recipients could be a reflection of a smaller storage capacity in this graft.
In 1971 Bouhasin and collaborators2 reported a rise in plasma factor VIII in a hemophilic child that developed lymphoblastic leukemia and postulated a role of the lymphocyte in antihemophilic globulin production. Recent investigations1, 8 indicate that factor VIII antigen can be localized to endothelial cells of blood vessels in many organs including lymph nodes and spleen. The lymphocyte could not be shown to have factor VIII antigens to any significant degree in these studies. In light of these new findings and the past publications, a unifying position might be that the degree of hematologic correction is proportional to the amount of tissue and/or blood vessels in grafts whether the grafts are primarily lymphatic (spleen, nodes) or not (liver). If this were true, it would not be surprising that the liver, spleen, and lymph node grafts were corrective in that order of effectiveness. Also, the finding of supernormal levels of factor VIII concomitant with very low levels of all other coagulation factors in a splenectomized liver transplant recipient with a failing graft indicates a site of synthesis of antihemophilic factor outside the liver and spleen.3
Of particular importance to our studies is the observations of Rickles and associates16 that the prolonged clotting time of factor VIII–deficient plasma can be corrected by a lymphocyte procoagulant material characterized as tissue factor and not factor VIII. These authors used a partial thromboplastin time (PTT) test to demonstrate their findings. Our factor VIII assays were all done by a thromboplastin generation time test (two-stage). Studies in our laboratory, which are being prepared in publication, indicate that the two-stage factor VIII assay is not altered by the presence of thrombin or tissue factor and, therefore, probably measures factor VIII clotting activity specifically. In contrast, the PTT type VIII assay is extremely sensitive to small amounts of tissue thromboplastin. These technical considerations must be noted in determination of factor VIII activity after tissue transplantation.
The results of the present study keep open the question of feasibility of tissue transplantation in patients with severe hemophilia. Previous attempts at splenic transplantation for various disease entities in man have all resulted in graft rejection within months,4, 7, 9 and the single attempt at transplantation for hemophilia was followed by graft rupture in seven days.7 Nonetheless, the improvements in management introduced into the field of transplantation over the last few years may warrant a trial in carefully selected cases.
Acknowledgments
The work was supported by research grants from the Denver Veterans Administration; by grants AI-AM-08898 and AM-07772 from the National Institutes of Health; and by grants RR-00051 and RR-00069 from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health.
Footnotes
The dogs were from the hemophilic colony developed by Dr. H. G. Downie of the Ontario Veterinary College, University of Guelph, Guelph. Ontario, by breeding which began in 1956. These dogs are not related to factor VIII–deficient dogs elsewhere in the United States or Canada. Pedigrees provided by Dr. F. Lotz of the Guelph Biomedical Science Department indicate a pattern of sex-linked inheritance identical to that seen in human classical hemophilia. The male dogs were hemizygous; the females were homozygous.
Contributor Information
Carl G. Groth, Department of Pathology, St. Mary’s Hospital and Medical School, London, England
William E. Hathaway, Department of Pathology, St. Mary’s Hospital and Medical School, London, England
Åke Gustafsson, Department of Pathology, St. Mary’s Hospital and Medical School, London, England
W. Peter Geis, Department of Pathology, St. Mary’s Hospital and Medical School, London, England
Charles W. Putnam, Department of Pathology, St. Mary’s Hospital and Medical School, London, England
Christer Björkén, Department of Pathology, St. Mary’s Hospital and Medical School, London, England.
K. A. Porter, Department of Pathology, St. Mary’s Hospital and Medical School, London, England
Thomas E. Starzl, Denver Veterans Administration Hospital, the Departments of Surgery and Pediatrics, Universary of Colorado School of Medicine, Denver, Colo.
REFERENCES
- 1.Bloom AL, Giddings JC, Wilks CJ. Factor VIII on the vascular intima: Possible importance in hemostasis and thrombosis. Nat. New Biol. 1973;241:217. doi: 10.1038/newbio241217a0. [DOI] [PubMed] [Google Scholar]
- 2.Bouhasin JD, Monteleone P, Altay C. Role of the lymphocyte in antihemophilic globulin production: A rise in antihemophilic globulin levels in a hemophilic subject with acute leukemia. J. Lab. Clin. Med. 1971;78:122. [PubMed] [Google Scholar]
- 3.Groth CG, Pechet L, StarzL TE. Coagulation during and after orthotopic transplantation of the human liver. Arch. Surg. 1968;98:31. doi: 10.1001/archsurg.1969.01340070049006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groth CG, Hagenfeldt L, Dreborg S, Löfström B, Öckerman PA, Samuelsson K, Svennerholm L, Werner B, Westberg G. Splenic transplantation in a case of Gaucher’s disease. Lancet. 1971;1:1260. doi: 10.1016/s0140-6736(71)91778-8. [DOI] [PubMed] [Google Scholar]
- 5.Groth CG, Kashiwagi N, Moore GE, Husberg B, Björkén C, Putnam CW, Starzl TE. Production of a standardized antilymphocyte globulin. In: Seiler FR, Schwick HG, editors. Behring Institute Mitteilungen. No. 51. Behringwerke AG: Marburg; 1972. pp. 86–89. (Behring Institute Workshop, Bad Soden [Ts], April 23–25, 1972) [PMC free article] [PubMed] [Google Scholar]
- 6.Hathaway WE, Belhasen LP, Hathaway HS. Evidence for a new plasma thromboplastin factor. I. Case report, coagulation studies, and physiochemical properties. Blood. 1965;26:521. [PubMed] [Google Scholar]
- 7.Hathaway WE, Mull MM, Githens JG, Groth CG, Marchioro TL, Starzl TE. Attempted spleen transplant in classical hemophilia. Transplantation. 1969;7:73. doi: 10.1097/00007890-196901000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoyer LW, de los Santos RP, Hoyer JR. Antihemophilic factor antigen: Localization in endothelial cells by immunofluorescent microscopy. J. Clin. Invest. 1973;52:2737. doi: 10.1172/JCI107469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchioro TL, Rowlands DT, Jr, Rifkind D, Waddell WR, Starzl TE, Fudenberg H. Splenic homotransplantation. Ann. N. Y. Acad. Sci. 1964;120:626. doi: 10.1111/j.1749-6632.1964.tb34757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchioro TL, Hougie C, Ragde H, Epstein RB, Thomas ED. Hemophilia: Role of organ homografts. Science. 1969;163:188. doi: 10.1126/science.163.3863.188. [DOI] [PubMed] [Google Scholar]
- 11.Marchioro TL, Hougie C, Ragde H, Epstein B, Thomas ED. Organ homografts for hemophilia. Transplant. Proc. 1969;1:316. [PubMed] [Google Scholar]
- 12.McKee PA, Coussons RT, Buckner RG, Williams GR, Hampton JW. Effects of the spleen on canine factor VIII levels. J. Lab. Clin. Med. 1970;75:391. [PubMed] [Google Scholar]
- 13.Niemetz J, Nossel HL. Activated coagulation factors: in vivo and in vitro studies. Br. J. Haematol. 1969;16:37. doi: 10.1111/j.1365-2141.1969.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 14.Norman JC, Covelli V, II, Sise HS. Transplantation of the spleen: Experimental cure of hemophilia. Surgery. 1968;64:1. [PubMed] [Google Scholar]
- 15.Pool JG, Robinson J. Assay of plasma antihaemophilic globulin (AHG) Br. J. Haematol. 1959;5:17. doi: 10.1111/j.1365-2141.1959.tb04009.x. [DOI] [PubMed] [Google Scholar]
- 16.Rickles FR, Hardin JA, Pittick FA, Hoyer LW, Conrad MD. Tissue factor activity in lymphocyte cultures from normal individuals and patients with hemophilia. Am. J. Clin. Invest. 1973;52:1427. doi: 10.1172/JCI107316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sise HJ, Joison J, Pegg CAS, Norman JC. Potential of the transplanted spleen in canine hemophilia. In: Brinkhous KM, editor. Hemophilia and new hemorrhagic states. Chapel Hill, N. C.: University of North Carolina Press; 1970. p. 106. [Google Scholar]
- 18.Webster WP, Penick GD, Peacock EE, Brinkhous KM. Allotransplantation of spleen in hemophilia. N. C. Med. J. 1967;28:505. [Google Scholar]
- 19.Webster WP, Zukoski CF, Hutchin P, Reddick RL, Mandel SR, Penick GD. Plasma factor VIII synthesis and control as revealed by canine organ transplantation. Am. J. Physiol. 1971;220:1147. doi: 10.1152/ajplegacy.1971.220.5.1147. [DOI] [PubMed] [Google Scholar]