Abstract
To study the cellular composition of human islet cell isolates for transplantation, formalin-fixed and paraffin-embedded cell pellets were stained by the immunoperoxidase method with a panel of antibodies characterising endocrine, epithelial, soft tissue and haematolymphoid components. Immediately after separation, the isolates contained 30–80% islet cells, differing mainly in the content of islet and acinar cells, whereas the soft tissue, ductal/ductular and haematolymphoid elements comprised a relatively constant 10–20%. After 1 week in culture the islet cell content of less highly purified isolates (30–40% islets) dropped dramatically to 5%. The highly purified isolates (70–80% islets) showed only a minimal change in cellular composition; however, approximately two-thirds of islet cells were degranulated and did not stain for insulin. Haematolymphoid components were still present in all cultured isolates. We conclude that primarily mechanical purification methods and short-term culture are not sufficient to eliminate highly immunogenic cells. In addition, short-term culture is deleterious to the isolate if a significant number of acinar cells is still present after enrichment.
Keywords: Islets of Langerhans, Transplantation, Cell isolation, Immunoperoxidase techniques
Introduction
Transplantation of pancreatic islet cells has been performed experimentally in animals and in limited clinical trials in humans for more than a decade [1]. Recent advances in isolation procedures and immunosuppression have led to human islet allotransplantation in patients receiving cluster organ transplants with good survival of functional islets for more than 6 months [2]. Rejection of the transplanted islet cells, however, remains a confounding problem, and precludes the use of islet cell allografts in not otherwise immunosuppressed hosts.
It has been shown that islet cells lack antigenicity if cell preparations are highly purified and devoid of stimulator cells which could initiate rejection [3]. Dendritic cells, which are present in the interstitium of almost all organs [4], are thought to be the primary stimulators of the rejection response [5]. Therefore, various in vitro methods, including graft or donor treatment with antibody and complement, or irradiation and cytotoxic chemotherapy have been used to deplete these cells before transplantation, and thus reduce the immunogenicity of the graft [6–9].
We are not aware of any study that investigated in detail the constituents of human islet cell preparation used for transplantation in clinical trials. In this study we investigated the degree of islet cell purification by our preparation procedure, at the time of isolation and after tissue culture for 1 week. The composition of these enriched cell suspensions was compared to normal pancreas.
Materials and methods
Preparation of samples
Human islets obtained from six different multi-organ donors were separated and purified as previously described [2, 10, 11]. A portion of the suspension was fixed in buffered formaldehyde and cell pellets obtained by centrifugation at 800 g for 3 min. In addition, a small fraction of the suspensions was fixed in glutaraldehyde for examination by transmission electron microscopy. The pellets were prepared immediately after isolation and after 1 week in CMRL-1066 culture medium (30 °C, 5% CO2 in air) of the remaining suspension.
Immunoperoxidase staining
Recognition of cell types was enhanced with a panel of immunoperoxidase stains using the avidin-biotin-complex (ABC) method of Hsu et al. [12] as summarised in Table 1. Specifically, islet cells were identified by staining with insulin, glucagon and chromogranin antibodies, soft tissue components by staining with actin (blood vessel walls, myoepithelial cells), vimentin (connective tissue) and factor VIII (endothelial cells) antibodies. Leucocytes were differentiated by staining with L60 (T-cells, macrophages), L26 (B-cells), MAC-387 and Lysozyme (macrophages) antibodies; dendritic cells were sought by means of S100 and HLA-DR antibodies. Ductal elements were highlighted by staining with AE-I and AE-3 antibodies. Staining with UCHL1 for better identification of T-cell lineage was attempted but was uninterpretable in any of the preparations. Normal non-disrupted pancreas served as a positive control and deletion or the primary antibody was used to detect non-specific staining.
Table 1.
Antibodies used for phenotypic analysis of cell populations in normal pancreas and islet cell suspensions
| Antibody | Dilution | Specificity |
|---|---|---|
| Insulina | 1 : 1067 | Islet cells (B) |
| Glucagona | Prediluted | Islet cells (A) |
| Chromogranina | 1 : 320 | Neuroendocrine cells |
| AE-lb | 1 : 1500 | Low molecular weight keratins |
| AE-3b | 1 : 1500 | Intermediate keratins |
| Vimentinc | 1 : 600 | Intermediate filaments |
| Actina | Prediluted | Smooth muscle cells, pericytes and myoepithelial cells |
| Factor VIIIR-Agd | 1 : 300 | Endothelial cells |
| S100d | 1 : 1000 | Neural and glial cells, melanocytes, myoepithelial cells, chondrocytes, fat cells. Langerhans cells, dendritic cell Subtype and some macrophages |
| LN3 (HLA-DR)e | 1 : 5 | B-cells, monocytes, macrophages, dendritic cells |
| MAC-387 d | 1 : 100 | Macrophages, granulocytes |
| Lysozymed | 1 : 500 | Myeloid cells, histiocytic cells, secretory epithelial cells |
| UCHL1 (CD45R)d | 1 : 10 | Most thymocytes and activated T-cells, “resting” T-cell subtype, macrophages, granulocytes |
| LCA (CD45)d | 1 : 50 | White blood cells |
| Leu-22 (CD43, L60)f | Prediluted | Most T-cells, macrophages, granulocytes |
| L26 (CD20)d | 1 : 200 | Pan B-cell |
Biogenex, San Ramon, Calif.
Boehringer Mannheim, Indianapolis, Ind.
Sigma, St. Louis, Mo.
Dako, Santa Barbara, Calif.
Biotest Diagnostics, Denville, N.J.
Becton Dickinson, Mountain View, Calif.
Electron microscopy
The 2% glutaraldehyde fixed cell pellets were post-fixed in 1% osmium tetroxide, dehydrated in a graded series of alcohols, and embedded in Epon-Araldite resin. The thin sections were cut at 75 nm, collected on 200 mesh copper grids and stained with 4% uranyl acetate followed by lead citrate. The sections were examined with a Philips EM 300 transmission electron microscope.
Experimental design
Islet cell isolation and culture
Freshly isolated human islets and those kept in culture for 1 week were pelleted by centrifugation, fixed in neutral buffered formalin and embedded in paraffin for light microscopy or fixed in glutaraldehyde for ultrastructural studies.
Immunohistochemical studies
A panel of monoclonal antibodies was used to enhance the recognition of cell type within the preparation (Table 1). An avidin-biotin-complex method was used on either formalin fixed or frozen tissue, depending on the antibody used. Normal pancreas and tonsils were used as positive controls and omission of the primary antibody from the procedure on the pellet sections were the negative controls. The slides were reviewed by two pathologists (CS and AJD) who subjectively estimated the relative percentage of the different cell types and staining intensity.
Results
Normal pancreatic tissue
As previously described, normal adult pancreas contained approximately 1 – 2% islet cells [8]. Small lymphoid aggregates and lymph nodes were regularly found in the peripancreatic fat and rarely within the parenchyma. In addition, scattered lymphoid cells primarily of T-cell type, a small number of mainly interstitial macrophages (Mac 387+) and HLA-DR positive dendritic-shaped cells were observed, similar to the observations of Hart and Fabre in rat pancreas [3]. Capillary endothelial cells were also regularly HLA-DR positive. Islets showed positive staining for insulin in 70–80% of the cells and 10–20% positivity for glucagon. Chromogranin was positive in about 95% of islet cells but was consistently weaker in intensity than the stains for specific hormones.
Islet cell preparations immediately after purification
The enriched cell suspensions showed a quantitatively different cellular composition, although all cell types present in normal pancreas were also observed in the cell suspensions. There was considerable variation in islet cell content of different preparations. In three samples, islets comprised approximately 30–40% of the cell suspension, based on positive staining for specific hormones and nonspecific neurosecretory granules (Fig. 1a). Insulin- and glucagon-producing cells were present in the same proportion as in normal islets. The remaining 60–70% of the cells were predominantly acini, often surrounded by a single layer of stromal cells. Occasional ducts and ductules with periductal non-parenchymal components were also observed. The other three preparations had an islet cell content of 80–90%, only occasional acinar clumps and roughly the same amount of ductal and soft tissue components (Fig. 1c). The staining characteristics of islets were similar to the preparations with fewer islets. In all preparations both islet and non-islet cells were mostly clustered in small to medium-sized cohesive cell clumps. Individual HLA-DR- and S100-positive cells with dendritic morphology were present in small lymphoid aggregates in all preparations (Fig. 1b). S100-positive spindle-shaped cells were observed around small ducts and occasionally within islets, which are interpreted as myoepithelial cells or Schwann cells. HLA-DR staining was also positive with intermediate intensity on capillaries, spindle cells near ducts and occasionally in small ductules with low staining intensity (summarised in Table 2).
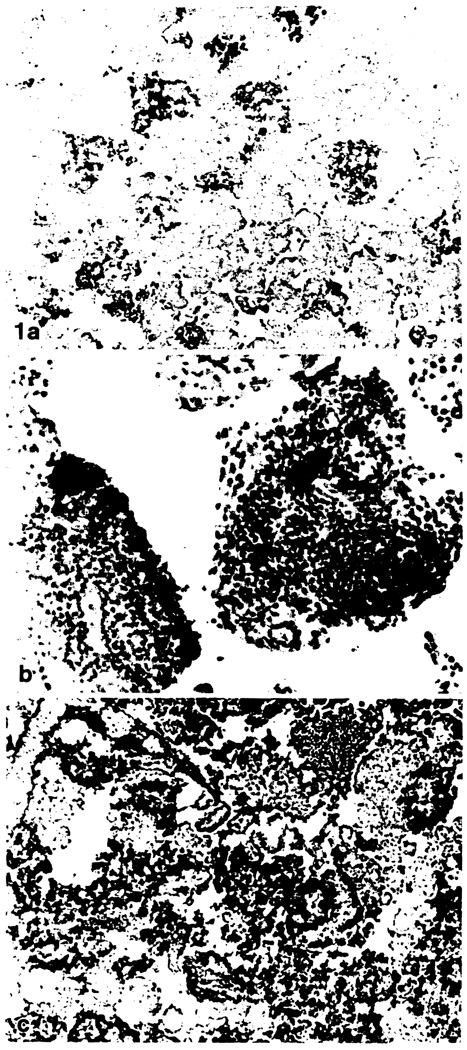
Fig. 1.
a–c. Islet cell isolates immediately after preparation (immunoperoxidase stains). a Moderately enriched isolate composed of 30% islets and acinar cells, stained for insulin. × 140. b Lymphoid aggregates stained for HLA-DR demonstrated dendritic cells in preparations of all degrees of purity. × 350. c Highly enriched isolate composed of 80% islets, stained for insulin. Also present are a segment of small blood vessel, few acinar cells, ribbons of ductal epithelium and a lymphoid aggregate in the top half of the picture. × 140
Table 2.
Composition of islet cell preparations before and after 1 week in culture (all percentages refer to estimated percentage of total cells)
| Cell type | Before culture | After culture | ||
|---|---|---|---|---|
| Higha | Lowa | Higha | Lowa | |
| Islet cells | 70–80% | 30–40% | 70–80% | 5% |
| Non-parenchymal cells | 10–20% | 10–20% | 10–20% | 5% |
| Ducts, ductules | Occasional ductal elements | Same as “high” | Same as pre-culture | Rare ductal elements |
| Acinar cells | 10% | 50–60% | 10% | 90% |
| Soft tissue | Scattered capillaries, venules with HLA-DR + endothelial cells, connective tissue around ductal elements | Same as “high” | Same as pre-culture | Scattered HLA-DR+single endothelial cells, marked loss of other soft tissue elements |
| Lymphoid cells | Few aggregates, few scattered single T-cells | Same as “high” | Same as pre-culture | Same as pre-culture |
| Macrophages, dendritic cells | Few MAC-387 + macrophages, occasional HLA-DR + and S100+ cells | Same as “high” | Same as pre-culture | Few MAC-387 + macrophages rare or absent dendritic cells |
Degree of purification: high, 70–80% islet cells; low, 30–40% islet cells
Ultrastructural examination confirmed the findings of routine light and immunoperoxidase studies. Acinar cells, most numerous in the moderately enriched preparations, were recognized by their abundant rough endoplasmic reticulum and zymogen granules. Islets with intact dense core neurosecretory granules were demonstrated in both moderately and highly enriched preparations (Fig. 2a). Some of the granules contained pleomorphic paracrystalline and rectangular cores, typical of insulin [13].
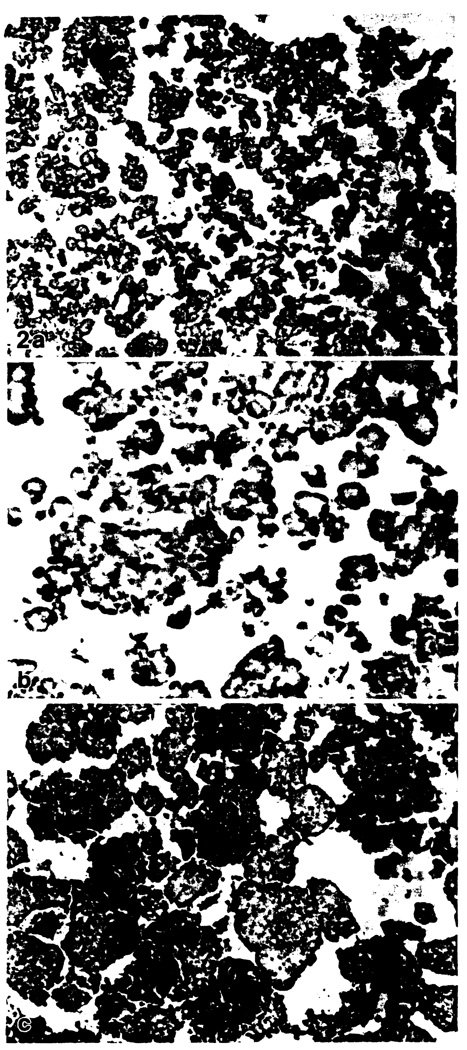
Fig. 2.
a–c. Islet cell isolates after one week culture (immunoperoxidase stains). a Moderately enriched isolate mostly composed of acinar cells and only few small residual clusters of islet cells, stained for insulin. × 140. b Residual islet cell cluster of initially moderately purified isolate with weak expression of MHC class II antigen, stained for HLA-DR. × 350. c Highly enriched isolate mostly composed of islet cell clusters, stained for insulin. Only one-third of the islets are stained, indicating degranulation during culture. × 140
Islet cell preparations after 1-week incubation
After 1 week of culture, all cells were much less tightly aggregated and cell clusters were of a more uniform, relatively decreased size. In the moderately enriched preparations there was a marked loss of islet cells (by morphology and neuroendocrine marker studies), which now constituted approximately 5% of all cells (Fig. 2a). Almost all remaining islet cells were beta-cells, as demonstrated by positive staining for insulin. Only rare glucagon-positive alpha-cells could be identified. Most of the non-islet cells were acinar cells by light and ultrastructural morphology. The amount of non-parenchymal tissue had declined in comparison with the preculture preparation. Small lymphoid aggregates were still present, HLA-DR-and/or S100-positive cells with dendritic morphology were either rare or entirely absent. In addition, a low-intensity HLA-DR positivity was also observed in some islet cell clusters (Fig. 2b). Because of the small number of islets, no ultrastructural examination could be performed. By contrast, the composition of highly purified isolates was changed very little by morphology after 1 week of culture (Fig. 2c). No staining for HLA-DR was observed in islets, although staining for insulin was positive in only 30% of islet cell clusters, indicating degranulation during culture. All other cell types showed only minor morphological changes but were not decreased.
Ultrastructurally, membrane-bound dense core granules could again be demonstrated, but mitochondrial swelling, dilatation of the rough endoplasmic reticulum, lipid vacuolisation and electron densities (? lysosomal bodies) indicative of islet cell degenerative changes were more prevalent than in pre-culture preparations (Fig. 3b).
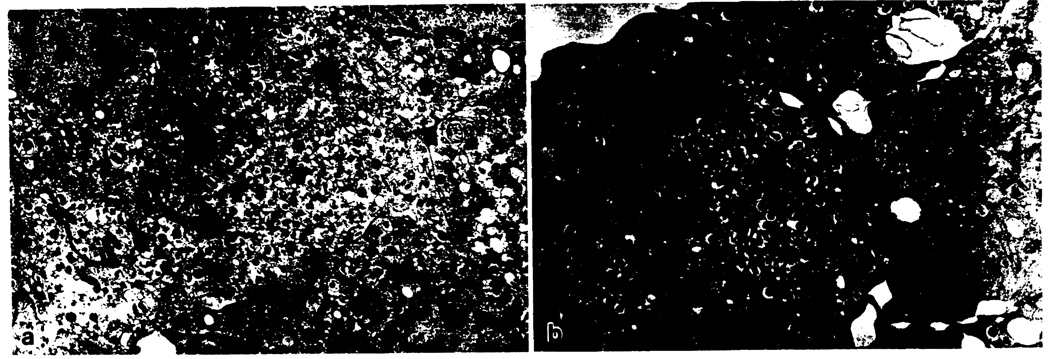
Fig. 3.
a–c. Transmission electron photoimicrographs from highly enriched isolates. a Islet cells immediately after purification containing abundant neurosecretory granules with pleomorphic paracrystalline inclusions characteristic of insulin. × 9100. b After 1 week in culture insulin granules are still present, in addition some mitochondrial swelling indicates mild degenerative change. × 11 060
Discussion
Our results demonstrate that, even in highly purified islet cell preparations, a significant percentage of acinar, nonparenchymal and ductal components, as well as cells of haematopoietic origin, remain in the cell suspension. This non-islet cell component can be as high as 70%. The invariably observed dendritic and lymphoid cells are of special concern, since these cells are increasingly viewed as central in the initiation of cellular rejection. These results indicate that reduction of immunogenicity of islet cell suspensions cannot be achieved solely by primarily mechanical purification procedures but requires additional measures.
One-week maintenance of the moderately enriched islet preparation in culture resulted in a significant decrease, but not elimination of, haematopoietic cells, which are thought to be of importance in initiation of the rejection response. However, any potential gain in reducing the immunogenicity compromised the number of islets, which dropped to as low as 5% after 1 week in culture. In addition, remaining islet cells weakly expressed HLA-DR antigen, which potentially increases islet cell immunogenicity. To date, similar phenomena have only been described in animals [14, 15], whereas human islets have not been found to express class II MHC [16]. The cause of the induced expression of HLA-DR on islet cells in our isolates is unclear. In our studies of in vitro cultures of human bile duct cells, we were able to determine that the fetal calf serum in the culture media was the inducing agent [17]. However, in highly purified preparations with 80% islet cells HLA-DR expression after culture with identical media was not observed. Possibly this phenomenon relates to severe damage to islet cells. It appears that the presence of acinar cells is particularly injurious to the islet cell isolates since the moderately and highly enriched preparations differed mainly in the content of acinar cells. Alternatively, low islet cell content could reflect harvesting injury occurring before the purification procedure and subsequent inferior survival of islet cells in culture because of the pre-existing damage.
In the clinical trials conducted to date, fresh isolates of islets with a similar range of purity were used for transplantation [2]. Whether the acinar tissue is capable of eliciting a potentially noxious non-specific inflammatory response because of enzyme release in humans is uncertain. However, we have seen such a response in syngeneic canine grafts, which simulated liver allograft rejection (unpublished observation).
In summary, the purity of human islet cell preparations used for cell transplantation in humans was investigated. Fresh isolates contained 30–80% islets with the remainder composed of acini, ducts, soft tissue elements and cells of haematopoietic origin, including interstitial dendritic cells. In vitro culture for 1 week resulted in a decrease in the populations which are potentially the most immunogenic. However, the week in culture also resulted in a significant decrease in the percentage of islets in the less purified isolates (as low as 5%), thereby compromising the functional component of the graft. These studies suggest that further advances in islet isolation methods are needed, as are more efficient means of depleting immunogenic cells.
Acknowledgements
Supported in part by Research Grant No. 1911421 from the Juvenile Diabetes Research International, New York. We would like to thank Ms. Sherri Moore for preparation of tissues from ultrastructural analysis. Ms. Beverly Gambrell for performance of the immunohistochemical studies and Mrs. Mary Ann Mient for editorial assistance.
References
- 1.Gray DWR, Morris PJ. Development in isolated pancreatic islet transplantation. Transplantation. 1987;43:311–331. doi: 10.1097/00007890-198703000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Tzakis AG, Ricordi C, Alejandro R, Zeng Y, Fung JJ, Todo S, Demetris AJ, Mintz DH, Starzl TE. Pancreatic islet cell transplantation after upper abdominal exenteration and liver replacement. Lancet. 1990;336:402–405. doi: 10.1016/0140-6736(90)91946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faustman DL, Steinman RM, Gebel HM, Hauptfeld V, Davie JM, Lacy PE. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci U.S.A. 1984;81:3864–3868. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart DNJ, Fabre JW. Demonstration and characterization of la-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981;154:347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Setum CM, Hegre OD, Serie JR, Moore MV. The potency of splenic dendritic cells as alloantigen presenters in vivo. Transplantation. 1990;49:1175–1177. doi: 10.1097/00007890-199006000-00031. [DOI] [PubMed] [Google Scholar]
- 6.McKenzie JL, Beard MEJ, Hart DNJ. Depletion of donor kidney dendritic cells prolongs graft survival. Transplant Proc. 1984;16:948–951. [PubMed] [Google Scholar]
- 7.Lloyd DM, Weiser MR, Kang DH, Budingham M, Stuart FP, Thistlethwarte JR. Does depletion of donor dendritic cells in an organ allograft lead to prolongation of graft survival on transplantation? Transplant Proc. 1989;21:482–483. [PubMed] [Google Scholar]
- 8.Dreger P, Steinmann J, Loffler H, Muller-Ruchholtz W. Depletion of accessory cells strongly reduces immunogenicity of T-depleted bone marrow and thus may facilitate its engraftment. Transplant Proc. 1989;21:2949–2951. [PubMed] [Google Scholar]
- 9.Hardy MA, Lau H, Weber C, Reemtsma K. Pancreatic islet transplantation. Induction of graft acceptance by ultraviolet irradiation of donor tissue. Ann Surg. 1984;200:441–450. doi: 10.1097/00000658-198410000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 11.Ricordi C, Alejandro R, Zeng K, Tzakis A, Casavilla A, Jaffe R, Mintz DH, Starzl TE. Human islet isolation and purification from pediatric-age donors. Transplant Proc. 1991;23:783–784. [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu SM, Raine L, Fanger H. The use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques. A comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 13.Kloppel G, Lenzen S. Anatomy and physiology of the endocrine pancreas. In: Kloppel G, Heitz PY, editors. Pancreatic pathology. New York: Churchill Livingstone; 1984. pp. 133–153. [Google Scholar]
- 14.Ulrichs K, Muller-Ruchholtz W. MHC class II antigen expression on the various cells of normal and activated isolated pancreatic islets. Diagn Immunol. 1985;3:47–55. [PubMed] [Google Scholar]
- 15.Yamada K, Miyajima E, Nohaka K. Inhibition of cytokine-induced MHC class II but not class I molecule expression on mouse islet cells by niacinamide an 3-aminobenzamide. Diabetes. 1990;39:1125–1130. doi: 10.2337/diab.39.9.1125. [DOI] [PubMed] [Google Scholar]
- 16.Shienvold FL, Alejandro R, Mintz DH. Identification of Ia-bearing cells in rat, dog, pig and human islets of Langerhans. Transplantation. 1986;41:364–372. doi: 10.1097/00007890-198603000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Saidman SL, Duquesnoy RJ, Zeevi A, Fung JJ, Starzl TE, Demetris AJ. Recognition of MHC antigens on cultured human biliary epithelial cells by alloreactive lymphocytes. Hepatology. (in press) [PMC free article] [PubMed] [Google Scholar]