Abstract
Physical exercise dampens an individual’s stress response and decreases symptoms of anxiety and depression disorders. While the extrinsic relationship of exercise and psychological state are established, their intrinsic relationship is unresolved. We investigated the potential intrinsic relationship of exercise with stress responsiveness using NIH rats bidirectionally selected for intrinsic endurance capacity. Selection resulted in two populations, one with high intrinsic endurance (high capacity runners; HCR) and one with low intrinsic endurance (low capacity runners; LCR). Animals from these populations were subjected to the elevated plus maze (EPM) and novel environment to assess levels of anxiety-like behavior, and to restraint stress to determine stress responsiveness. Pre-test plasma corticosterone levels and the response of plasma corticosterone to exposure to the EPM and restraint were analyzed using ELISA. A dexamethasone suppression test was performed to assess negative feedback tone of corticosterone release. Pre-test plasma corticosterone levels were similar between LCR and HCR, and these populations had similar behavioral and corticosterone responses to the EPM. Following restraint, HCR animals exhibited more anxiotypic behavior than LCR animals on the EPM, and exhibited an increase in plasma corticosterone following EPM and restraint that was not observed in LCR animals. HCR animals also exhibited more anxiotypic behavior in the novel environment compared to LCR animals. Plasma corticosterone levels were equally reduced in both populations following dexamethasone administration. Overall, our data suggest a positive genetic relationship between exercise endurance and stress responsiveness, which is at odds with the established extrinsic relationship of these traits.
Keywords: exercise endurance, stress, anxiety, selective breeding
Introduction
The ability of an organism to perform physical activity is a measure central to its survival. Every aspect of an organism’s life, whether it involves pursuing prey, evading predators or engaging in social activities requires some level of physical activity. This capacity to perform physical activity is often termed endurance capacity, and is determined by both genetic (innate or intrinsic) and environmental (trained or extrinsic) factors. As may be expected, multiple physiological and psychological systems influence an organism’s endurance capacity, and one of the primary contributors to this measure are the glucocorticoids (Nybo, 2003; Tharp, 1975).
Glucocorticoids (cortisol and corticosterone) are hormones produced by the hypothalamic-pituitary-adrenal (HPA) axis that strongly influence an organism’s ability to engage in prolonged physical activity (i.e. its endurance capacity) by promoting the release of energy stores (primarily glucose) to the body (Sellers et al., 1988; Tharp, 1975; Wilmore and Costill, 1994). The importance of these hormones in determining endurance capacity is demonstrated by the observations that adrenalectomy significantly decreases treadmill endurance scores in rats, and replacement of glucocorticoids restores levels of endurance (Sellers et al., 1988). By mobilizing energy stores, glucocorticoids enable an organism to physically respond to a stressor (Tharp, 1975). Complimenting this function, glucocorticoids also stimulate neural systems (e.g. hippocampus and amygdala) that facilitate both the awareness and memory of a stressor (Korte, 2001; Overton et al., 1991; Sauro et al., 2003). By influencing both the psychological and physiological aspects of a stress response, glucocorticoids allow for coordinated and appropriate responses to environmental stressors.
The relationship between exercise and glucocorticoids is reciprocal: performing physical exercise influences the glucocorticoid system. For example, rats that experience four weeks of wheel running express decreased light phase plasma ACTH (the primary secretogogue of glucocorticoids), and increased dark phase basal plasma corticosterone levels (Droste et al., 2003). Furthermore, exercising rats exhibit dampened ACTH and corticosterone responses to a novel environment (Droste et al., 2003) and restraint stress (Fediuc et al., 2006), while having more robust corticosterone responses to swim stress compared to sedentary controls. These results, which illustrate both the enhancing and detracting effects of exercise on stress response, demonstrate the complex nature of this relationship and suggest that exercise modulates not only the release of glucocorticoids from the HPA axis, but also the sensitivity of the elements within the HPA axis to stressors.
The relationship between glucocorticoids and stress response is also reflected in the observation that psychological disorders such as generalized anxiety and depression are commonly associated with dysfunctions of the HPA axis (O’Brien et al., 1993). These diseases involve inappropriate, often exaggerated responses of this system to environmental stressors (Young et al., 2004). Symptoms of these disorders, including an exaggerated stress responses, are often normalized following successful clinical treatment, which includes exercise therapy (O’Brien et al., 1993; Salmon, 2001). The HPA axis is thought to be one of the systems through which these benefits of physical exercise are mediated (Biddle et al., 2000; Fox, 1999; Salmon, 2001)
Data from human and animal studies suggest an inverse relationship between the extrinsic (i.e. environmentally influenced) factors of exercise endurance and stress responsiveness, where individuals with higher attained endurance capacity express dampened stress responses. These studies have examine exercise mediated changes in behavior (Binder et al., 2004; Breus and O’Conner, 1998; Brosse et al., 2002; Salmon, 2001), elements of the HPA axis (Cotman and Engesser-Cesar, 2002; Droste et al., 2003), central neurotransmitter systems (Dey, 1994; Dishman, 1996; Dishman et al., 2006; Greenwood and Fleshner, 2008) and molecular markers such as BDNF in the central nervous system (Adlard and Cotman, 2004; Cotman and Engesser-Cesar, 2002). The intrinsic, or innate, relationship of these traits has not been investigated as thoroughly however. A series of studies that investigates this innate relationship of exercise and stress response uses mice selectively bred for intrinsically high levels of motivation to (as opposed to ability to) exercise (Swallow et al., 1998a). Mice were selectively bred for an increased motivation to exercise in home cage running wheels (Swallow et al., 1998a), and exhibited positively correlated changes in VO2max (a measure of exercise endurance (Swallow et al., 1998b), as well as a more robust response to restraint stress (Waters et al., 2010). While these studies imply a positive relationship between intrinsic endurance and intrinsic stress responsiveness (i.e., mice that express intrinsically higher levels of exercise endurance also exhibit more robust responses to stress), some experiments using these selected mouse lines do not support these correlations (Rezende et al., 2005). At any rate, more direct evidence of a relationship between intrinsic endurance capacity and intrinsic stress response is called for.
To directly investigate the nature of an intrinsic relationship between an organism’s ability to exercise and its stress responsiveness, we use two populations of female rats that differ in their intrinsic levels of endurance capacity (one with high endurance capacity [HCR], and one with low endurance capacity [LCR];(Koch and Britton, 2001). The occurrence of disorders that involve an exaggerated stress response, such as generalized anxiety and depression, is more prevalent in women. Similarly, female laboratory animals exhibit more robust behavioral and physiological responses to stressors (reviewed in Palanza, 2001). To both facilitate the ability to observe stress responses in our subjects, as well as for the purpose of translating this research to the clinical field; we feel that the use of female subjects herein is appropriate and relevant.
We hypothesize an intrinsic (i.e. innate) relationship between endurance capacity and stress responsiveness that reflects the observed extrinsic (i.e. environmentally influenced) relationship between these traits. In other words, we predicted animals with higher innate levels of endurance capacity (HCR) to exhibit decreased stress responsiveness, less anxiotypic behaviors, and dampened corticosterone responses to stressors compared to individuals with innately low levels of endurance (LCR) (Fediuc et al., 2006; Rodgers et al., 1999). Finally, we predicted that HCR rats would exhibit greater pharmacological (dexamethasone) suppression of plasma corticosterone than LCR animals, indicating a higher level of HPA axis tone in the HCR animals (Cole et al., 2000).
Results
One HCR animal from Group A was removed from basal plasma corticosterone assessments on the basis that it was a statistical outlier (Grubs outlier test). Three animals from Group B (2 LCR, 1 HCR) were excluded from the restraint plus EPM behavior assessment due to a failure of the restraint protocol resulting from human error. Plasma corticosterone assessment from four animals was excluded due to insufficient blood collection: one animal following EPM trial (Group A: 1 LCR), two animals following restraint (Group B: 1 LCR, 1 HCR), and one animal following restraint + EPM (Group B: 1 LCR).
Basal Plasma Corticosterone Concentration
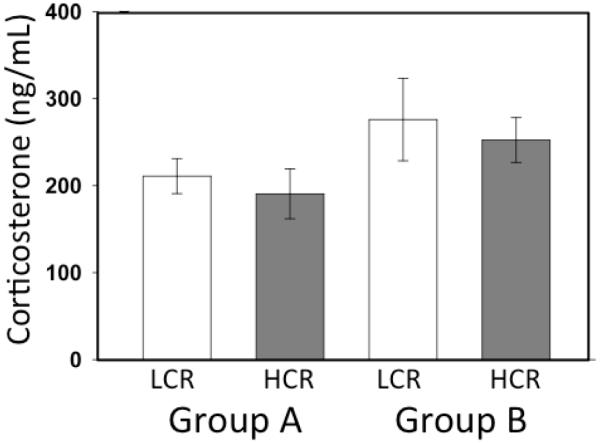
Basal levels of plasma corticosterone were similar between all cohorts of animals. Neither group designation (A or B) nor selected line (HCR or LCR) affected this measure (mean ± SE - LCR: 255.5±133.81 ng/ml v HCR: 220.1±96.03 ng/ml; F3,45= 1.164; P > 0.335; Fig. 1).
Figure 1.
Basal plasma corticosterone. Basal plasma corticosterone levels were not different between Groups A and B or between LCR (n=24) and HCR (n=23) animals.
Group A
Elevated Plus Maze
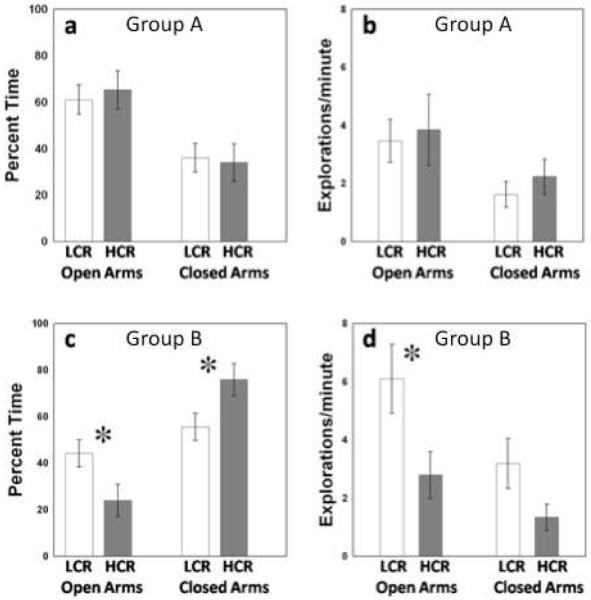
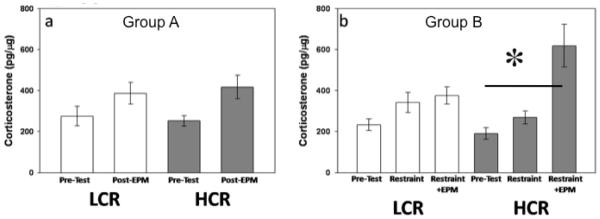
These populations of rats did not differ in their behavioral response to the EPM. Populations spent similar amounts of time in open arms (t22 = 0.305; P > 0.763; Fig. 2a) and closed arms (t22 = 0.305; P > 0.763; Fig. 2a), and exhibited similar numbers of entrances into open (t22 = 0.270; P > 0.790; Fig. 2b) and closed arms (t22 = 0.816; P > 0.423; Fig. 2b) of the maze. Time spent in the hub was minimal for all animals (5.3 + 11.95 seconds [mean ± SEM]). Elevated plus maze exposure did not elicit a plasma corticosterone response in these animals (F1,21= 0.269; P > 0.609; Fig. 3a).
Figure 2.
EPM Behavior. Within group A, LCR (n=12) and HCR (n=12) populations spent similar amounts of time in open and closed arms (a), and entered open and closed arms of the maze with similar frequency (b). Group B animals were exposed to restraint stress prior to EPM exposure. HCR (n=11) animals expressed higher levels of anxiety-like behavior, spending less time in open arms, more time in closed arms (c), and entering the open arms of the EPM fewer times than LCR (n=10) animals (d). [* - p < 0.05]
Figure 3.
Plasma corticosterone response to EPM and restraint. EPM exposure alone did not elicit a plasma corticosterone response in either population (LCR [n=11]; HCR [n=12]) (a). Following restraint stress, plasma corticosterone was not significantly increased in either population (LCR - n=11; HCR - n=11). Twenty-four hours later, HCR (n=11) animals exhibited a significant increase in plasma corticosterone following EPM exposure compared to basal levels and levels following restraint stress. LCR (n=9) animals do not show a significant corticosterone response in either condition. [* - p < 0.05]
Dexamethasone Suppression Test
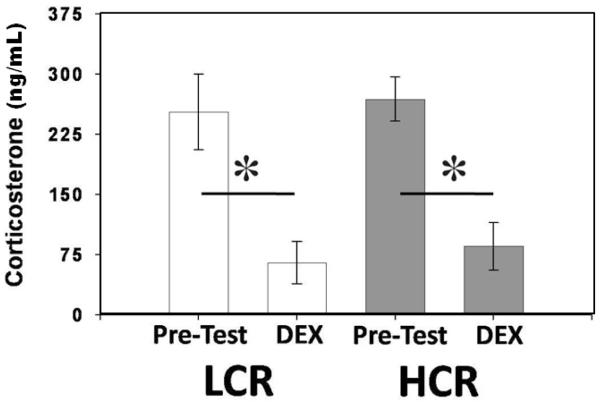
In both HCR and LCR animals, DEX administration suppressed plasma corticosterone concentrations (F1,20= 18.157; P < 0.001; Fig. 4). Suppression of plasma corticosterone concentration was equivalent between lines (F1,21= 0.831; P > 0.373; Fig. 4).
Figure 4.
Plasma corticosterone response to DEX. DEX administration successfully suppressed plasma corticosterone equally in both LCR (n=12) and HCR (n=12) animals. [* - p < 0.05]
Group B
Restraint Stress + Elevated Plus Maze
Following 1 h of restraint stress and a 24-hour recovery period HCR and LCR animals exhibited significant differences in their behavior on the EPM. Low capacity runners spent more time in the open arms (t19 = 2.222; P < 0.039; Fig. 2c), less time in the closed arms (t19 = 2.222; P < 0.039; Fig. 2c), and entered the open arms more often than HCR animals (t19 = 2.359; P < 0.029; Fig. 2d). Time spent in the hub was negligible (1.0 ± 2.36 seconds [mean ± SEM]). Selection for endurance also affected plasma corticosterone response to restraint + EPM (Line X Sample Time effect; F2,32 = 3.259; P < 0.05; Fig. 3b). Post-hoc analysis with Student-Newmann-Keuls revealed that EPM exposure following restraint elicited an increase in plasma corticosterone in HCR animals that was not exhibited by LCR animals.
Novel Environment
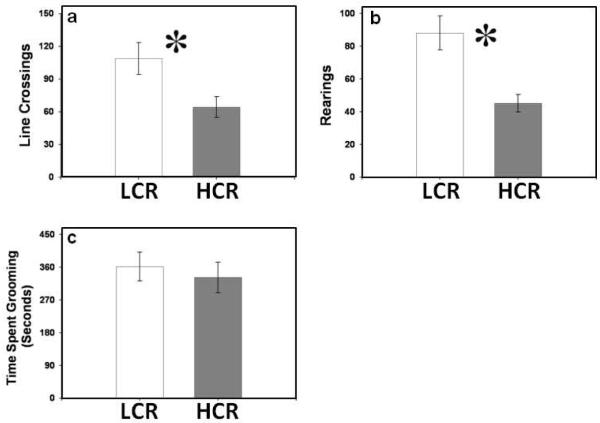
Low capacity runners exhibited higher levels of line crossing than HCR animals in the novel environment (t20= 5.302; P<0.032; Fig. 5a). Low capacity runners also reared more than HCR animals in this apparatus (t20=14.950; P < 0.001; Fig, 5b). Grooming time did not differ between HCR and LCR animals (t19= 0.232; P > 0.635; Fig, 5c).
Figure 5.
Behavior in the novel environment. (a) Low capacity runners (LCR; n=12) exhibit higher levels of locomotion than HCR (n=12) animals in the novel environment. (b) Low capacity runners (LCR) exhibit higher levels of rearing than HCR animals in the novel environment. (c) Low capacity runners (LCR) and HCR animals spend similar amounts of time grooming in the novel environment. [* - p < 0.05]
Discussion
Performing physical exercise increases endurance capacity, and dampens behavioral (Crews and Landers, 1987) and endocrine response (Korte, 2001; Sellers et al., 1988) to mild stressors. As well, a meta-analysis in humans demonstrates a negative relationship between psychosocial stress responsiveness and attained aerobic capacity (Crews and Landers, 1987). While the majority of these data have been gathered from studies using males, these trends are also observed in female animals (White-Welkley et al., 1995; White-Welkley et al., 1996) as well as in women (Loucks et al., 1989). These studies suggest an inverse relationship between stress response and exercise in both males and females, however the data presented herein suggest an apparent positive relationship between the intrinsic determinants of endurance capacity and stress responsiveness in female rats selectively bred for divergent endurance capacity (Koch and Britton, 2001). Specifically, these lines exhibited differences in their behavior in the novel environment, and differences in their behavior and corticosterone response to the EPM following restraint stress. These findings suggest that this selection protocol resulted in a divergence in the magnitude of the response of these animals to a novel environment and to restraint stress.
Animals from the HCR and LCR populations behaved similarly on the EPM and exhibited similar corticosterone responses to this environment. However, following restraint stress and 24 hours of recovery, animals selected for increased intrinsic endurance capacity (HCR animals) spent less time in the open arms of the EPM, and entered this open area less than animals selected for low intrinsic endurance capacity (LCR animals). The 24-hour recovery period was chosen to minimize the immediate effects of the stressor on behavior, while maximizing the psychological effects of the restraint (Kavushansky et al., 2009). Ultimately, our results suggest higher levels of anxiety-like behavior in HCR animals following restraint stress (Hogg, 1996), suggesting a greater persistent stress response in the HCR animals compared to the LCR animals. Corresponding with these behavioral differences, HCR animals also exhibit a significant increase in plasma corticosterone in the restraint+EPM condition that was not observed in LCR rats, and there was a trend for plasma corticosterone levels of HCR rats to be higher than LCR rats following restraint+EPM (P < 0.062). This divergence in the magnitude of the behavioral and hormonal responses to the EPM following restraint appears comparable to the changes achieved by direct selection for social anxiety or stress responsiveness in rats and mice (Landgraf et al., 1999). These include a divergence in anxiotypic behavior on the EPM and glucocorticoid responses to psychological stressors (Benus et al., 1991; Landgraf and Wigger, 2002; Veenema et al., 2003). The similarity of the behavioral and physiological changes induced by our selective breeding to those of selection programs aimed specifically at manipulating stress responsiveness support a correlation between the intrinsic determinants of endurance capacity and stress responsiveness.
The differences in anxiotypic behavior expressed by these animals are not limited to their performance on the EPM. The novel environment is an established measure of general anxiotypic behavior, and levels of locomotion, rearing and grooming in this paradigm can be used as indices of an anxiety-like state in rats (Courviosier et al., 1996). Locomotion and rearing are investigative behaviors, and high levels of these behaviors suggest a low anxious state in rodents (Courviosier et al., 1996). We observed lower levels of locomotion and rearing in HCR animals than in LCR animals, consistent with higher levels of anxiety-like behavior in HCR individuals compared to LCR animals. Interestingly, this difference in locomotion was not observed in behavior on the EPM, as indicated by similar numbers of closed arm entries by the two populations (figure 2b; Pellow et al., 1985). The observation that HCR animals responded more robustly to the novel environment than the (unstressed) EPM, in terms of decreased locomotion, suggests that the novel environment might serve as a better paradigm to detect basal differences in anxiety state than the EPM. To our knowledge these are the first data toward this point to be reported.
Dexamethasone (DEX) is a synthetic ligand that artificially suppresses endogenous glucocorticoid (i.e. corticosterone) secretion by binding to inhibitory glucocorticoid receptors throughout the HPA axis. Disorders that include exaggerated stress responsiveness as a symptom, such as anxiety and depression disorders are typically associated with a diminished suppressiblility of the HPA axis, a condition termed HPA non-suppression (Halbreich et al., 1989; O’Brien et al., 1993). Due to the relationship between stress response and HPA non-suppression, DEX administration is often used to determine if a dysfunction of negative feedback within the HPA axis is responsible for exaggerations of the stress response. We found that both LCR and HCR animals responded to DEX administration with normal suppression of plasma glucocorticoid concentrations, indicating that both of these populations possess similarly functional negative feedback regulation of HPA activity. We therefore conclude that any physiological differences contributing to the observed differences in corticosterone release and behavior between HCR and LCR groups do not include differences in negative feedback of the HPA system. Data are currently being analyzed that will examine possible differences in monoaminergic activity in select brain regions, including the bed nucleus of the stria terminalis, amygdala, and hippocampus. Monoamine neurotransmitter activity in these brain regions in is known to influence both behavioral responses to stress as well as HPA axis activity (Choi et al., 2007; Emerson et al., 2000; Lourenco Joca et al., 2007; Walker et al., 2003).
Herein, we suggest an intrinsic relationship between an organism’s exercise ability and stress responsiveness that is at odds with the direction of the established relationship between performing exercise and magnitude of stress response. Interestingly, previous data from our lab have demonstrated that HCR rats exhibit lower restraint stress-induced corticosterone responses than LCR rats following chronic physical exercise (however it is important to note that HCR females engaged in significantly more voluntary wheel running activity than LCR females; Waters et al., 2007). In the current study, no exercise outlet, such as a running wheel, was available to any rats, and HCR rats exhibited higher restraint stress-induced corticosterone responses than LCR rats (following a 24 hour recovery period and EPM). Together, these data suggest a complex interaction of the innate and training effects of exercise ability on stress responsiveness: animals with high intrinsic exercise ability (HCR) exhibit exaggerated responses to environmental stressors, but also experience an enhanced dampening of the stress response following exercise training, compared to LCR females. The intrinsic and extrinsic interactions of exercise with stress responsiveness seem to involve the HPA axis, and suggest the possibility of a heightened exercise induced training effect on this system when an organism expresses intrinsically hypersensitive responses to environmental stressors. To our knowledge, our data are the first to suggest this complex relationship.
In conclusion, our results indicate an intrinsic relationship between endurance capacity and stress responsiveness. This relationship is in a positive direction, and potentially involves the HPA axis, but also higher brain systems such as the hippocampus or extended amygdala. Future studies using these animals will examine monoamine neurotransmitter levels in these and other brain regions that are implicated in regulating HPA activity as well as central neural responses to stressors. These studies will aim to determine if there are inherent neurochemical differences between these populations that could account for the behavioral and hormonal differences that we observe between LCR and HCR animals.
Experimental Methods
Animals
Bi-directionally selected lines were generated from a founder population of 80 male and 88 female NIH stock rats (Hansen and Spuhler, 1984) based on intrinsic aerobic treadmill running capacity, as described in detail previously (Koch and Britton, 2001). Thirteen families of each line were set up for a within-family rotational breeding paradigm to maintain a heterogeneous genetic substrate within each selected line. The subset of animals used in this study averaged (mean ± S.E.) 1512.1±67.59m (HCR; n=24) and 393.2±53.70m (LCR; n=24) run to exhaustion.
The rats used in the current study were females maintained from LCR and HCR populations of rats from generations 16 (Group A; n=12 HCR, n=12 LCR) and 10 (Group B; n=12 HCR, n=12 LCR). Groups exhibited similar within line endurance scores. Animals were air shipped to the University of South Dakota (Vermillion, SD) animal facility at 196 days ± 3.1 and 145 days ±0.4 old for Groups A and B respectively. Rats were group housed (3-4 per cage) in clear plastic Nalge™ cages (43”× 27”× 15”) with cedar bedding and wire lids. Animals from Group A and Group B were not cross-housed. Teklad Rodent Diet (8604; Harland Laboratories, Madison, WI) and water were available ad libitum. Rats were maintained on a reverse 12:12 dark:light cycle (dark-08:00-20:00), and bedding, food and water were changed once weekly. Rats were given at least 60 days to acclimate to this environment before testing began. All procedures used in these studies were carried out according to the Guide for the Care and Use of Laboratory Animals, and were approved by the University of South Dakota Institutional Animal Care and Use Committee.
Starting at approximately 255 (255.4 ± 1.53; mean ± S.E.) days of age, all animals were subjected to a series of tests to assess stress responsiveness and anxiety-like behavior (Cole et al., 2000; Hogg, 1996; Korte, 2001). The tests administered were the elevated plus maze (EPM) and DEX administration (in stated order) for Group A rats, and the novel locomotion chamber and restraint stress plus EPM (in stated order) for Group B rats. Following all testing procedures, animals were placed in an individual holding cage for 20 minutes (min) prior to being returned to the home cage. All tests were conducted at least seven days apart to minimize interaction of the tests. Testing was carried out in the animal testing facility at the University of South Dakota between 10:00 h and 14:00 h (in the dark phase).
All tests were performed during the diestrous II phase of the estrus cycle. Cycle stage was determined by vaginal cytology. Vaginal lavage was performed on all animals between 0:800 and 0:900 h, once daily for at least five days prior to the scheduled testing day to establish a cycling pattern and acclimate rats to this treatment.
Animal Testing
Group A
Anxiety-like behavior in the Elevated Plus Maze
We used a standard elevated plus maze (Actimetrics™, Willmette, IL) made of opaque plastic, with a central hub (10 cm × 10 cm) and four arms (50 cm × 10 cm) extending out horizontally at 90° to each other, to assess differences in anxiety-like behaviors between HCR and LCR groups (Hogg 1996). Animals were given 5 min to explore the EPM; time spent in the open and closed arms, and the hub, as well as the numbers of entries into each arm were recorded via computer (Actimetrics ™ software). The order of testing the two populations of animals was counterbalanced to eliminate order effects, and the apparatus was thoroughly cleaned using 70% ethanol between tests. Following a 20 min recovery period in an individual cage, blood was collected for assessment of plasma corticosterone levels (Sakakura et al., 1976).
Dexamethasone Suppression Test
To assess negative feedback within the HPA axis, HCR and LCR rats were subcutaneously injected with 50 mg/kg dexamethasone (Sigma, St. Louis, MO), and placed back into their home cages for 90 min (Cole et al., 2000). As this was the final test performed, they were killed by rapid decapitation and trunk blood was collected for analysis of plasma corticosterone.
Group B
Responses to Novel Environment
The novel environment used in this test was an opaque black plastic chamber 52 cm × 33.5 cm and 30 cm deep. A wire lid was placed on top of the chamber during trials. The floor of the chamber was divided with white paint into a 2 × 3 grid. Rats were allowed 60 min to explore this environment and their behavior was video-recorded. The order of testing the two populations of animals was counterbalanced to eliminate order effects, and the apparatus was thoroughly cleaned using 70% ethanol between tests. A scorer, blind to the HCR and LCR designation, evaluated videotapes. Line crossings were recorded as a measure of locomotor activity. The time spent grooming and the number of rearings were also recorded (Courviosier et al., 1996).
Response to the Elevated Plus Maze following Restraint Stress
Animals were subjected to 1 h of restraint stress using an opaque plastic cylinder (6 cm diameter × 20 cm length) capped on one end with wire gauze, and the other with a rubber stopper. Rats were placed into individual cages for 20 min following restraint, after which blood samples were collected. To assess the animal’s ability to recover from this stressor, animals were placed on the EPM and allowed 10 minutes to explore the maze following a 24 hour recovery period. Behavior was scored via computer, recording time spent in the central hub, open arms and closed arms, as well as the number of entries into each arm. The order of testing the two populations of animals was counterbalanced to eliminate order effects, and the apparatus was thoroughly cleaned using 70% ethanol between tests. Rats were placed into individual cages for 20 min following the EPM exposure and blood samples were collected. Post-restraint and post-EPM blood samples were assessed for plasma corticosterone levels.
Plasma Corticosterone Collection and Analysis
Animals were restrained, supine on a platform and a heparinized 28.5 G needle and 1mL syringe was used to draw approximately 0.5ml of blood from the jugular vein; the entire handling process took less than 90 seconds. Following blood collection, samples were expelled into heparinized 1.5 ml eppendorf tubes and centrifuged. Plasma was drawn off and stored at −80°C until analysis.
Plasma corticosterone was measured using a corticosterone enzyme-linked immunoassay kit, following instructions from the manufacturer (R&D Systems, Minneapolis, MN, USA). Assay procedure was performed according to the directions included with the kit. The assay plate was placed into an automated microplate reader (Bio-Tek Instruments, Winooska, VT, USA), and detection of plasma corticosterone was performed by measuring the absorbance of samples at 405 nm (wavelength correction set at 595 nm) with automated plate reader software (KinetiCalc Jr., Bio-Tek Instruments, Winooska, VT, USA). From the absorbance values obtained from samples, we calculated maximum binding percentage (average 14.8%) and non-specific binding percentage (average 5.2%), both of which were within the manufacturer’s range. The detection limit sensitivity of this assay was 27.0 pg/ml. Corticosterone levels obtained from this assay were expressed as ng corticosterone/ml plasma.
Statistical Analyses
Pre-test plasma corticosterone levels were compared using a two way ANOVA (between subject factors - Group X Line). For Group A, behavior of LCR and HCR animals on the EPM was compared using one-way ANOVA. Pre-test plasma corticosterone from LCR and HCR rats was compared to levels following EPM and DEX administration using two-way repeated measures ANOVA (rmANOVA; Line X Sample Time [repeated factor]). Equality of variance was verified for repeated measures data sets using Box’s test of Equality of Covariance Matices (SPSS 18.0).
For Group B, behavior of LCR and HCR animals on the EPM following restraint, and in the novel environment was compared using a one-way ANOVA. Pre-test plasma corticosterone levels were compared to levels following restraint and restraint + EPM, using two-way rmANOVA (Line X Sample Time [repeated factor]).
Upon finding significance with two-by-two ANOVA, t-tests were used to determine differences between groups. Upon significant differences with rmANOVA analysis of plasma corticosterone concentration, one-way ANOVA was performed to determine differences between sampling time and line. In the analysis of Group B animals that compared pre-test plasma corticosterone levels to those following restraint and restraint + EPM, a Student-Newman-Keuls posthoc test was run to determine within line effects of sampling time on plasma corticosterone level. Significance was set at α=0.05 for all tests.
Acknowledgements
Special thanks go to Ms. Brittany Seiler and Ms. Jamie Scholl for technical assistance with the completion of this project. We would also like to thank Dr. Yuhlong Lio and Dr. Mark Dixon for their immense help with statistical analysis. This project was supported by grants from NSF (IOB-0448060), NSF EPSCoR in South Dakota (0091948), the USD JF and MP Nelson Endowment to JGS, the Center of Biomedical Research Excellence (COBRE – NIH P20 RR15567) to JGS, RPW, and KJR, and by NIH grant HL6427, and Grant Number RR17718 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) to LGK and SLB. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985–992. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Benus RF, Bohus BG, Koolhaas JM, van Oortmerssen GA. Heritable variation for aggression as a reflection of individual coping strategies. Cellular and Molecular Life Sciences (CMLS) 1991;47:1008–1019. doi: 10.1007/BF01923336. [DOI] [PubMed] [Google Scholar]
- Biddle SJH, Fox KR, Boutcher KR. Physical Activity and Psychological Well-Being. Routledge; NY: 2000. Emotion, mood and physical activity; pp. 63–117. NY. [Google Scholar]
- Binder E, Droste SK, Ohl F, Reul J. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behavioural Brain Research. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Breus ME, O’Conner PJ. Exercise-induced anxiolysis: A test of the “time-out” hypothesis in high anxious females. Medicine and Science in Sports and Exercise. 1998;30:1107–1112. doi: 10.1097/00005768-199807000-00013. [DOI] [PubMed] [Google Scholar]
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the Treatment of Clinical Depression in Adults. Sports Medicine. 2002;32:741–760. doi: 10.2165/00007256-200232120-00001. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–34. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MA, Kim PJ, Kalman BA, Spencer RL. Dexamethasone suppression of corticosteroid secretion: evaluation of the site of action by receptor measures and functional studies. Psychoneuroendocrinology. 2000;25:151–167. doi: 10.1016/s0306-4530(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exercise and Sports Science Reviews. 2002;30:75–79. doi: 10.1097/00003677-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Courviosier H, Moisan MP, Sarrieao A, Hendsley ED, de Morme P. Behavioral and neuroendocrine reactivity to stress in the WKHA/WKY inbred rat strains: a multifactorial and genetic analysis. Brain Research. 1996;743:77–85. doi: 10.1016/s0006-8993(96)01023-2. [DOI] [PubMed] [Google Scholar]
- Crews DJ, Landers DM. A meta-analytic review of aerobic fitness and reactivity to psychosocial stressors. Medicine and Science in Sports and Exercise. 1987;19:s114–s120. [PubMed] [Google Scholar]
- Dey S. Physical exercise as a novel antidepressant agent: Possible role of serotonin receptor subtypes. Physiology & Behavior. 1994;55:323–329. doi: 10.1016/0031-9384(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Medicine and Science in Sports and Exercise. 1996;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–56. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Muller MB, Lindthorst ACE, Reul J. Effects of Long-Term Voluntary Exercise on the Mouse Hypothalamic-Pituitary-Adrenocortical Axis. Endocrinology. 2003;144:3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Emerson AJ, Kappenman DP, Ronan PJ, Renner KJ, Summers CH. Stress induces rapid changes in serotonergic activity: restraint and exertion. Behavioural Brain Research. 2000;111:83–92. doi: 10.1016/s0166-4328(00)00143-1. [DOI] [PubMed] [Google Scholar]
- Fediuc S, Campbell JE, Riddell MC. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Spraque-Dawley rats. Journal of Applied Physiology. 2006;100:1867–1875. doi: 10.1152/japplphysiol.01416.2005. [DOI] [PubMed] [Google Scholar]
- Fox KR. The influence of physical activity on mental well-being. Public Health Nutrition. 1999;2:411–418. doi: 10.1017/s1368980099000567. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. NeuroMolecular Medicine. 2008;10:81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Olympia J, Carson S, Glogowski J, Ching-Ming Y, Axelrod S, Desu MM. Hypothalamo-pituitary-adrenal activity in endogenously depressed post-traumatic stress disorder patients. Psychoneuroendocrinology. 1989;14:365–370. doi: 10.1016/0306-4530(89)90006-1. [DOI] [PubMed] [Google Scholar]
- Hansen C, Spuhler K. Development of the National Institutes of Health genetically heterogeneous rat stock. Alcoholism, clinical and experimental research. 1984;8:477. doi: 10.1111/j.1530-0277.1984.tb05706.x. [DOI] [PubMed] [Google Scholar]
- Hogg S. A Review of the Validity and Variability of the Elevated Plus-Maze as an Animal Modal of Anxiety. Pharmacology Biochemistry and Behavior. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Kavushansky A, Ben-Shachar D, Richter-Levin G, Klein E. Physical stress differs from psychosocial stress in the pattern and time-course of behavioral responses, serum corticosterone and expression of plasticity-related genes in the rat. Stress. 2009;12:412–25. doi: 10.1080/10253890802556081. [DOI] [PubMed] [Google Scholar]
- Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiological Genomics. 2001;5:45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neuroscience and Biobehavioral Reviews. 2001;25:117–142. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Wigger A, Holsboer F, Neumann ID. Hyper-Reactive Hypothalamo-Pituitary-Adrenocortical Axis in Rats Bred for High Anxiety-Related Behaviour. Journal of Neuroendocrinology. 1999;11:405–407. doi: 10.1046/j.1365-2826.1999.00342.x. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Wigger A. High vs Low Anxiety-Related Behavior Rats: An Animal Model of Extremes in Trait Anxiety. Behavior Genetics. 2002;32:301–314. doi: 10.1023/a:1020258104318. [DOI] [PubMed] [Google Scholar]
- Loucks A, Mortola J, Girton L, Yen S. Alterations in the hypothalamic-pituitary-ovarian and the hypothalamic-pituitary-adrenal axes in athletic women. Journal of Clinical Endocrinology & Metabolism. 1989;68:402. doi: 10.1210/jcem-68-2-402. [DOI] [PubMed] [Google Scholar]
- Lourenco Joca S, Ferreira F, Guimaraes F. Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress: The International Journal on the Biology of Stress. 2007;10:227–249. doi: 10.1080/10253890701223130. [DOI] [PubMed] [Google Scholar]
- Nybo L. CNS Fatigue and Prolonged Exercise: Effect of Glucose Supplementation. Medicine and Science in Sports and Exercise. 2003;35:589–594. doi: 10.1249/01.MSS.0000058433.85789.66. [DOI] [PubMed] [Google Scholar]
- O’Brien JT, Ames D, Schweitzer I. HPA Axis function in depression and dementia: A review. International Journal of Geriatric Psychiatry. 1993;8:887–898. [Google Scholar]
- Overton JM, Kregen KC, Davis-Gorman G, Seals DR, Tipton CM, Fisher LA. Effects of exercise training in responses to central injection of CRF and noise stress. Physiology & Behavior. 1991;49:93–98. doi: 10.1016/0031-9384(91)90237-i. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neuroscience & Biobehavioral Reviews. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SF, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. Journal of Neuroscience Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Rezende EL, Chappell MA, Gomes FR, Malisch JL, Garland T., Jr. Maximal metabolic rates during voluntary exercise, forced exercise, and cold exposure in house mice selectively bred for high wheel-running. J Exp Biol. 2005;208:2447–58. doi: 10.1242/jeb.01631. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Haller J, Holmes A, Halasz J, Walton TJ, Brain PF. Corticosterone response to the plus-maze: High correlation with risk assessment in rats and mice. Physiology & Behavior. 1999;68:47–53. doi: 10.1016/s0031-9384(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Sakakura M, Saito Y, Takebe K, Yamashita I, Ishii K. Time course of hypothalamic CRF activity after the administration of two different stresses. Endocrinol Jpn. 1976;23:413–6. doi: 10.1507/endocrj1954.23.413. [DOI] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: A unifying theory. Clinical Psychology Review. 2001;21:33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- Sauro MD, Jorgensen RS, Pedlow CT. Stress, Glucocorticoids, and Memory: A Meta-analytic Review. Stress. 2003;6:235–245. doi: 10.1080/10253890310001616482. [DOI] [PubMed] [Google Scholar]
- Sellers TL, Jaussi AW, Yang HT, Heninger RW, Winder WW. Effect of the exercise-induced increase in glucocorticoids on endurance in the rat. Journal of Applied Physiology. 1988;65:173–178. doi: 10.1152/jappl.1988.65.1.173. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Carter PA, Garland T., Jr Artificial selection for increased wheel-running behavior in house mice. Behavior Genetics. 1998a;28:227–237. doi: 10.1023/a:1021479331779. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Garland T, Jr, Carter PA, Zhan W, Sieck GC. Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus) Journal of Applied Physiology. 1998b;84:69–76. doi: 10.1152/jappl.1998.84.1.69. [DOI] [PubMed] [Google Scholar]
- Tharp GD. The role of glucocorticoids in exercise. Medicine and Science in Sports and Exercise. 1975;7:6–11. [PubMed] [Google Scholar]
- Veenema AH, Meijer OC, De Kloet ER, Koolhaas JM, Bohus BG. Differences in basal and stress-induced HPA regulation of wild house mice selected for high and low aggression. Hormones and Behavior. 2003;43:197–204. doi: 10.1016/s0018-506x(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Waters R, Pringle R, Summers C, Renner K, Watt M, Forster G, Britton S, Koch L, Swallow J. Society for Neuroscience. 2007. Selection for endurance influences behavior, central monoamine and endocrine responses. [Google Scholar]
- Waters R, Pringle R, Forster G, Renner KJ, Malisch JL, Garland T, Jr, Swallow JG. Effects of selection for increased voluntary wheel running on behavioral activity, plasma corticosterone and brain monoamines in mice. Journal of Applied Physiology. 2010 In Review. [Google Scholar]
- White-Welkley JE, Bunnell BN, Mougey EH, Meyerhoff JL, Dishman RK. Treadmill Exercise Training and Estradiol Differentially Modulate Hypothalamic-Pituitary-Adrenal Cortical Responses to Acute Running and Immobilization. Physiology & Behavior. 1995;57:533–540. doi: 10.1016/0031-9384(94)00348-9. [DOI] [PubMed] [Google Scholar]
- White-Welkley JE, Warren GL, Bunnell BN, Mougey EH, Meyerhoff JL, Dishman RK. Treadmill exercise training and estradiol increase plasma ACTH and prolactin after novel footshock. Journal of Applied Physiology. 1996;80:931–939. doi: 10.1152/jappl.1996.80.3.931. [DOI] [PubMed] [Google Scholar]
- Wilmore JH, Costill DL. Physiology of sport and exercise. Human Kinetics; Champaign, IL: 1994. [Google Scholar]
- Young EA, Abelson JL, Cameron OG. Effect of comorbid anxiety disorders on the Hypothalamic-Pituitary-Adrenal axis response to a social stressor in major depression. Biological Psychiatry. 2004;56:113–120. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]