Abstract
Purpose
We previously showed that overexpression of epidermal growth factor receptor (EGFR) is associated with malignant grade in childhood glioma. The objective of this study was to determine whether protein expression of EGFR or platelet-derived growth factor receptor (PDGFR) and their active signaling pathways are related to malignant histology, progression of disease, and worse survival.
Experimental Design
Tissue microarrays were prepared from untreated tumors from 85 new glioma patients [22 high-grade gliomas (HGG) and 63 low-grade gliomas (LGG)] diagnosed at this institution from1989 to 2004. Immunohistochemistry was used to assess total expression of EGFR, PDGFRβ, and PTEN and expression of phosphorylated EGFR, phosphorylated PDGFRα (p-PDGFRα), phosphorylated AKT, phosphorylated mitogen-activated protein kinase, and phosphorylated mammalian target of rapamycin. These results were correlated with clinicopathologic data, including extent of initial tumor resection, evidence of dissemination, tumor grade, proliferation index, and survival, as well as with Affymetrix gene expression profiles previously obtained from a subset of these tumors.
Results
High expression of p-PDGFRα, EGFR, PDGFRβ, and phosphorylated EGFR was seen in 85.7%, 80.0%, 78.9%, and 47.4% of HGG and 40.0%, 87.1%, 41.7%, and 30.6% of LGG, respectively. However, high expression of p-PDGFRα and PDGFRβ was the only significant association with malignant histology (P = 0.031and 0.005, respectively); only the loss of PTEN expression was associated with worse overall survival. None of these targets, either alone or in combination, was significantly associated with progression-free survival in either LGG or HGG.
Conclusions
High PDGFR protein expression is significantly associated with malignant histology in pediatric gliomas, but it does not represent an independent prognostic factor. Deficient PTEN expression is associated with worse overall survival in HGG.
Gliomas are the most common central nervous system tumors in childhood, accounting for over 1,000 new cases each year in the United States. Approximately 30% of these are classified as high-grade malignant tumors (WHO grade 3 or 4; refs. 1, 2). Although recent therapeutic advances have been reported for the majority of pediatric cancers, treatment options for malignant childhood gliomas remain limited and prognosis continues to be dismal (3, 4).
Substantial evidence supports that malignant glial transformation results from overexpression of growth factor receptors with activation of downstream signaling pathways that control cell proliferation, differentiation, invasion, and cell death (5–11). The receptors for epidermal growth factor (EGFR) and platelet-derived growth factor (PDGFR) are closely linked to malignant glioma progression and have thus served as the mainstay for molecular-targeted therapies in adult patients (12–15). Glioblastomas in adults arise either de novo (primary) or by secondary progression from a lower histologic grade. The molecular profile differs greatly between these two types: >70% of primary adult glioblastomas overexpress EGFR and 30% to 40% show amplification of EGFR, whereas EGFR over-expression or amplification in secondary adult glioblastoma is rare (16–18).
PDGFRα overexpression has been reported in ~60% of secondary adult glioblastomas, with amplification noted in a small subset (<10%; refs. 1, 18). Varela and colleagues (19) noted high expression of PDGFRα in ~50% of adult malignant gliomas (n = 78) and 48% of grade 2 gliomas (n = 25). They also reported shorter survival in patients with grade 2 gliomas with overexpression of PDGFR (relative risk, 5.03; P = 0.03).
A limited number of studies have evaluated the role of the EGFR and PDGFR downstream signaling cascades, RAS/MAPK and phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR, in adult gliomas (11, 20, 21). Chakravarti and colleagues assessed levels of expression of PI3K pathway members in 92 adult gliomas [70 high-grade gliomas (HGG) and 22 low-grade gliomas (LGG)] and correlated these data with clinical outcome. PI3K pathway activation was significantly associated with increasing tumor grade and reduced overall survival (OS; ref. 21). Additionally, PTEN, which inhibits PI3K/AKT signaling, is frequently deleted in adult HGG and is a significant poor prognostic finding (22, 23).
Several studies of childhood HGG have confirmed that PTEN deletions and EGFR amplification are rare, although EGFR overexpression is common (24–28). However, to date, a comprehensive analysis of the concomitant protein expression pattern of EGFR/PDGFR and the downstream PI3K/AKT and MAPK signaling pathways, and the relationship of expression to histology and outcome has not been investigated in childhood gliomas. This gap in understanding is important due to the potential lack of concordance between DNA, RNA, and protein expression and the contribution that each target member may have on the overall activity of the pathways. We hypothesized that pediatric gliomas showing high expression of EGFR or PDGFR and their active signaling pathways would be more likely to exhibit aggressive behavior and worse clinical outcome. Second, we propose that the protein expression of these pathways in childhood gliomas is distinct from that of adults. Finally, our goal in this study was to map the EGFR/PDGFR signaling pathway expression in a large cohort of pediatric glioma patients using tissue microarray (TMA) protein immunohistochemistry to identify suitable prognostic targets for future therapeutic interventions.
Materials and Methods
Study population
All but one of 164 glioma patients treated at Children’s National Medical Center between 1989 and 2004 was under 21 y of age. TMAs were prepared from the 103 paraffin-embedded tissue specimens available from these patients. Eighty-five of 103 tumors were from untreated patients with newly diagnosed glioma (22 HGG and 63 LGG; Table 1). Informed consent was obtained from patients or guardians at time of surgical or medical treatments. Children’s National Medical Center Institutional Review Board approval was obtained for chart review and tissue analysis.
Table 1.
Clinical characteristics of the pediatric glioma patients analyzed
| WHO tumor grade (total) | Mean age in years at Dx (range) | Gender | Location | M(+) at Dx | Mean PFS in years (range), total progressed | Mean OS in years (range), total died |
|---|---|---|---|---|---|---|
| 1 (42)* | 10.2 (0.5–27.8) | 18 M | ST = 18 | 2 | 2.9 (0.1–9.1), 12 | 4.13 (0.1–10.0), 1 |
| 2 (21)† | 13.0 (1.4–20.8) | 10 M 11 F |
ST = 18 PF = 3 |
1 | 3.9 (0.2–7.7), 5 | 3.9 (0.1–15.9), 2 |
| 3 (13) | 9.2 (0.4–18.0) | 8 M 5 F |
ST = 10 BS/Sp = 3 |
3 | 0.58 (0.1–4.9), 10 | 2.1 (0.1–10.9), 6 |
| 4 (9) | 14.3 (6.0–18.3) | 4 M 5 F |
ST = 9 | 2 | 0.5 (0–1.5), 8 | 1.5 (0.1–1.6), 7 |
| Total (85) | 10.4 (0.2–27.8) | 40 M 45 F |
ST = 55 PF = 21 BS/Sp = 9 |
8 | 2.3 (0–9.1), 35 | 3.5 (0–15.9), 16 |
Abbreviations: Dx, diagnosis; M, male; F, female; ST, supratentorial; PF, posterior fossa (including cerebellum); BS, brain stem; Sp, spine; M(+), number of patients with metastasis at diagnosis.
Thirty-nine juvenile pilocytic astrocytoma and 3 pilomyxoid astrocytoma.
Thirteen diffuse astrocytoma, 5 oligodendroglioma, and 3 mixed oligoastrocytoma.
Tissue specimens
Before construction of the TMA block, a neuropathologist (M.S.) marked a representative tumor core within each formalin-fixed, paraffin-embedded tissue block. Subsequently, a de-identified pediatric glioma TMA block was constructed, holding 278 representative 0.6-mm tumor cores, from 103 patients. Twenty-one tumor specimens had more than one sample in a separate location within the TMA block to assess reliability and validity of the immunohistochemistry.
Immunohistochemistry
Standard immunohistochemistry was applied to stain TMA sections to map the protein expression of EGFR, p-EGFR, PDGFRβ, p-PDGFRα, phosphorylated AKT (p-AKT), phosphorylated mitogen-activated protein kinase (p-MAPK), phosphorylated mammalian target of rapamycin (p-mTOR; also known as FRAP1), and PTEN. Immunohistochemistry for p-PDGFRβ and total PDGFRα was not done with the TMA because reliable results for antibodies for these targets in control paraffin-embedded tissues could not be obtained. Optimization for each antibody was done using positive and negative control specimens before doing immunohistochemistry of TMA slides, and all TMA immunohistochemistry was done in duplicate in two separate experiments. TMA slides, as well as positive and negative control slides for each protein target, were simultaneously deparaffinized in changes of CitriSolve (Fischer Scientific) followed by washes in 100% and 95% ethanol. Antigen retrieval was done either with 1 mmol/L EDTA (pH 8.0) followed by heating in a microwave (EGFR, p-EGFR, and PDGFRβ) or with DAKO antigen retrieval solution (pH 6.0; sodium citrate solution, DAKO) that was heated until boiled followed by 5 min at sub-boiling temperature (p-PDGFRα, p-MAPK, p-AKT, p-mTOR, and PTEN). Endogenous peroxidase activity was quenched using 3% hydrogen peroxide (H2O2). Sections were incubated for 1 h using recommended serum block to eliminate non-specific staining and subsequently incubated for 18 to 22 h overnight at 4°C using one of eight primary antibodies: EGFR (1:25; Cell Signaling Technology, Inc.), p-EGFR (Tyr1068) mouse monoclonal antibodies (1:200; Cell Signaling Technology), PDGFRβ (1:50; Santa Cruz Biotechnology), p-AKT (Ser473; 1:25; Cell Signaling Technology), p-mTOR (Ser2448; 1:50; Cell Signaling Technology), p-MAPK (44/42; 1:100; Cell Signaling Technology), PTEN mouse monoclonal antibodies (1:400; clone 6H2.1; Cascade Bioscience), and p-PDGFRα (Tyr754; 1:50; Santa Cruz Biotechnology). Biotinylated secondary antibody was applied to the sections [mouse monoclonal secondary antibodies, ImmunoCruz kit (p-EGFR), rabbit polyclonal secondary antibodies from avidin-biotin complex method kit (EGFR, PDGFRβ, p-mTOR, p-MAPK, and p-AKT), mouse monoclonal antibodies from VectaKit (PTEN), and rabbit polyclonal anti-goat antibodies from Vector Laboratories (p-PDGFRα)]. Subsequently, peroxidase-conjugated streptavidin [ImmunoCruz kit (p-EGFR), VectaKit (PTEN and p-PDGFRα), or avidin-biotin complex method staining kit (other targets)] was applied for 1 h to the sections. The labeled antibody was localized with 3,3′-diaminobenzidine solution (3,3′-diaminobenzidine in peroxidase substrate buffer from Santa Cruz Biotechnology staining kits) and counterstained with Gill’s hematoxylin (Fischer Scientific). Osteosarcoma tissue sections served as a positive control for PDGFRβ, chordoma tissue for p-PDGFRα, and p53+ breast cancer sections for the other targets (Newcomer Supply, Inc.). Childhood nontumor brain cortex sections served as a negative control for all protein targets.
Protein expression profiling
For each of the 278 tumor tissue cores per TMA slide, a protein expression score was assigned by four investigators independently for all eight protein targets using light microscopy with >40-fold magnification to evaluate membrane (EGFR, PDGFR, and mTOR), cytoplasmic (EGFR, PDGFR, AKT, mTOR, MAPK, and PTEN), and nuclear (AKT) staining within the tumor cells. Investigators were blinded as to the clinical history relating to each microarray. The principal investigator and two neuropathologists (R.M. and E.J.R.) scored the protein expression separately. Microarrays scored differently by these investigators (19% of the microarrays) were subsequently scored by a fourth investigator (T.J.M.). This additional score served as the deciding one and was in accordance with the pathologists’ score in 85% of arrays scored. PTEN staining was scored according to a previously established scale of 0 to 2 (20). Tumor cells were graded “2” if their staining intensity was equal or greater to that of vascular endothelium and “1” if diminished relative to the endothelium. Expression score of “0” was given if staining was absent compared with control sections. For other targets, score 1 was assigned for weak focal staining, score 2 for weak diffuse staining, score 3 for strong focal staining, and score 4 for strong diffuse staining within the tumor core specimen on the microarray, similar to what has previously been described (29). High expression was defined as score of 3 to 4, low expression as score 1 to 2, and absent expression was scored as 0. Intergrading and intragrading agreement was 81% and 76%, respectively, between tumor duplicates.
The expression score for each protein target was correlated with clinical data, including survival, tumor grade, dissemination, proliferation index, age of patient, and extent of initial tumor resection. Progression-free survival (PFS) and/or OS were calculated from the day of diagnosis to the date of tumor progression (PFS) or death (OS). Patients who were alive and free from tumor progression were censored at the date of last follow-up. The proliferation index (MIB index) was reported (M.S.) for a subset of the tumors at diagnosis using MIB-1 monoclonal antibody immunohistochemical staining directed toward the Ki-67 nuclear antigen. Gross-total or near-total resection represented removal of >80% of tumor volume at time of diagnosis but subtotal resection or biopsy removal of <80% of disease. Disseminated disease (M+) was defined as having tumor seeding into a separate location within the central nervous system as determined by neuroimaging or colony-stimulating factor cytology.
RNA profiling (historical comparison)
Gene expression profiles were obtained before construction of the TMA from nine of the LGG and six HGG tumors as previously described (26). In this previously reported cohort, total RNA was isolated from the tissues and hybridized to an Affymetrix U95Av2 array containing ~12,000 human transcripts (26).
Statistics
The univariate Pearson correlation model was applied to determine the association between protein expression of EGFR, p-EGFR, PDGFRβ, p-PDGFRα, p-mTOR, p-AKT, p-MAPK (score 0–2 or score 3–4), or PTEN (score 0–1 or score 2) and tumor grade (WHO grade 1–2 or 3–4). Kaplan-Meier time-to-event analysis was evaluated for the proportional hazard assumption before proceeding with Cox regression analysis to assess the risk of progression or death.
A linear regression model was developed to compare the gliomas and estimate the correlation between age at diagnosis, extent of resection, and level of expression of specific targets. By using linear regression, we were able to evaluate whether the correlation differed from zero while holding other confounding factors constant (age of patient, tumor grade, and extent of resection). The EGFR and p-AKT targets violated somewhat the proportional hazards assumption. The Cox multivariate model was therefore reapplied after removing either or both of these targets from the model.
Results
Patient clinical characteristics
The pediatric glioma TMA constructed contained tumor duplicates from 85 newly diagnosed pediatric glioma patients treated at Children’s National Medical Center from 1989 to 2004 (63 LGG and 22 HGG) as well as 17 pretreated patients with progressive disease (13 HGG and 4 LGG) that were excluded from further analysis. Table 1 summarizes the untreated patients’ clinical characteristics. Mean age and gender did not differ significantly between LGG and HGG patients. Primary tumor location was predominantly supratentorial for HGG and only marginally more supratentorial versus infratentorial for LGG. All of the analyzed HGG tumors were uniformly classified as astrocytic and without significant oligodendroglioma or ependymal components, as were the majority of the LGG.
Patient outcomes associated with clinical characteristics
Overall, 35 of 85 (41%) patients had tumor progression (27% LGG and 82% HGG; median, 10 months after diagnosis; range, <1–87 months) and 16 patients (18%) died (4% LGG and 60% HGG; median, 14 months after diagnosis; range, <1–42 months; Table 1). Patients with grade 1 and 2 gliomas were treated similarly, 31 of 42 with grade 1 had surgery only, 4 had focal radiation therapy, and 7 had adjuvant chemotherapy (most often using carboplatin/vincristine) in addition to radiation therapy and surgery, but 17 of 21 with grade 2 had resection only, 2 had focal radiation therapy, and 2 had adjuvant chemotherapy. The overall outcome data were similar to that observed in historical national clinical trial data for the respective histologies (30–35).
Tumor grade
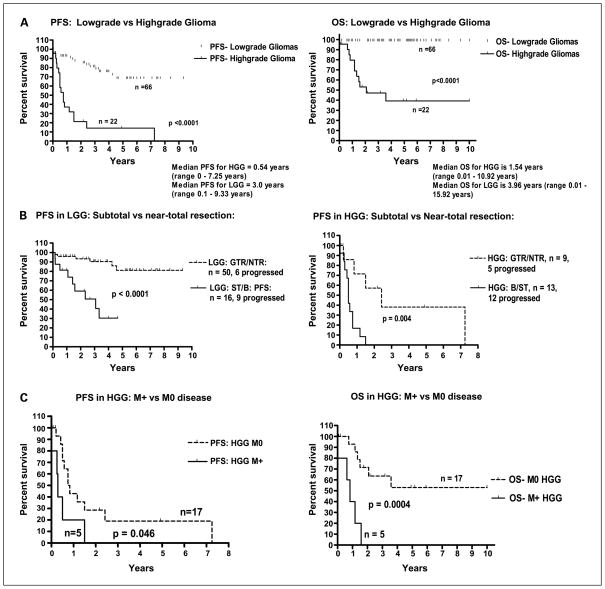
As expected, patients with HGG had significantly shorter PFS and OS compared with patients with LGG (P < 0.0001, respectively; Fig. 1A). PFS of patients with grade 1 gliomas did not differ significantly from that of patients with grade 2 (P = 0.178).
Fig. 1.
Relationship of pediatric glioma patient survival to histology (A), extent of surgical resection (B), and presence of metastasis at diagnosis in HGG (C). PFS and OS are worse for high-grade histology and the presence of metastasis at diagnosis in HGG. PFS is worse for subtotal resection in either LGG or HGG. GTR/NTR, gross-total/near-total resection; B/ST, biopsy/subtotal resection; M+, presence of metastasis at diagnosis; M0, no metastasis at diagnosis.
Extent of resection
Similar to that observed in cooperative group clinical trials, in the logistic regression analysis for LGG and HGG, extent of resection, subtotal resection/biopsy or near-total/gross-total, was an important predictor of progression [LGG: hazard ratio, 18.91; 95% confidence interval (95% CI), 3.84–93.15; HGG: hazard ratio, 24.09; 95% CI, 2.44–238.03]. In the unadjusted analysis, 16 of 63 LGG patients, who had biopsy/subtotal resection at diagnosis, had significantly worse PFS compared with 50 LGG patients after near-total/gross-total resection (P = 0.004; Fig. 1B). Furthermore, 13 of 22 HGG patients, with history of biopsy/subtotal resection at diagnosis, had significantly worse PFS than 9 HGG patients with near-total/gross-total resection (P = 0.004; Fig. 1B). Evaluating OS, 2 of 9 HGG patients with history of near-total/gross-total resection died compared with 9 of 13 HGG with history of subtotal resection/biopsy resection (P = 0.080).
Patient age
Median PFS for 5 HGG patients <5 years old at diagnosis was 1.2 years (range, 0.7–7.3 years) compared with 0.4 years (range, <0.1–2.4 years) for 17 HGG patients >5 years old (P = 0.09). Median OS in HGG patients <5 years old was 4.9 years (range, 1.5–10.9 years) compared with 1.2 years (range, <0.1–5.9 years) for >5 years old (P = 0.22). For 9 LGG patients <3 years old, the PFS and OS were not significantly different from that of 54 LGG patients >3 years old (P = 0.90 and 1.0, respectively). Hazard ratio for age in LGG was 0.91 (95% CI, 0.84–0.99) and 0.94 (95% CI, 0.85–1.03) for HGG.
Tumor dissemination
Eight patients (five HGG and three LGG) had leptomeningeal spread and metastasis when diagnosed (M+). Five HGG patients, M+ at diagnosis, had significantly lower PFS and OS compared with that of 17 M0 HGG patients (P = 0.046 and 0.0004, respectively; Fig. 1C).
Proliferation index
MIB index was reported at time of diagnosis for 9 HGG patients (MIB index range, >5–50%) and 27 LGG patients (range, <1–5%). Five LGG patients with known MIB index had progression of disease, thereof four had MIB <2%. Consistent with the findings of a German study in 1996 (36), the PFS and OS of 6 LGG patients with MIB index >2% did not differ significantly from that of 21 LGG patients with index <2% (P = 0.950 and 1.0, respectively). However, the OS of six HGG patients with MIB index >10% was significantly lower than that of three HGG patients with MIB index <10% (P = 0.034) but with no statistical difference when evaluating PFS (P = 0.153).
EGFR and PDGFR pathway protein expression profiling
Protein target expression and histology
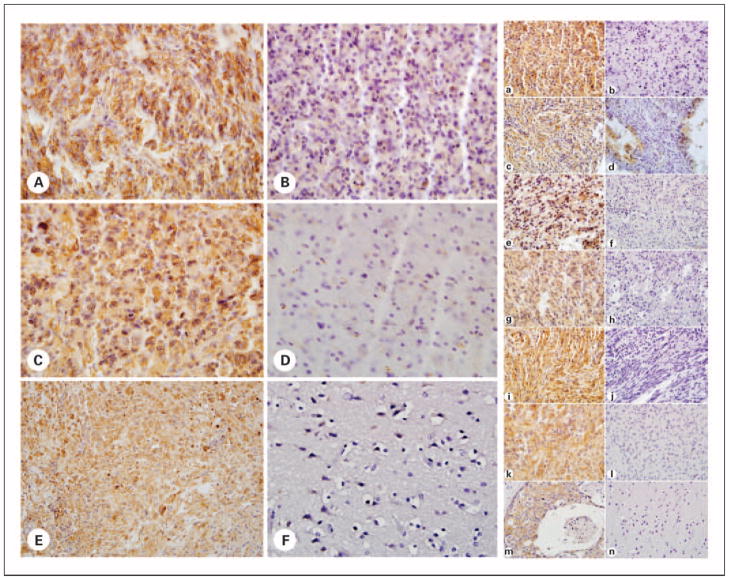
The justification for combining the grade 1 pilocytic astrocytomas with the molecularly and histologically distinct grade 2 pediatric astrocytomas into one group (LGG) for comparison with the HGG group with respect to EGFR and PDGFR pathway expression was based on a separate analysis (data not shown) that showed no significant difference between the two grades in the expression of the specific targets analyzed as well as our results indicating that there were no significant differences in the treatment nor clinical outcomes for our two cohorts. High expression, defined as grade 3 or 4 positive immunostaining, of EGFR, p-EGFR, p-PDGFRα, and PDGFRβ was seen in 80.0%, 47.4%, 85.7%, and 78.9% of HGG and 87.1%, 30.6%, 40.0%, and 41.7% of LGG, respectively. Only high expression of PDGFRβ and p-PDGFRα was significantly associated with malignant histology (P = 0.005 and 0.031, respectively; Table 2). PTEN staining that was less than that of the vascular endothelium was noted in 32.1% of LGG and in 40% of HGG (Pearson χ2 = 0.32; P = 0.568; Table 2). High expression of each target was observed diffusely, but staining clearly localized predominantly to individual tumor cells and to the appropriate cellular compartment of the cells as described (Fig. 2).
Table 2.
Association between EGFR and PDGFR pathway protein expression and glioma grade
| Target | LGG |
HGG |
Score 3–4: (all gliomas) | Pearson correlation between score 3–4 and HGG (P) | Odds ratio (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Score 0–2 | Score 3–4 | Score 0–2 | Score 3–4 | ||||
| PDGFRβ | 58.3% | 41.7% | 21.1% | 78.9% | 50.6% | 8.02 (0.005) | 5.25 (1.56–17.73) |
| p-PDGFRα | 40.0% | 60.0% | 14.3% | 85.7% | 65.0% | 4.63 (0.031) | 4.00 (1.06–15.08) |
| EGFR | 12.9% | 87.1% | 20.0% | 80.0% | 85.4% | 0.82 (0.366) | 0.55 (0.14–2.06) |
| p-EGFR | 69.4% | 30.6% | 52.6% | 47.4% | 34.6% | 1.79 (0.180) | 2.04 (0.71–5.82) |
| p-MAPK | 29.5% | 70.5% | 30.0% | 70.0% | 70.4% | <0.01 (0.967) | 0.98 (0.32–2.95) |
| p-AKT | 33.9% | 66.1% | 15.8% | 84.2% | 70.4% | 2.28 (0.131) | 2.73 (0.71–10.44) |
| p-mTOR | 22.6% | 77.4% | 30.0% | 70.0% | 76.8% | 0.91 (0.340) | 0.57 (0.18–1.81) |
| PTEN* | 32.1% | 67.9% | 40.0% | 60.0% | 66.2% | 0.33 (0.568)* | 0.71 (0.22–2.30) |
For PTEN, in place of score 0 to 2 and 3 to 4 as for the other targets, a score of 0 to 1 was used to represent loss of PTEN expression and a score of 2 was used to represent no loss of PTEN expression compared with that of the vascular endothelium.
Fig. 2.
Representative immunohistochemical TMA protein analysis of the EGFR and PDGFR pathway in pediatric gliomas. Left, A to D, representative TMA of pediatric glial tumors stained for PDGFR markers. p-PDGFRα – positive (A), p-PDGFRα – negative (B), PDGFRβ-positive (C), and PDGFRβ-negative (D) glial tumors are shown. Osteosarcoma positive control (E) and cerebral cortical gray matter negative control (F) were used for both PDGFR markers. Right, A to L, representative TMA of pediatric glial tumors stained for EGFR and PDGFR pathway markers. Positive (A, C, E, G, I, and K) and negative (B, D, F, H, J, and L) glial tumors staining for EGFR, p-AKT, p-EGFR, p-MAPK, p-MTOR, and PTEN, respectively, are shown. Breast carcinoma positive control (M) and cerebral cortical gray matter negative control (N) for EGFR staining are shown and the same tissues were used as controls for all markers analyzed. Magnification is ×200 for all TMA images, except the p-AKT – negative glial tumor (right, D) shown is ×400 to show that no tumor was completely negative for this marker. Controls are ×400 magnification, except the EGFR-negative control (right, N) shown is ×100.
Relationship between protein targets
There was an association between high expression of EGFR and high expression of p-AKT, p-mTOR, and p-MAPK by Pearson’s correlation analysis [Pearson χ2 = 22.9 (P < 0.001), 23.7 (P < 0.001), and 15.7 (P < 0.001), respectively] for each target. However, there was no relationship between high PDGFRβ expression and high expression of p-AKT, p-mTOR, or p-MAPK [Pearson χ2 = 1.09 (P = 0.297), 0.32 (P = 0.574), and 2.38 (P = 0.123), respectively]. Likewise, there was no relationship observed between p-PDGFRα and other targets.
Protein target expression and clinical outcome
Forty patients with high PDGFRβ expression had, when evaluated individually, significantly worse OS compared with that of 39 patients with low expression [4-year OS, 73.9% (95% CI, 57.1–90.8%) and 96.9% (95% CI, 91.1–100%), respectively; P = 0.008], but in a multivariate analysis adjusting for presence of other targets and for extent of tumor resection, this difference disappeared. When analyzing LGG and HGG patients separately, there was no statistical difference in OS or PFS of patients when evaluating individually high PDGFRβ expression compared with low expression [for HGG 4-year OS was 33.9% (95% CI, 0.9–67.0%) and 75.0% (95% CI, 32.6–100%), respectively; P = 0.131]. When pairing high PDGFRβ expression with expression of each of the other targets, coexpression of p-MAPK and PDGFRβ revealed borderline worse PFS in LGG patients after adjusting for extent of tumor resection (hazard ratio, 2.68; 95% CI, 0.89–8.09; P = 0.079). High p-PDGFRα expression was not associated with worse PFS or OS in LGG or HGG.
In LGG patients, when using univariate analysis for evaluation of individual targets, there was borderline evidence that high expression of p-mTOR and p-AKT was associated with greater risk of progression (P = 0.109 and 0.242, respectively). However, this relationship disappeared in the multivariate model when controlling for effect of other targets and extent of initial resection.
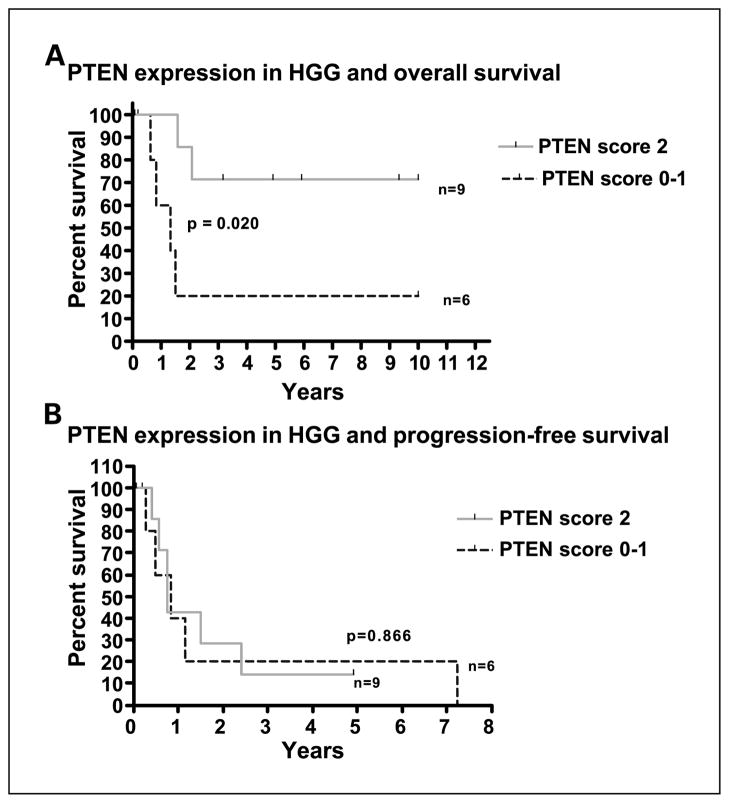
Loss of PTEN tumor suppressor expression was associated with worse OS in HGG patients (P = 0.020). However, loss of PTEN expression did not seem to be associated with risk of progression in the same group of patients (P = 0.867; Fig. 3).
Fig. 3.
Association between patient survival and protein expression of PTEN in pediatric HGG. Loss of PTEN is associated with worse OS (A) but not with PFS (B). A score of 0 to 1was used to represent loss of PTEN expression and a score of 2 was used to represent no loss of PTEN expression compared with that of the vascular endothelium.
High expression of EGFR, p-EGFR, and their active signaling pathways was not associated with worse PFS or OS in either LGG or HGG patients. Multivariate models containing EGFR or p-EGFR paired with other targets did not reveal statistical differences in PFS or OS. Furthermore, when evaluating progression in either LGG or HGG, there was no statistically significant evidence that having high expression of EGFR, p-EGFR, PDGFRβ, p-MAPK, p-mTOR, and p-AKT compared with having low expression of all of these targets, or any other combination (high or low) of expression levels, was related to progression, whether adjusting for extent of initial tumor resection or not.
RNA profiling (historical data)
Table 3 shows the RNA expression profiles and average intensity of the EGFR and PDGFR pathway members previously obtained in 13 tumors from our pediatric glioma cohort (9 HGG and 6 LGG). EGFR was significantly up-regulated in the HGG (permutational P for all < 0.004) and AKT1 and PDGFA up-regulation seemed borderline associated with HGG (permutational P = 0.095 and 0.056, respectively). In the LGG tumors, PI3K was significantly up-regulated (permutational P = 0.015, 0.010, and 0.024, respectively). In this small subset of LGG, PDGFRα gene expression seemed to be evident in all the LGG and HGG tumor samples, although with greater intensity in the HGG, but EGFR gene expression was only evident in the HGG tumors. For each tumor that was analyzed by mRNA for markers that were also examined by immunohistochemistry for total protein expression (EGFR, PDGFRβ, and PTEN), there was a direct correlation for all three markers between the mRNA and total protein expression levels; however, as a group, only PDGFRβ (higher mRNA and protein levels in HGG) and PTEN (lower mRNA and protein in HGG) expression directly correlated.
Table 3.
RNA expression of targets assessed by gene expression profiling of 9 LGGs and 6 HGGs
| Target gene (probe ID) | Average intensity HGG | Average intensity LGG | Present in HGG | Present in LGG | Permutation P |
|---|---|---|---|---|---|
| PDGFRβ (1771_at) | 600.4 | 488.8 | 67% | 43% | 0.457 |
| PDGFRα (1731_at) | 9,957.8 | 6,896.0 | 100% | 100% | 0.218 |
| EGFR (1537_at) | 2,389.2 | −41.3 | 67% | 0% | 0.003 |
| EGFR (37327_at) | 987.6 | −39.1 | 33% | 0% | 0.003 |
| AKT1 (1564_at) | 2,402.6 | 388.9 | 67% | 43% | 0.095 |
| p44 MAPK (1000_at) | 1,685.6 | 2,034.0 | 83% | 100% | 0.111 |
| p42 MAPK (976_s_at) | 227.4 | 226.8 | 50% | 57% | 0.994 |
| FRAP1 (267_at) | 111.6 | 98.9 | 50% | 57% | 0.823 |
| FRAP1 (40139_at) | 851.0 | 891.8 | 100% | 100% | 0.772 |
| PDGFB (1573_at) | 48.3 | −110.9 | 0% | 14% | 0.319 |
| PDGFA (35703_at) | 2,388.0 | 828.4 | 83% | 86% | 0.056 |
| PDAP1 (38757_at) | 293.7 | 221.4 | 83% | 14% | 0.372 |
| PDAP1 (38758_at) | 5,055.5 | 2,822.5 | 100% | 100% | 0.001 |
| EGF (1542_at) | 399.0 | 409.9 | 17% | 14% | 0.914 |
| PTEN (1434_at) | 183.9 | 203.9 | 50% | 86% | 0.587 |
| PTEN (39552_at) | 283.0 | 316.3 | 67% | 71% | 0.536 |
| PI3K (36287_at) | 109.3 | 120.4 | 33% | 14% | 0.726 |
| PI3K (35373_at) | 533.5 | 1,742.7 | 67% | 100% | 0.015 |
NOTE: Shaded areas indicate genes with significant (P < 0.01, dark shade) or near-significant (P < 0.10, light shade) differential overexpression by HGG or LGG.
Discussion
In our prior study, we showed that RNA overexpression of the EGFR and PDGFR pathways best distinguishes childhood HGG from LGG (26). Because RNA levels may not correlate directly with protein expression and can be a product of both tumor cells and their surrounding microenvironment, we used immunohistochemical analysis to validate protein overexpression and to determine localization of expression of specific EGFR and PDGFR pathway members to establish suitable candidates for drug targeting in childhood glioma. Herein, we show for the first time that high expression of p-PDGFRα and PDGFRβ is significantly associated with HGG, whereas relatively decreased PTEN expression by HGG is associated with worse OS. By comparison, on the protein level, EGFR and almost all members of the downstream pathways analyzed are similarly abundantly expressed by the tumor cells of both childhood HGG and LGG. These findings suggest that the expression of PDGFR not only characterizes the malignant phenotype of childhood glioma but may also represent an important clinical target for therapeutic intervention in HGG. A recent study comparing pediatric gliomas found no significant difference in the total expression of PDGFRα between grade 2 (n = 16), grade 3 (n = 10), and grade 4 (n = 18) tumors; however, in that report, the active form (p-PDGFRα) was not analyzed (28). This important distinction may account for the seemingly discrepant results. Indeed, we show that mRNA levels of the receptor do not differ between tumor grades; however, given that the activation of the receptor is dependent on the presence and binding efficiency of the PDGF ligands AA and CC as well as other factors (i.e., integrin expression), it is quite possible that the total receptor expression may be equivalent, whereas the expression of the more biologically relevant activated form of the receptor may differ between the histologic grades.
Analyzing coexpression of pairs of protein targets in relation to outcome may ultimately provide more useful information for stratifying targeted therapeutics. For example, PDGFR overexpression alone was not significantly associated with PFS or OS in our HGG cohort; however, if the HGG sample size were expanded, expression of PDGFR alone or in combination with additional targets may correlate with outcome. It can also be argued that it is unlikely that there would be observable differences in survival between PDGFR high- and low-expressing tumors until a similar analysis is done on HGG specimens derived from patients in clinical trials using drugs that are designed to specifically target PDGFR. Several studies investigating the PDGFR inhibitor imatinib mesylate for recurrent malignant glioma, including a phase I trial in children, have reported a small incidence of objective responses (37–39). The responses observed to the PDGFR inhibitor in these recent studies may even be better appreciated if tumors were separated based on PDGFR expression level. An illustration of this includes a study of 186 prospectively collected adult malignant gliomas in which EGFR was the most frequent oncogene amplification yet without apparent correlation with patient survival (18). Of interest, reanalysis of these tumors by Mellinghoff and colleagues (13) recently showed that coexpression of the active EGFR mutant, EGFRvIII, and PTEN by glioblastomas is associated with improved clinical responsiveness to EGFR kinase inhibitors.
The only outcome association seen in our LGG patients was coexpression of p-MAPK and PDGFRα, having borderline worse PFS on multivariate analysis (P = 0.079). However, in the HGG cohort, we found that a relative deficiency in PTEN expression, a negative regulator of PDGFR and EGFR signaling, is associated with worse OS but not to PFS. A similar finding was noted in a cohort of 39 pediatric malignant gliomas, where PTEN mutation was associated with decreased survival (40). This finding needs to be confirmed in a larger cohort of pediatric HGG. If the prognostic significance of the lack of PTEN expression is validated in a larger study, with or without PDGFR and/or EGFR overexpression, it invites the stratification of pediatric HGG patients at diagnosis into prognostic risk groups, with the goal of tailoring treatment. Elucidating a mechanistic role for reduced PTEN expression in HGG is a potentially important future direction that should be undertaken in subsequent analyses.
Our findings also illustrate the importance of protein validation, as gene expression studies alone would not have predicted that PDGFR is more differentially expressed than EGFR on the protein level in HGG compared with LGG. Despite the limited sample size of our study, the clinical characteristics of our cohort seem to be nearly identical to that of the astrocytoma population observed in the larger pediatric cooperative group clinical trials (30–35). Accordingly, our results should be highly representative of childhood gliomas in general, although a larger prospective clinical trial would be necessary for confirmation. Interestingly, despite increased active PDGFR expression by HGG, overexpression of the active downstream targets of the pathway was not found. A plausible explanation for this is that the downstream targets are common to many different receptor tyrosine kinase pathways, as well as other upstream signaling molecules, and thus the ability to detect subtle differences in downstream signal amplitude by protein immunohistochemistry may be quite limited. Nonetheless, identifying a possible critical initiating molecule, such as PDGFR, may still have important clinical implications for altering the pathway activity. A randomized clinical trial using inhibitors to the receptors identified and linking the effect on pathway activation to changes in tumor progression would be needed to validate this concept.
Finally, our results further highlight an interesting distinction that has been noted between primary pediatric and adult malignant gliomas. Whereas adult HGG can be either primary (de novo) tumors or secondary tumors progressing from LGG, pediatric HGGs do not typically arise from LGG, yet pediatric HGGs seem to encompass a biology resembling that of both primary and secondary adult HGG. For example, we found high protein expression of both PDGFR and EGFR in pediatric HGG but showed that PDGFR, rather than EGFR, expression is more closely associated with pediatric HGG. In comparison, PDGFRα expression is noted in 60% of secondary adult glioblastomas and in only a small fraction of primary adult glioblastomas. However, >70% of primary adult glioblastomas overexpress EGFR, which is only rarely seen in secondary glioblastomas. This supports our hypothesis that the molecular biology of pediatric gliomas is distinct, and as such, therapeutic interventions targeting specific biological modifiers should be tailored to pediatric glioma biology as opposed to exactly replicating clinical trials of these agents that were designed specifically for adult-type gliomas. Furthermore, it is recognized that grade 1 tumors are distinct, both histologically and molecularly, from grades 2 to 4, and yet our results indicate that if one focuses solely on the EGFR/PDGFR pathways, grade 1 tumors have similar activity to grade 2 tumors and can only be distinguished from grades 3 and 4 by virtue of PDGFR expression. This finding has potentially important clinical implications in that it implies that activation of these pathways is characteristic of all pediatric astrocytic tumors, and in this particular regard, grade 1 tumors are not distinct.
In conclusion, only limited studies have correlated protein or gene expression profiles in pediatric astrocytomas with clinical outcome. Our study cohort was relatively large compared with prior publications on the expression profiles of EGFR/PDGFR relating histology to outcome in pediatric gliomas. High expression of p-PDGFRα and PDGFRβ was found to be significantly associated with HGG, whereas loss of PTEN expression was associated with worse OS. Large multicenter studies are needed to evaluate the prognostic significance of coexpression of signaling cascade targets within tumor cells and to evaluate to full extent the effects on therapeutic response when applying tyrosine kinase or signaling inhibitors.
Acknowledgments
We thank Robert McCarter for statistical support.
Grant support: Children’s National Medical Center’s Research Advisory Council Award (H.K. Thorarinsdottir), Fighting Children’s Cancer Foundation (H.K. Thorarinsdottir), and Friends of Ian Foundation (T.J. MacDonald).
References
- 1.Kleihues P, Cavanee WK. World Health Organization classification of tumours. Lyon (France): IARC; 2000. Pathology and genetics of tumours of the nervous system; pp. 9–54. [Google Scholar]
- 2.Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17:503–11. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 3.Finlay JL, Anderson JR, Cecalupo AJ, et al. Pre-irradiation chemotherapy in children with high-grade astrocytoma: tumor response to two cycles of the ‘8-drugs-in-1-day’ regimen. A Childrens Cancer Group study, CCG-945. J Neurooncol. 1994;21:255–65. doi: 10.1007/BF01063775. [DOI] [PubMed] [Google Scholar]
- 4.MacDonald TJ, Arenson EB, Ater J, et al. Phase II study of high-dose chemotherapy before radiation in children with newly diagnosed high-grade astrocytoma: final analysis of Children’s Cancer Group Study 9933. Cancer. 2005;104:2862–71. doi: 10.1002/cncr.21593. [DOI] [PubMed] [Google Scholar]
- 5.Newton HB. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. Part 1: growth factor and Ras signaling pathways. Expert Rev Anticancer Ther. 2004;3:105–28. doi: 10.1586/14737140.3.5.595. [DOI] [PubMed] [Google Scholar]
- 6.Lassman A. Molecular biology of gliomas. Curr Neurol Neurosci Rep. 2004;4:228–33. doi: 10.1007/s11910-004-0043-3. [DOI] [PubMed] [Google Scholar]
- 7.Mischel PS, Shai R, Shi T, et al. Identification of molecular subtypes of glioblastoma by gene expression profiling. Oncogene. 2003;22:2361–73. doi: 10.1038/sj.onc.1206344. [DOI] [PubMed] [Google Scholar]
- 8.Biernat W, Huang H, Yokoo H, Kleihues P, Ohgaki H. Predominant expression of mutant EGFR (EGFRvIII) is rare in primary glioblastomas. Brain Pathol. 2004;14:131–36. doi: 10.1111/j.1750-3639.2004.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFR-vIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462–66. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Yuan M, Kim IA, Chang CM, Bernhard EJ, Shu HKG. Mutant epidermal growth factor receptor displays increased signaling through the phosphatidyli-nositol-3 kinase/AKT pathway and promotes radioresistance in cells of astrocytic origin. Oncogene. 2004;23:4594–602. doi: 10.1038/sj.onc.1207602. [DOI] [PubMed] [Google Scholar]
- 11.Feldkamp MM, Lala P, Lau N, Roncari L, Guha A. Expression of activated epidermal growth factor receptors, Ras-guanosine triphosphate, and mitogen-activated protein kinase in human glioblastoma multiforme specimens. Neurosurgery. 1999;45:1442–50. doi: 10.1097/00006123-199912000-00034. [DOI] [PubMed] [Google Scholar]
- 12.Mischel PS, Cloughesy TF. Targeted molecular therapy of glioblastoma. Brain Pathol. 2003;13:52–61. doi: 10.1111/j.1750-3639.2003.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. New Engl J Med. 2005;353:2012–24. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 14.Besson A, Yong VW. Mitogenic signaling and the relationship to cell cycle regulation in astrocytomas. J Neurooncol. 2001;51:245–64. doi: 10.1023/a:1010657030494. [DOI] [PubMed] [Google Scholar]
- 15.Rao RD, James CD. Altered molecular pathways in gliomas: an overview of clinically relevant issues. Semin Oncol. 2004;31:595–604. doi: 10.1053/j.seminoncol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Kleihues P, Ohgaki H. Primary and secondary glioblastoma: from concept to clinical diagnosis. Neurooncol. 1999;1:44–51. doi: 10.1093/neuonc/1.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biernat W, Tohma Y, Yonekawa Y, Kleihues P, Ohgaki H. Alterations of cell cycle regulatory genes in primary (de novo) and secondary glioblastomas. Acta Neuropathol. 1997;94:303–9. doi: 10.1007/s004010050711. [DOI] [PubMed] [Google Scholar]
- 18.Galanis E, Buckner J, Kimmel D, et al. Gene amplification as a prognostic factor in primary and secondary high-grade malignant gliomas. Int J Oncol. 1998;13:717–24. [PubMed] [Google Scholar]
- 19.Varela M, Ranuncolo SM, Morandi A, et al. EGF-R and PDGF-R, but not bcl-2, overexpression predict overall survival in patients with low-grade astrocytoma. J Surg Oncol. 2004;86:34–40. doi: 10.1002/jso.20036. [DOI] [PubMed] [Google Scholar]
- 20.Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–46. [PubMed] [Google Scholar]
- 21.Chakravarti A, Zhai G, Suzuki Y, et al. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004;22:1926–33. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 22.Ushio Y, Tada K, Shiraisi S, et al. Correlation of molecular genetic analysis of p53, MDM2, p16, PTEN, EGFR and survival of patients with anaplastic astrocytoma and glioblastoma. Front Biosci. 2003;8:e281–8. doi: 10.2741/865. [DOI] [PubMed] [Google Scholar]
- 23.Smith JS, Tachibana I, Passe SM, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246–56. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- 24.Bredel M, Pollack IF, Hamilton RL, James CD. Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clin Cancer Res. 1999;5:1786–92. [PubMed] [Google Scholar]
- 25.Di Sapio AJ, Morra I, Pradotto L, Guido M, Schiffer D, Mauro A. Molecular genetic changes in a series of neuroepithelial tumors of childhood. J Neurooncol. 2002;59:117–22. doi: 10.1023/a:1019697117253. [DOI] [PubMed] [Google Scholar]
- 26.Khatua S, Peterson KM, Brown KM, et al. Over-expression of the EGFR/FKBP12/HIF-2α pathway identified in childhood astrocytomas by angiogenesis gene profiling. Cancer Res. 2003;63:1865–70. [PubMed] [Google Scholar]
- 27.Pollack IF, Hamilton RL, James CD, et al. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children’s Cancer Group 945 cohort. J Neurosurg. 2006;105:418–24. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura M, Shimada K, Ishida E, et al. Molecular pathogenesis of pediatric astrocytic tumors. Neuro-Oncol. 2007;9:113–23. doi: 10.1215/15228517-2006-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams EJ, Green JA, Clark AH, Youngson JH. Comparison of different scoring systems for immunohistochemical staining. J Clin Pathol. 1999;52:75. doi: 10.1136/jcp.52.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollack IF, Finklestein SD, Woods J, et al. Expression of p53 and prognosis in children with malignant gliomas. N Engl J Med. 2002;346:420–7. doi: 10.1056/NEJMoa012224. [DOI] [PubMed] [Google Scholar]
- 31.Pollack IF, Hamilton RL, Burnham J, et al. Impact of proliferation index on outcome in childhood malignant gliomas: results in a multi-institutional cohort. Neurosurgery. 2002;50:1238–32. doi: 10.1097/00006123-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Fouladi M, Hunt DL, Pollack IF, et al. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children’s Cancer Group high-grade glioma study CCG-945. Cancer. 2003;98:1243–52. doi: 10.1002/cncr.11637. [DOI] [PubMed] [Google Scholar]
- 33.Cohen KJ, Broniscer A, Glod J. Pediatric glial tumors. CurrTreat Options Oncol. 2001;2:529–36. doi: 10.1007/s11864-001-0074-9. [DOI] [PubMed] [Google Scholar]
- 34.Shaw EG, Wisoff JH. Prospective clinical trials of intracranial low-grade glioma in adults and children. Neuro Oncol. 2003;5:153–60. doi: 10.1215/S1152-8517-02-00060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broniscer A, Gajjar Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist. 2004;9:197–206. doi: 10.1634/theoncologist.9-2-197. [DOI] [PubMed] [Google Scholar]
- 36.Wolff JE, Boos J, Kuhl J. HIT-GBM: multicenter study of treatment of children with malignant glioma. Klin Padiatr. 1996;208:193–6. doi: 10.1055/s-2008-1046473. [DOI] [PubMed] [Google Scholar]
- 37.Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric BrainTumor Consortium report. Neuro Oncol. 2007;9:145–60. doi: 10.1215/15228517-2006-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desjardins A, Quinn JA, Vredenburgh JJ, et al. Phase II study of imatinib mesylate and hydroxyurea for recurrent grade III malignant gliomas. J Neurooncol. 2007;83:53–60. doi: 10.1007/s11060-006-9302-2. [DOI] [PubMed] [Google Scholar]
- 39.Reardon DA, Egorin MJ, Quinn JA, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol. 2005;23:9359–68. doi: 10.1200/JCO.2005.03.2185. [DOI] [PubMed] [Google Scholar]
- 40.Raffel C, Frederick L, O’Fallon JR, et al. Analysis of oncogene and tumor suppressor gene alterations in pediatric malignant astrocytomas reveals reduced survival for patients with PTEN mutations. Clin Cancer Res. 1999;5:4085–90. [PubMed] [Google Scholar]