Summary
Activation of the Jun-N-terminal kinase (JNK) signaling cascade by phorbol esters (TPA) or protein kinase C (PKC) is well documented, although the underlying mechanism is not known. Here, we demonstrate that the receptor for activated C kinase 1 (RACK1) serves as an adaptor for PKC-mediated JNK activation. Phosphorylation of JNK by PKC occurs on Ser129 and requires the presence of RACK1. Ser129 phosphorylation augments JNK phosphorylation by MKK4 and/or MKK7 and is required for JNK activation by TPA, TNFα, UV irradiation, and PKC, but not by anisomycin or MEKK1. Inhibition of RACK1 expression by siRNA attenuates JNK activation, sensitizes melanoma cells to UV-induced apoptosis, and reduces their tumorigenicity in nude mice. In finding the role of RACK1 in activation of JNK by PKC, our study also highlights the nature of crosstalk between these two signal-transduction pathways.
Introduction
A variety of extracellular stimuli—growth factors, cytokines, tumor promoters, UV radiation, and hormones— induce activation of stress-activated protein kinases and, in particular, JNK. In turn, JNK causes concomitant activation of its substrates, including c-Jun, ATF2, p53, and Bad, which play key roles in the cellular response to stress and the cell’s ability to undergo apoptosis (Davis, 2000). JNK is activated by sequential protein phosphorylation through a MAP kinase module, i.e., MAP3K→MAP2K→MAPK. Two MAP2Ks (JNKK1/MKK4/SEK1 and JNKK2 [also called MKK7]) have been identified for JNK. Phosphorylation of JNK by these dual-specificity protein kinases on Thr183 and Tyr185 are necessary for JNK activation (Davis, 2000). Although both MKK4 and MKK7 may be required for full activation of JNK, the differential phosphorylation of JNK by MKKs provides a molecular basis for differential activation of JNK by various stimuli (Fleming et al., 2000). For example, simultaneous disruption of both Mkk4 and Mkk7 genes was required to block JNK activation by UV or anisomycin, whereas disruption of Mkk7 alone was sufficient to prevent JNK activation by proinflammatory cytokines (Tournier et al., 2001). Several MAP3Ks, including members of the MEKK family, ASK1, MLK, TAK1, and TPL-2, have been reported to act as MAP3Ks for JNK (Davis, 2000). JNK activity is also regulated by scaffold proteins such as JIP, β-arrestin, and JSAP1 (Morrison and Davis, 2003) as well as by the protein phosphatase MKP5 (Theodosiou et al., 1999).
Among the extracellular stimuli that activate MAPK pathways are phorbol esters. Phorbol esters such as 12-O-tetradecanoylphorbol-13-acetate (TPA) and their derivatives are able to induce signaling events mimicking those triggered by activated growth-factor receptors, resulting in diverse effects ranging from proliferation and cell survival to differentiation and cell death (Kazanietz, 2000). Members of the Protein Kinase C (PKC) family serve as key mediators of phorbolester actions. The activation of PKC by phorbol esters or diacylglycerol results in concomitant activation of at least two MAPK pathways: the extracellular signal-regulated kinase (ERK) and the JNK pathways. The ERK signaling cascade, which is composed of Raf kinase, ERK kinase (MEK1/2), and ERK1/2, is ubiquitously expressed in mammalian cells (Schonwasser et al., 1998). Unlike ERKs, which are primarily activated by mitogens, JNKs are potently and preferentially activated by cellular stress and by inflammatory cytokines (Ip and Davis, 1998). Consistent with this finding, TPA effectively activates JNK in certain cell types including hematopoietic (Werlen et al., 1998), lung (Lang et al., 2004), epithelial (Werlen et al., 1998), cardiac (Ping, 2003), rhabdomyosarcoma (Mauro et al., 2002), and mast cells (Kawakami et al., 1998). The role of the Ras-Raf-MEK pathway in JNK activation has been proposed (Buchner, 2000); however, the underlying mechanism and the proximal kinases involved in this pathway remain elusive.
We have identified RACK1 as a novel ATF2-interacting protein in a yeast two-hybrid screen by using ATF2 as bait. RACK1 is a highly conserved, 36 kDa protein originally identified on the basis of its ability to bind to the activated form of protein kinase C (Ron et al., 1994). RACK1 belongs to a family of proteins containing different numbers of structural Trp-Asp (WD) repeats; RACK1 contains seven consecutive 41–48 amino acids-long WD repeats (Ron et al., 1994). RACK1 is thought to interact only with activated PKC and to serve both as an anchor protein for PKC and as a scaffold protein that recruits PKCs (and other proteins) into a signaling complex (Schechtman and Mochly-Rosen, 2001). Here, we report the identification and characterization of RACK1 as a key factor in the activation of JNK by various stimuli that activate PKC.
Results
RACK1 Associates with JNK
The finding that RACK1 is an ATF2-associated protein was confirmed in vitro and in vivo, pointing to a previously undetected layer in the regulation of this transcription factor (P.L.-B., A.B., and Z.R., unpublished data). This observation led us to explore the possibility that JNK, both as ATF2 bound protein and as kinase, may also be associated with—and affected by—RACK1. Coimmunoprecipitation experiments revealed that exogenously expressed JNK1 and JNK2 bind to endogenous RACK1 (Figure 1A), indicating that JNK1 and JNK2 associate with RACK1 in vivo.
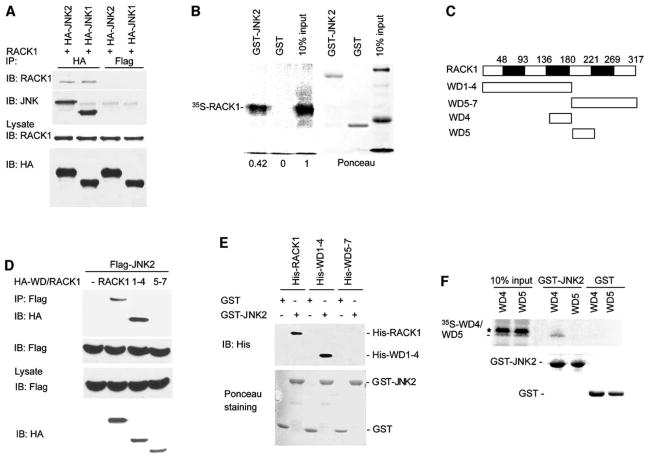
Figure 1. RACK1 Interacts with JNK.
(A) RACK1 interacts with JNK in vivo. HEK293 T cells were transfected with 1μg of pEF plasmid encoding HA-JNK1 or HA-JNK2. Whole-cell lysates (500 μg) were subjected to IP with anti-HA or anti-Flag Ab (used as a control). Immunoprecipitates were analyzed by immunoblotting (IB) with anti-JNK or anti-RACK1 Ab (upper). Lower panels show IB analysis of protein lysates (80 μg) with anti-HA or anti-RACK1 Ab to monitor protein levels.
(B) RACK1 associates with JNK2 in vitro. Beads containing GST or GST-JNK2 (2 μg) were incubated with 35S-labeled RACK1. After washing, bead bound material was subjected to SDS-PAGE, transferred to nitrocellulose, and analyzed with a phosphorimager (left). Right panel shows Ponceau S staining of the membrane.
(C) Schematic diagram of RACK1 and its mutants. The seven WD domains of RACK1 and their relative position within the protein are indicated. All constructs have an HA-tag on their NH2 terminus end.
(D) JNK2 interacts with WD1–4. HEK293 T cells were cotransfected with Flag-JNK2 (1 μg) and wt HA-RACK1 or mutant forms of RACK1 (HA-WD1–4 or HA-WD5–7) (2 μg). Whole-cell lysates (500 μg) were subjected to IP by using anti-Flag Ab followed by IB with anti-HA or anti-Flag Ab (upper). Lower panels show IB analysis of protein lysates (50 μg) with the indicated antibody to monitor the protein levels.
(E) JNK2 interacts directly with RACK1 and WD1–4 in vitro. Beads containing GST or GST-JNK2 (4 μg) were incubated with His-RACK1, His-WD1–4, or His-WD5–7 (2 μg). Bead bound material was subjected to IB with anti-His Ab. Lower panel shows Ponceau S staining of the membrane.
(F) WD4 associates with JNK2 in vitro. Beads containing GST or GST-JNK2 (4 μg) were incubated with 35S-labeled WD4 or WD5. After washing, bead bound proteins were subjected to SDS-PAGE, transferred to nitrocellulose, and analyzed with a phosphorimager (upper). Lower panel shows Ponceau S staining of the membrane. The asterisk indicates a nonspecific band.
To further confirm the interaction between these proteins, we carried out in vitro pull-down assays. For this purpose, GST-JNK2 bound to glutathione beads was incubated with 35S-labeled, in vitro-translated RACK1. This analysis revealed that RACK1 interacts with JNK2, confirming the association observed in vivo (Figure 1B).
To map the domains required for association of RACK1 with JNK, we generated several RACK1 deletion mutants that included WD domains one to four (WD1–4) and WD domains five to seven (WD5–7) (Figure 1C). Coimmunoprecipitation assays demonstrated that WD1–4 interacts with JNK2 (Figure 1D). In contrast, the WD5–7 form of RACK1 was not found in association with JNK (Figure 1D). In vitro binding assays with bacterially expressed and purified GST-JNK2 and His-RACK1 revealed a direct association between the two proteins (Figure 1E). A smaller portion of RACK1 that consisted only of the WD4 domain exhibited poor in vivo expression, probably because of a short half-life. Nevertheless, in vitro association assays with a 35S-labeled WD4 fragment revealed association of WD4 with JNK2. In contrast, a 35S-labeled fragment consisting of the WD5 domain failed to interact with JNK2 (Figure 1F). These data suggest that the WD4 domain is the primary region required for RACK1 association with JNK2.
RACK1 Contributes to Activation of JNK by TPA, TNFα, and UV Irradiation
We next assessed whether RACK1 association with JNK affects its kinase activities. Immunokinase assays that used GST-c-Jun1–89 as a substrate revealed increased JNK activity (10.4-fold) in cells that were co-transfected with full-length RACK1 (Figure 2A). Similarly, the WD1–4 fragment, which interacted with JNK, also increased its activity (7.9-fold). Conversely, RACK1 fragment WD5–7, which does not bind to JNK, did not affect JNK activity (Figure 2A). Because WD1–4 can associate with JNK and increase its activity, the data suggest that it is sufficient for functional association and activation of the JNK signaling cascade.
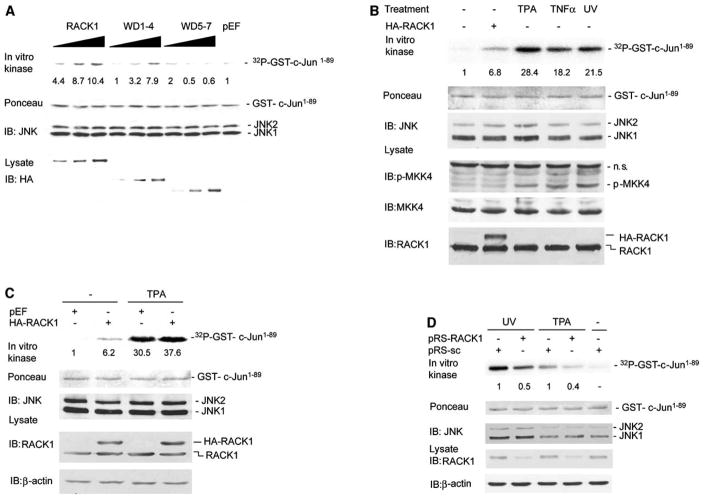
Figure 2. RACK1 Participates in TPA- and UV-Induced JNK Activation.
(A) RACK1 and WD1–4 increase JNK activity. HEK293 T cells were transfected with control vector (pEF, 2 μg), HA-RACK1 (0.5 μg, 1 μg, and 2 μg), HA-WD1–4 (0.5 μg, 1 μg, and 2 μg), or HA-WD5–7 (1.5 μg, 3 μg, and 6 μg). Cells were harvested 48 hr after transfection, and protein extracts (400 μg) were subjected to IP by using antibodies to JNK1 followed by an in vitro kinase assay with GST-c-Jun1–89 as a substrate. Quantification of JNK activity is shown (relative fold increase). Lower panel shows IB analysis of cell lysates (40 μg) with anti-HA Ab.
(B) Comparison of JNK activation achieved by TPA, UV, TNFα, and RACK1. HEK293 T cells were transfected with control vector (pEF) or HA-RACK1 (2 μg). Forty-eight hr after transfection, cells were UV irradiated (45 J/m2) and treated with TPA (20 ng/ml, 60 min) or TNFα (40 ng/ml, 20 min). Protein extracts (400 μg) were subjected to JNK immunokinase reaction as detailed in (A). Protein lysates (80 μg) were subjected to IB with the indicated antibodies.
(C) RACK1 potentiates TPA-induced JNK activation. HEK293 T cells were transfected with control vector (pEF) or HA-RACK1 (2 μg). Forty-eight hr after transfection, cells were treated with TPA (20 ng/ml) for 60 min. Protein extracts were subjected to JNK immunokinase reaction as detailed in (A). Protein lysates (50 μg) were subjected to IB with the indicated antibodies.
(D) siRNA of RACK1 efficiently inhibits RACK1 expression and both TPA- and UV-induced JNK activation. MeWo cells were stably transfected with pRS-scramble (pRS-sc) or pRS expressing siRNA for RACK1 (pRS-RACK1). Cells were serum-starved for 12 hr, treated with TPA or UV irradiated (45 J/m2), and harvested after 60 min and 30 min, respectively. Proteins (20 μg) were analyzed by IB with antibodies to RACK1 and β-actin. A JNK immunokinase reaction was carried out as detailed in (A). Relative change in JNK activity was determined by comparison with pRS-sc transfected cells (set as 1).
We next compared RACK1 effect on JNK activation by TPA, TNFα, and UV irradiation, each a potent activator of JNK signaling. JNK immunokinase reactions revealed that each of these stimuli efficiently increased JNK activity (Figure 2B). Cells transfected with RACK1 also exhibited an increase in JNK activity, albeit to a lesser degree (2- to 4-fold) compared with TPA, TNFα, or UV (Figure 2B). These data suggest that on its own RACK1 cannot elicit the degree of activation seen after physiological stimuli. Indeed, TPA treatment of RACK1-transfected cells caused a marked increase in JNK activity, suggesting that RACK1 contributes to activation of JNK by TPA (Figure 2C).
To monitor possible changes in the association between endogenous RACK1 and JNK after TPA or UV irradiation, antibodies against JNK1 and JNK2 were used to determine changes in association with RACK1 before and after treatment. Of note, TPA treatment caused an increase in the amount of RACK1 bound JNK (see Figure S1A in the Supplemental Data available with this article online). Confocal microscopy-based immunofluorescence revealed colocalization of RACK1-JNK within the cytoplasm (Figure S1B), which was confirmed by immunoblot analysis (Figure S1C). In line with former studies (Ron et al., 1999), RACK1 localized throughout the cytoplasm in nonstimulated cells and exhibited perinuclear localization after TPA treatment (Figure S1B).
To further assess the effect of RACK1 on JNK activity, we generated siRNA for RACK1 (pRS-RACK1). Stable expression of pRS-RACK1 in MeWo (melanoma) cells caused 50%–70% inhibition of RACK1 expression (Figure 2D). Immunokinase reactions revealed 50%–60% inhibition of UV- and TPA-dependent JNK activation in the cells that were inhibited for RACK1 expression (Figure 2D and Figure S1D). MKK4 and/or MKK7 activation by UV irradiation was not affected by RACK1 siRNA (Figure S1D). Overexpression of exogenous RACK1 in cells that were inhibited for endogenous RACK1 expression (via siRNA to 5′UTR) increased JNK activity after TPA treatment (Figure S2A). These data provide direct evidence for the role of RACK1 in the activation of JNK by two quite different stimuli, TPA and UV irradiation.
Classical Isoforms of PKC Are Involved in TPA-Induced JNK Activation
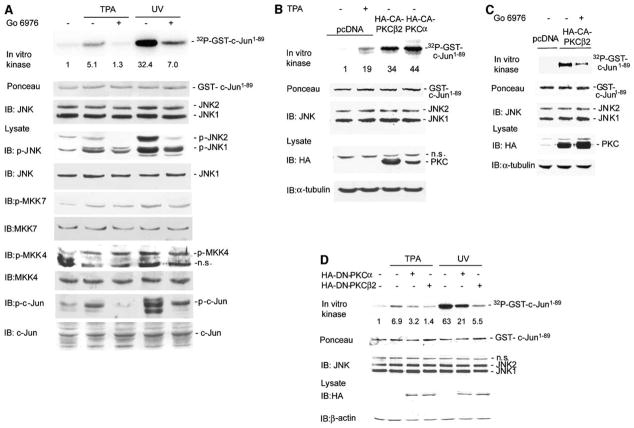
UV- and TPA-dependent activation of JNK1 and JNK2, monitored by immunokinase assays and by endogenous phosphorylation of c-Jun, was efficiently attenuated by Go6976, an inhibitor of classical PKC isoforms (Figure 3A and Figure S2B), although the activity of JNK upstream kinases MKK4 and/or MKK7 remained mostly unaffected (Figure 3A). Forced expression of constitutively active forms of PKCα (CA-PKCα) or PKCβII (CA-PKCβII) effectively induced JNK activation, as measured by immunokinase reactions (Figure 3B). Addition of Go6976 attenuated activation of JNK by CA-PKCβII (Figure 3C), further implicating classical PKC isoforms in the activation of JNK. Similarly, expression of dominant-negative PKCα (DN-PKCα) or PKCβII (DN-PKC-βII) attenuated UV- and TPA-dependent activation of JNK (Figure 3D). These observations are consistent with reports that established JNK activation by PKC (Werlen et al., 1998; Kawakami et al., 1998; Lang et al., 2004), although the underlying mechanism has remained obscure.
Figure 3. Classical Forms of PKC Mediate Activation of JNK by TPA.
(A) Go6976 blocks JNK activation by TPA and UV. MeWo cells were pretreated with Go6976 for 60 min followed by addition of TPA or UV irradiation. JNK in vitro kinase reactions and IB analysis were performed as described in Figure 2B. Quantification of JNK activity is shown (relative fold increase).
(B) PKCα and PKCβII increase JNK activity. HEK293 T cells were transfected with control vector (pcDNA) or 2 μg of HA-tagged constitutive active PKCβII or PKCα (HA-CA-PKCβII, HA-CA-PKCα). The JNK immunokinase reaction was performed as described. Protein lysates (40 μg) were subjected to IB with the indicated antibodies.
(C) Go6976 partially blocks activation of JNK by PKCβII. HEK293 T cells were transfected with control vector (pcDNA) or HA-CA-PKCβII (2 μg). Cells were treated with Go6976 for 60 min before harvesting. JNK immunokinase reactions were performed as described. IB with anti-HA antibody was performed to monitor the HA-CA-PKCβII expression level.
(D) Dominant negative PKC inhibits JNK activation. HEK293 T cells were transfected with 2 μg of HA-tagged dominant-negative PKCβII or PKCα (HA-DN-PKCβII, HA-DN-PKCα). Control cells were transfected with empty vector (pcDNA). Before harvesting, the cells were stimulated with UV or TPA. JNK immunokinase reaction was performed as described. Protein lysates (40 μg) were subjected to IB with the indicated antibodies.
RACK1 Mediates PKCβII Association with, and Phosphorylation of, JNK
To assess the role of RACK1 in PKC-mediated activation of JNK, we next performed in vitro binding assays with GST-JNK and 35S-labeled CA-PKCβII in the presence or absence of RACK1. Such association was not seen between CA-PKCβII and GST-JNK by themselves, but was observed upon inclusion of RACK1 in the reaction (Figure 4A). In vitro kinase reactions with active PKC and His-JNK2 as its substrate resulted in weak phosphorylation of JNK, which was markedly increased in the presence of RACK1 (Figure 4B). These data provide direct support for the role of RACK1 in JNK’s association with, and activation by, PKC. These observations are consistent with colocalization of JNK, RACK1, and PKC (Figures S1B and S1C). Further analysis was carried out by using the WD1–4 of RACK1, which was found to serve as a JNK docking site. Immunoprecipitation of JNK identified PKC and WD1–4 of RACK1 in the immunoprecipitated material (Figure S2C), implying that WD1–4 associates with both JNK and PKC.
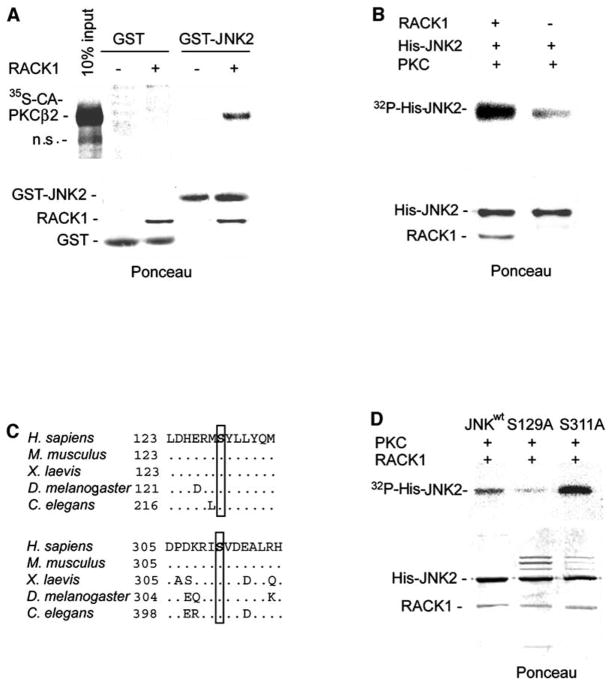
Figure 4. RACK1 Mediates PKC Binding and Phosphorylation of JNK.
(A) RACK1 is required for association between JNK and PKC in vitro. Beads containing GST or GST-JNK2 proteins (4 μg) were incubated with 35S-labeled CA-PKCβII in the presence or absence of His-RACK1 (2 μg). After washing, beads were subjected to SDS-PAGE, transferred to nitrocellulose, and analyzed with a phosphorimager (upper). Lower panel shows Ponceau S staining of the membrane.
(B) PKC phosphorylates JNK2 in vitro. An in vitro phosphorylation reaction was performed with active PKC (BioMol) and His-JNK2 (2 μg) as substrate in the presence or absence of His-RACK1 (1 μg). Ponceau S staining of the membrane is shown (lower).
(C) Consensus sites for PKC phosphorylation are conserved. Amino-acid sequences corresponding to the two putative phosphoacceptor sites for PKC are shown for JNK proteins of different species. Ser129 and 311 (H. sapiens numbering) are highlighted. Accession numbers are as follows: H. sapiens, AAC50609; M. musculus, AAD22579; X. laevis, BAB85483; D. melanogaster, AAB48381; C. elegans, NP741434.
(D) In vitro phosphorylation of JNK mutants by PKC. An in vitro kinase reaction was carried out by using purified PKC (BioMol) and the indicated His-JNK2 mutants (2 μg) in the presence of His-RACK1 (1 μg) and kinase buffer. Nickel bead bound JNK was washed and subjected to SDS-PAGE, transferred to nitrocellulose, and analyzed with a phosphorimager (upper). Lower panel shows Ponceau S staining of the membrane.
PKC Phosphorylates JNK on Ser129
Search of the JNK amino acid sequence for consensus sites for PKC phosphorylation revealed two putative PKC phosphoacceptor sites on Ser129 and Ser311, which are conserved (Figure 4C). To assess whether these residues serve as the PKC phosphoacceptor sites on JNK, we generated His-JNK2 mutated in those sites (JNKS129A and JNKS311A) and tested their phosphorylation by PKC in vitro. JNKS311A exhibited an increase in phosphorylation compared to JNKwt, but JNKS129A was poorly phosphorylated by PKC in vitro (Figure 4D). These data establish that S129 is the primary site of PKC phosphorylation on JNK.
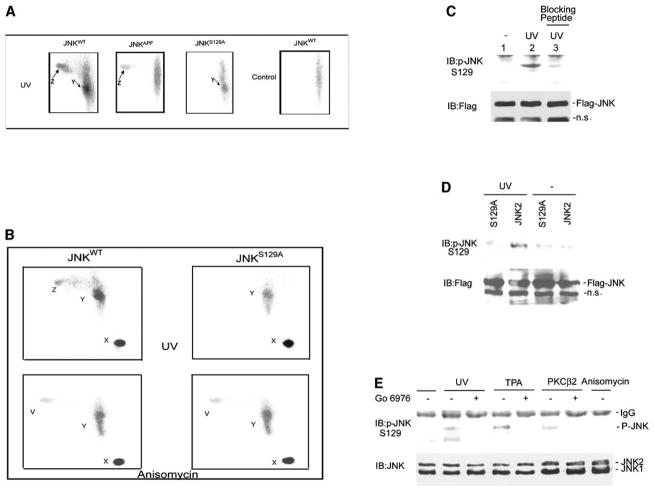
Detection of S129 Phosphorylation by Phosphopeptide Mapping and S129-Specific Phosphoantibodies
To confirm JNK phosphorylation by PKC in vivo, we monitored JNK phosphorylation in cells metabolically labeled with [32P]orthophosphate followed by trypsin phosphopeptide mapping. To identify peptides phosphorylated by PKC, but not by MKK4 and/or MKK7, patterns of phosphorylation of JNKwt, JNKS129A, and JNKAPF (which contain T183A and Y185A substitutions within the phosphoacceptor sites for MKK4 and/or MKK7) were compared.
Trypsin digest of JNKwt in nonstimulated cells identified phosphopeptide X, which represents undigested JNK molecules labeled under basal conditions (Figure 5A and Figure S2D). After UV treatment, two additional peptides were found to be phosphorylated in JNKwt (peptide Y and Z, Figure 5A). Parallel analysis of mutant JNKS129A revealed phosphorylation of the Y-, but not the Z-, peptide (Figure 5A). Conversely, peptide mapping of JNKAPF identified the Z-, but not the Y-, peptide. These data suggest that the Z peptide contains the S129 site, which is phosphorylated in vivo after UV stimulation.
Figure 5. Detection of S129 Phosphorylation In Vivo by Phosphopeptide Mapping and S129-Specific Phosphoantibodies.
(A and B) Mapping the site of PKC phosphorylation of JNK in vivo. Tryptic phosphopeptide mapping of JNK forms was carried out with JNKwt, JNKAPF, or JNKS129A transfected into 293 HEK cells that were incubated with [32P]orthophosphate followed by UV irradiation ([A] and [B]) or anisomycin treatment ([B]). Phosphorylated proteins were IP with anti-Flag Ab followed by their separation on SDS-PAGE, and corresponding bands were cut and subjected to in-gel digestion with trypsin. The tryptic digests were separated with thin-layer electrophoresis (horizontal dimension) followed by ascending chromatography (vertical dimension) and visualized by phosphorimager. The phosphopeptides X, Y, Z, and V are indicated. Note that running conditions differ among (A) and (B).
(C) JNK is phosphorylated in S129 after UV irradiation. HEK293 T cells were transfected with JNKwt (JNK2) (1 μg). Cells were serum starved and treated with UV as described in Figure 2B. Protein extracts (800 μg) were subjected to IP with anti-Flag Ab and blotted with the p-JNKS129 antibody. The antibody used to develop the membrane in lane 3 was preincubated with the phosphopeptide used for immunization. The membrane was reprobed with rabbit anti-Flag Ab to reveal quantities of Flag-JNK in the immunoprecipitates (lower).
(D) Antibodies to phosphorylated S129 do not recognize JNKS129A. HEK293 T cells were transfected with JNKwt or JNKS129A (1 μg). The experiment was performed as indicated in (C).
(E) Phosphoantibodies to S129 identify JNK phosphorylation upon exposure to PKC-dependent stimuli.
HEK293 T cells were subjected to the indicated treatment (see Experimental Procedures for details), and proteins were prepared 30 min later. Where indicated, cells were transfected with HA-CA-PKCβII (2 μg). Protein extracts (1 mg) were subjected to IP with mouse anti-JNK1 antibodies and blotted by using the p-JNKS129 antibody. The membrane was reprobed with rabbit anti-JNK antibodies to reveal quantities of JNK in the immunoprecipitates (lower).
Further analysis with TPA stimulation also revealed selective phosphorylation of both Y- and Z-peptides in JNKwt. As seen with UV, only the Z-peptide was phosphorylated in the JNKAPF form and the Y peptide in the JNKS129A form, further suggesting that the S129 site is within the Z peptide, which is phosphorylated by PKC in response to TPA and UV stimuli (Figure 5A and Figure S2D).
We next compared phosphorylation of peptides obtained from wild-type (wt) and S129A forms of JNK after either UV or anisomycin treatment. Anisomycin was selected for this comparison because its activation of JNK does not depend on PKC nor does it require S129 (Figure 6B). This comparison confirmed the presence of Z peptide after UV, but not after anisomycin treatment. The Z peptide was no longer seen in JNKS129A-derived fragments after UV or anisomycin treatment (Figure 5B). Of note, anisomycin treatment resulted in the formation of a new peptide (V), suggesting independent phosphorylation that is induced by this stimulus (Figure 5B). These data demonstrate that Z peptide is modified upon TPA and UV irradiation and is no longer observed after anisomycin treatment or with S129A mutant, suggesting that this peptide contains the Ser129 phosphoacceptor site.
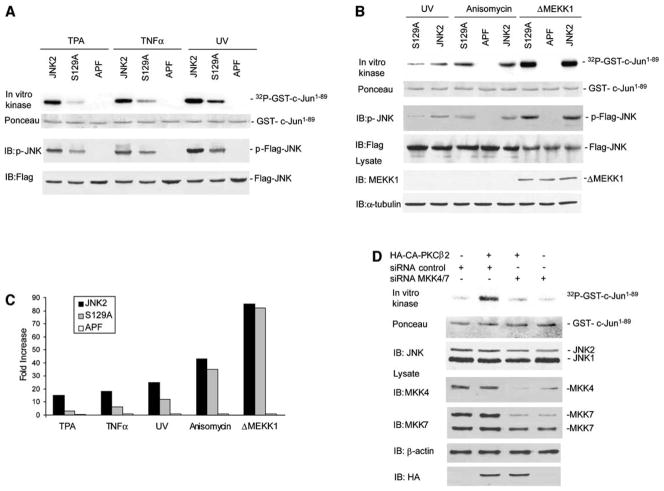
Figure 6. Ser129 Is Required for JNK Activation by PKC and Augments JNK Activation by MKK4 and MKK7.
(A) Mutation of Ser129 decreases JNK activation after TPA, TNFα, and UV stimulation. HEK293 T cells were transfected with JNKwt (JNK2), JNKAPF (APF), or JNKS129A (S129A) (1 μg). Cells were treated with TNFα, TPA, or UV as described in Figure 2B. Protein extracts (400 μg) were subjected to IP with anti-Flag Ab and to a JNK in vitro kinase assay. Middle panel shows IB with antibodies to JNK phosphorylated on T183 and Y185. The membrane was reprobed with rabbit anti-Flag Ab to reveal quantities of JNK variants in the immunoprecipitates (lower).
(B) Ser129 mutation does not affect JNK activation by anisomycin and ΔMEKK1. The experiment was performed as described in (A) except that, where indicated, cells were transfected with 1 μg of ΔMEKK1. Anisomycin (10 μg/ml) was added 60 min before harvesting the cells. ΔMEKK1 expression was assessed by IB with 40 μg of cell lysate.
(C) Activation of JNK variants by several stimuli. Graph shows relative activation of JNKwt (JNK2), JNKAPF (APF), and JNKS129A (S129A) by TPA, TNFα, UV, anisomycin, and ΔMEKK1, as shown in (A) and (B).
(D) MKK4 and MKK7 are required for JNK activation. U2OS cells were transfected with HA-tagged CA-PKCβII and a pool of siRNA oligos against MKK4 and MKK7 (Dharmacon). Cells were harvested after 60 hr, and a JNK immunokinase reaction was performed as described. Cell lysates (50 μg) were analyzed by IB with the indicated antibodies.
To further establish the phosphorylation of endogenous JNK on S129, we have generated antibodies that detect the phosphorylated form of S129 (p-JNKS129). The p-JNKS129 antibodies detected phosphorylation on S129 after UV, which was inhibited by the phosphorylated peptide corresponding to this domain (Figure 5C). Further, p-JNKS129 antibodies did not detect the S129A mutant form of JNK (Figure 5D), thereby establishing the specificity of these phospho-antibodies. The p-JNKS129 antibodies identified changes in S129 phosphorylation of endogenous JNK after TPA, UV treatment, or expression of CA-PKCβII, but not after exposure to anisomycin (Figure 5E). The phosphorylation of S129 induced by CA-PKCβII, TPA, or UV treatment was inhibited when cells were pretreated with the PKC inhibitor Go6976 (Figure 5E). Collectively, the phosphomapping combined with the use of p-JNKS129 antibodies establish the phosphorylation of S129 in vivo after physiological stimuli that involve PKC.
S129 Is Required for JNK Activation by TPA, TNFα, and UV, but Not Anisomycin or MEKK1
To further assess the importance of S129 phosphorylation for JNK activation, we compared the levels of JNKwt, JNKS129A, and JNKAPF activity in HEK293 cells after TPA, TNFα, or UV irradiation. Immunokinase reactions revealed that substitution of Ala for Ser129 reduced the degree of JNK activation by these treatments, although the degree of decrease varied among the different stimuli (Figure 6A). The most significant decrease was seen after TPA treatment, where JNK activity was reduced by over 80% relative to that of JNKwt (Figures 6A and 6C), compared with 60% inhibition after TNFα treatment and 40% inhibition after UV treatment. Decrease in JNK activity was also reflected in reduced JNK phosphorylation by MKK4 and/or MKK7 on 183 and 185 phosphoacceptor sites (Figure 6A).
However, the activity of JNKS129A was only marginally reduced after anisomycin treatment (a PKC-independent JNK activator, see below) and was equal to that seen with JNKwt after expression of the constitutively active form of MEKK1 (ΔMEKK1) (Figures 6B and 6C). Anisomycin treatment or ΔMEKK1 expression also induced a similar level of phosphorylation on residues 183 and 185 in both JNKwt and JNKS129A (Figure 6B), which is in contrast to the decrease in phosphorylation and activity of JNKS129A seen after treatment by TPA, TNFα, or UV irradiation. The difference observed in JNK activation was also seen under conditions in which the degree of JNK activation by anisomycin and UV were comparable, thereby excluding the possibility that such differences are due to the different strength of these stimuli (Figure S3A). These findings suggest that Ser129 is required for JNK activation by stimuli that also activate PKC. Indeed, common to UV, TNFα, and TPA is their reported activation of PKC (Laouar et al., 1999; Fukunaga et al., 2001).
To substantiate the role of PKC in the activation of JNK by TNFα, but not by anisomycin, we monitored changes in JNK activation by each of these stimuli in cells subjected to treatment with PKC inhibitor Go6976. Immunokinase assays revealed that the inhibitor efficiently inhibited JNK activation by TNFα but had no effect on JNK activation by anisomycin (Figure S3B).
Together, these results suggest that phosphorylation on S129 is important for JNK activation by certain stimuli, probably through augmenting subsequent JNK phosphorylation by MKK4 and/or MKK7 on T183 and Y185, resulting in an increase of overall JNK activity.
PKC Activation of JNK Requires MKK4 and/or MKK7
The observation that phosphorylation on Ser129 of JNK augments its phosphorylation on T183 and Y185 by MKK4 and/or MKK7 led us to evaluate the role of MKK4 and/or MKK7 in PKC activation of JNK. To this end, we inhibited expression of both MKK4 and MKK7 by corresponding RNAi. Transfection of RNAi oligos directed to MKK4 and MKK7 caused about 50% inhibition in their expression (Figure 6D). Whereas expression of CA-PKCβII induced JNK activation in control-transfected cells, inhibition of MKK4 and/or MKK7 expression markedly inhibited JNK activation by PKC, as measured by immunokinase assays and by monitoring phosphorylation of JNK on T183 and Y185 (Figure 6D). These data indicate that MKK4 and/or MKK7 is required for JNK activation by PKC. Given the notion that phosphorylation of Ser129 may augment MKK4 and/or MKK7 phosphorylation of JNK, our data suggest that PKC phosphorylation of JNK on S129 serves to increase MKK4- and/or MKK7-dependent JNK activation.
RACK1 Is Important for Protection of Melanoma Cells from UV-Induced Apoptosis
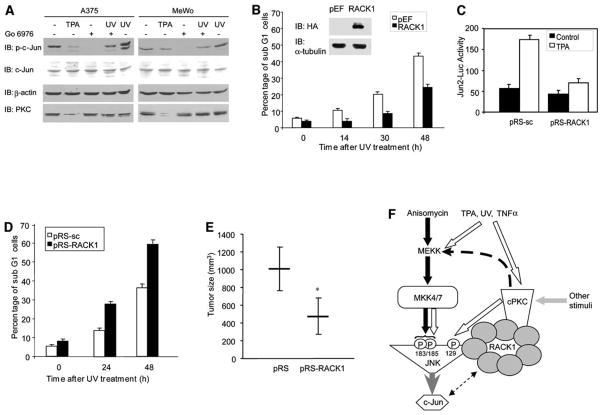
To further assess the role of RACK1 in PKC-mediated activation of JNK, we used melanoma cells. Analysis of RACK1 expression revealed low levels in melanocytes compared with a higher level in melanoma-derived cells (Figure S3C). Further, compared with melanocytes, RACK1 levels increased (2-fold) in early-stage melanoma cells, whereas a greater increase (up to 6-fold) was observed in late-stage and metastatic melanoma cell lines (Figure S3C). The melanoma cells also exhibited elevated basal levels of Jun phosphorylation, which was attenuated after their treatment with Go6976 or prolonged (24 hr) incubation with TPA (which down-regulates PKC after prolonged exposure; Figure 7A), pointing to the role of PKC in JNK-Jun activities in these cells.
Figure 7. RACK1 Is Important in Melanoma Resistance to Apoptosis and Tumorigenesis.
(A) PKC is involved in activation of c-Jun in melanoma cells. MeWo and A375 cells were serum-starved for 12 hr or, where indicated, treated with TPA for 24 hr. Before harvesting, cells were pretreated with Go6976 for 60 min followed by UV irradiation. Protein extracts (40 μg) were analyzed by IB with the indicated antibodies.
(B) Overexpression of RACK1 increases resistance of melanoma cells to UV-induced apoptosis. MeWo cells stably transfected with control pEF or HA-RACK1 were harvested 14 hr, 30 hr, or 48 hr after UV irradiation (60 J/m2), and the degree of apoptosis was monitored via FACS analysis. Inset: Protein extracts from control cells and cells overexpressing HA-RACK1 were blotted with antibodies to HA and α-tubulin. Data represent the mean ± SD from three independent experiments.
(C) siRNA of RACK1 attenuates Jun2-mediated transcription. MeWo cells stably transfected with pRS-sc and pRS-RACK1 (as shown in Figure 2D) were transiently transfected with Jun2-Luc construct. 40 hr after transfection, cells were treated with TPA for 8 hr, and proteins were prepared and used for luciferase and β-gal assays. Data represent the mean ± SD from three independent experiments.
(D) siRNA of RACK1 sensitizes melanoma cells to UV-induced apoptosis. MeWo cells stably transfected with pRS-sc and pRS-RACK1 (as shown in Figure 2D) were subjected to UV irradiation (60 J/m2), and the degree of apoptosis was determined 24 hr and 48 hr later via FACS. Data represent the mean ± SD from three independent experiments.
(E) Reduced RACK1 expression attenuates tumorigenesis of melanoma cells. MeWo cells (3 × 106) that stably express control vector (pRS) or pRS-RACK1 was injected subcutaneously into groups of NCR/Nu mice (five per group), and tumor growth was monitored for 35 days. The average tumor growth 35 days after injection is shown. Error bars represent standard deviation; *p < 0.001 (t test).
(F) Proposed model. Activation of JNK by certain stimuli (i.e., anisomycin or MEKK activation) proceeds through MKK4 and/or MKK7 activation and phosphorylation of JNK in residues 183 and 185 (filled arrows). Other stimuli (i.e., TPA, UV, TNFα) activate classical PKC (cPKC) isoforms as well as MKK4 and MKK7 (clear arrows). By serving as a docking site for PKC and JNK (via different WD40 domains), RACK1 mediates PKC phosphorylation of JNK on S129, which augments JNK phosphorylation by MKK4 and MKK7. Accordingly, RACK1 emerges as a regulatory protein that enables crosstalk between PKC and MKK4- and/or MKK7-signaling cascades in the course of JNK activation.
To elucidate the possible role of RACK1 expression in transcription and sensitization of melanoma cells to apoptosis (which are mediated by JNK substrates), MeWo cells were transfected with RACK1, and stable clones that overexpress this protein were selected (Figure 7B, inset). Because TPA treatment does not induce cell death in melanoma cells, these cells were subjected to UV irradiation. Elevated RACK1 expression in the cells markedly increased (up to 50%) their resistance to UV-induced apoptosis (Figure 7B).
Given the direct relationship among RACK1 expression, c-Jun activity, and radiation resistance, we performed the reciprocal experiment in which RACK1 expression was inhibited by pRS-RACK1. Cells that express pRS-RACK1 were transfected with Jun2-Luc, enabling monitoring of AP-1 transcriptional activities. TPA treatment resulted in efficient activation of Jun2-Luc, which was attenuated in cells that express pRS-RACK1 (Figure 7C). This finding points to the role of RACK1 in PKC-mediated activation of JNK-Jun. Opposite to what was observed upon overexpression of RACK1, the expression of selective siRNA for RACK1 markedly sensitized melanoma cells to UV-induced apoptosis (Figure 7D). Together, these findings establish the role of RACK1 in the resistance of melanoma to UV-induced cell death.
The sensitization of human melanoma cells to UV-induced apoptosis upon inhibition of RACK1 expression led us to evaluate possible changes in the tumorigenicity of melanoma cells whose RACK1 expression was inhibited. Subcutaneous injection of MeWo cells that stably express pRS control vector resulted in moderate growth, producing 1012 mm3 tumors after 35 days. A marked reduction (50%) in tumor growth was observed in mice injected with MeWo cells that stably express pRS-RACK1 (474 mm3; Figure 7E). These data point to the role of RACK1 in melanoma cell tumorigenicity and substantiate earlier studies that established the role of JNK and ATF2 in these processes (Bhoumik et al., 2004).
Discussion
The participation of PKC and phorbol esters in upstream regulation of JNK has been well documented, although the precise mechanism remained elusive. Earlier studies pointed to the possible role of Rac-cdc42, MEKK, and MKK4/MKK7 (Kawakami et al., 1998; Lang et al., 2004); yet, other pathways independent of MKK4 and MKK7 have also been proposed (Kawakami et al., 1998; Mauro et al., 2002; Lang et al., 2004). The present study identifies RACK1 as a key component in PKC-mediated JNK activation and demonstrates that PKC phosphorylation of JNK on Ser129 augments JNK activation by MKK4 and/or MKK7, pointing to the mechanism underlying PKC activation of the JNK signaling cascade. Our findings also provide understanding of the mechanism underlying the crosstalk between PKC and MKK4 and/or MKK7 in response to diverse stimuli.
Our studies reveal the role of RACK1 in JNK activation by classic PKC isoforms. Yet, other members of the PKC family are likely to also use the pathway identified in the present study. Consistent with this possibility is the finding that PKCε forms functional signaling complexes with JNK (Ping, 2003) and that both PKCβII and PKCε were shown to associate with RACK1 (Mochly-Rosen and Gordon, 1998).
Given the role of MKK4 and/or MKK7 in the activation of JNK, it is important to define the relationship between these kinases and PKC in JNK activation. Inhibition of MKK4 and/or MKK7 expression by corresponding RNAi attenuated PKC’s ability to activate JNK, suggesting that MKK4 and MKK7 are required for PKC-dependent JNK activation, in agreement with former studies (Mitsutake et al., 2001; Kaneki et al., 1999). Because RACK1 does not appear to serve as a docking site for the MEKK1-MKK4/7 complex (data not shown), it is likely that MKK4- and/or MKK7-independent phosphorylation of JNK by PKC serves to augment the degree of JNK activation.
Indeed, mutation of S129 attenuates JNK activation by PKC-activating stimuli. Furthermore, certain stimuli, including anisomycin (which does not mediate activation of PKC) and MEKK1, efficiently activate wt and S129A forms of JNK. These findings point to cooperation between PKC and MKK4 and/or MKK7 in JNK activation (Figure 7F). The newly identified relationship between diverse signaling pathways engaged in JNK activation may function as a mechanism that regulates the degree and possibly the duration of JNK activity as a kinase. Accordingly, RACK1 emerges as an important regulator of JNK signaling, as it converges both MKK4 and/or MKK7 and PKC components, thereby establishing the means for crosstalk among diverse signal-transduction pathways.
Our findings also define the minimal domain required for RACK1’s association with JNK. The fourth WD40 domain is only one of the seven WD40 domains of RACK1, raising the possibility that different domains may be occupied by other signaling components. Whereas WD4 appears to be the primary site for JNK association (this study), WD2, WD3, and WD6 were suggested to mediate association with PKC (Ron et al., 1994).
Relative levels of RACK1 appear to be important to the degree of PKC-dependent JNK activation. Changes in RACK1 expression were reported in different types of human tumors and are illustrated in the present study through the changes observed in human melanoma-derived cell lines. High levels of RACK1 expression were reported in nonsmall-cell lung (Berns et al., 2000) and colon carcinoma (Berns et al., 2000; Saito et al., 2002), implying that RACK1 may contribute to the resistance as well as tumorigenicity of other tumor types. Our data provide initial support for this possibility, because forced expression of RACK1 renders melanoma cells more resistant to apoptosis, whereas inhibition of RACK1 expression sensitizes them to treatment and reduces their tumorigenicity.
Our finding of Ser phosphorylation on JNK, in addition to its phosphorylation on Tyr183 and Thr185, are in line with Ser phosphorylation that regulates the activity of other members of the MAPK family, which are structurally similar to JNK. ERK1 and ERK5 as well as SEK1 are subjected to Ser phosphorylation in addition to Tyr and/or Thr (Zheng et al., 2004; Arcand et al., 2004; Park et al., 2002). Of further interest is that JNK was originally reported as having constitutive Ser phosphorylation (Derijard et al., 1994). The degree of PKC activity will determine the level of basal or inducible Ser phosphorylation on JNK, which is expected to differ among tissue and cell type. Similarly, it is possible that RACK1 will also mediate PKC phosphorylation of selective JNK isoforms in a cell-type-dependent manner.
It is intriguing to note that the original finding of c-Jun phosphorylation upon UV irradiation implicated Src-Ras as the signaling cascade (Devary et al., 1992). Given the role of Src in the phosphorylation of RACK1, which has been implicated in the regulation of PKC activity (Chang et al., 2001, 2002), it is plausible that this regulatory module will also regulate the phosphorylation of JNK on S129 by PKC, thereby providing mechanistic insight for the original observation that Src is important to the cellular response to UV irradiation. The link established between PKC-RACK1 and JNK in the present studies also provides plausible explanations for the mechanisms underlying RACK1 contribution to cell adhesion. JNK phosphorylation of paxillin (Huang et al., 2003) may be mediated through RACK1, which was shown to play a Src-dependent role in focal adhesion assembly and in cell motility (Cox et al., 2003).
Of interest is that S129 is partly buried and/or slightly exposed and would not be accessible to a kinase in the conformation observed in the available JNK3 structure (Xie et al., 1998). It may be significant that this region has been shown crystallographically to be the binding site for the inhibitory (by an allosteric mechanism) protein JIP1 (Heo et al., 2004). Because binding to this region has been shown to control JNK kinase activity allosterically, it is possible that RACK1 association-dependent phosphorylation at S129 is having an allosteric (in this case activating) effect on JNK kinase activity.
Collectively, the current study identifies RACK1 as a hitherto unrecognized component in JNK signaling that mediates JNK activation by PKC, resulting in JNK phosphorylation by PKC on Ser129. Such phosphorylation serves to augment JNK phosphorylation by MKK4 and/or MKK7 on T183 and Y185, resulting in its increased activity in response to physiological stimuli that activates PKC.
Experimental Procedures
Constructs
Constructs encoding Flag-JNK2, GST-Jun1–89, and His-JNK2 were previously described (Fuchs and Ronai, 1999). The plasmids encoding ΔMEKK and Flag-JNKAPF (containing T183A and Y185A mutations in the MKK4 and/or MKK7 phosphoacceptor sites) were kindly provided by M. Karin. Plasmids encoding for constitutive active and dominant-negative PKCβII and PKCα were described (Soh and Weinstein, 2003). Human RACK1 (Hermanto et al., 2002) was cloned into the mammalian expression vector pEF-HA to generate pEF-HA-RACK1. RACK1 was subcloned into the bacterial expression vector pTAT (kindly provided by S. Dowdy, UCSD) to generate TAT-His-RACK1-tagged protein (His-RACK1). pEF-HA-WD1-4 and pEF-HA-WD5–7 contain the WD domains one to four (residues 1–180) and five to seven (residues 181 to 317), respectively, of RACK1. These constructs were generated by PCR amplification followed by cloning into the BamHI–NotI sites of the pEF-HA plasmid. These fragments were also cloned into pTAT. WD domains four (residue 134 to 180) and five (residues 181 to 230) were cloned in the BamHI–NotI sites of the pcDNA3 vector. Mutations on Flag- JNK2 and His-JNK2 expression vector at S129A and S311A were introduced by using the Quick Change Site-Directed Mutagenesis Kit (Stratagene) and confirmed by DNA sequencing.
Cell Lines, Reagents, and Transfection
Melanoma cell lines were kindly provided by Dr. M. Herlyn and were maintained as indicated (Satyamoorthy et al., 1997). HEK293 T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with fetal bovine serum (10%) and antibiotics. Cells were transfected by calcium phosphate or by using LipofectAMINE PLUS Reagent (Invitrogen) according to the manufacturer’s protocol. Antibodies and reagents were purchased as follows: anti-RACK1 antibody (Ab), Transduction Technologies; anti-JNK1 and anti-JNK2 Ab, Santa Cruz; anti-phospho-JNK Ab, Promega; anti-MKK7 Ab, anti-MKK4 Ab, anti-phospho-MKK7 Ab, anti-phospho-MKK4 Ab, and anti-phospho-cJun Ab, all from Cell Signaling; anti-cJun, Santa Cruz; anti-HA Ab, 12CA5, Babco; and anti-Flag Ab, Sigma. Phosphoantibodies to S129 of JNK (p-JNKS129) were generated by immunizing rabbits with the peptide DANLCQVIHMELD HERMSPYLLYQMLCGIKHLHSAG followed by selective purification on phosphopeptide columns (PhosphoSolutions, Aurora, CO). Antibodies were used in immunoblots at a 1/50 dilution in 3% BSA in TBS. In the competition experiment, the antibody was preincubated with a 5-fold excess of blocking peptide in a small volume (500 μl) of PBS for 2 hr at room temperature. Final concentrations of the reagents were as follows: TPA (Sigma), 20 ng/ml; hTNFα (R&D), 40 ng/ml; anisomycin (Sigma), 10 μg/ml; and Go6976 (Calbiochem), 3 μM. When Go6976 or anisomycin were used, control reactions were performed with DMSO.
Luciferase Assays
Cells cultured in six-well plates were transiently transfected with 0.2 μg of 5X-Jun2-Luc and pCMV–gal (0.1 μg). At 36 hr after transfection, cells were treated with or without TPA (20 ng/ml), and protein samples were prepared 8 hr after treatment. Luciferase activity was measured with the luciferase assay system (Promega) in a luminometer and normalized to the β-galactosidase activity in the same sample.
RNA Interference
A pair of oligonucleotides containing 19 bp of human RACK1 (accession no. M24194) from nt 228–246 (CTGACCAGGGATGAGA CCA) was generated as follows: 5′-gatccccCTGACCAGGGATGAGACCAttcaagagaTGGTCTCATCCCTGGTCAGttttggaaa-3′ and 5′-agcttttccaaaaACTGACCAGGGATGAGACCAtctcttgaaTGGTCTCATCCCTGGTCAGggg-3′. Oligonucleotides to target the 5′UTR region of RACK1 were as follows: 5′-gatccccGCCATCCAGTGCCATCCTCtcaagagaAGGATGGCACTGGATGGCTTttggaaa-3′ and 5′-agcttttccaaaaAGCCATCCAGTGCCTCCTCctcttgaaGAGGATGGCACTGGATGGCggg-3′. To construct pRS-RACK1, the oligos were annealed and ligated into BglII and HindIII sites of pRetroSuper vector (pRS, Brummelkamp et al., 2002). Construct integrity was confirmed by direct sequencing of the plasmid. Packaging of retroviral constructs was carried out in HEK293 T cells. MeWo cells were infected for 18 hr in the presence of 4 μg/ml polybrene (Sigma). pBabe-puro-EGFP was used to monitor the efficiency of transfection to 293T cells and infection. A pRS-scramble plasmid (pRS-sc) was used as a control by cloning the sequence GGCAGTTCC ACCCCAGTGC into pRS as described for pRS-RACK1. To target MKK4 and MKK7 expression, corresponding SMARTpool siRNA reagents were used. As a control, the corresponding siRNA control oligo was used (Dharmacon).
In Vivo 32P-Labeling and Tryptic Peptide Analysis
Metabolic 32P-labeling was performed in HEK293 T cells transfected with Flag-JNK mutants as described (Adler et al., 1995). JNK mutants were IP with anti-Flag antibodies and resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The band corresponding to Flag-JNK was excised and digested with the In-gel Tryptic Digestion kit (Pierce, IL) according to the manufacturer’s protocol. The 32P-labeled peptides were then separated in two dimensions on thin-layer cellulose plates by electrophoresis at pH 1.9 and chromatography in phosphochromatography buffer (Boyle et al., 1991) by using the Hunter Thin Layer Peptide Mapping Electrophoresis System (CBS Scientific, CA).
Apoptosis Analysis
Apoptosis was assessed by quantifying the percentage of hypodiploid nuclei undergoing DNA fragmentation to the left of the diploid G0/1 peak (Ivanov et al., 2001).
Supplementary Material
Acknowledgments
We thank I.B. Weinstein for PKC constructs; R. Davis and M. Karin for MKK4, MKK7, and JNK constructs; S. Dowdy for pTAT plasmid; and R. Agami for the pRS plasmid. Support from a National Cancer Institute grant (CA51995 to Z.R.) is gratefully acknowledged.
Footnotes
Supplemental Data including three figures, Supplemental Experimental Procedures, and Supplemental References are available with this article online at http://www.molecule.org/cgi/content/full/19/3/309/DC1/.
References
- Adler V, Schaffer A, Kim J, Dolan L, Ronai Z. UV irradiation and heat shock mediate JNK activation via alternate pathways. J Biol Chem. 1995;270:26071–26077. doi: 10.1074/jbc.270.44.26071. [DOI] [PubMed] [Google Scholar]
- Arcand M, Coulombe P, Dumas F, Meloche S. Phosphorylation of serine 273 is required for the nuclear function of Erk1. 12th International Conference on Second Messengers and Phosphoproteins.2004. [Google Scholar]
- Berns H, Humar R, Hengerer B, Kiefer FN, Battegay EJ. RACK1 is up-regulated in angiogenesis and human carcinomas. FASEB J. 2000;14:2549–2558. doi: 10.1096/fj.99-1038com. [DOI] [PubMed] [Google Scholar]
- Bhoumik A, Jones N, Ronai Z. Transcriptional switch by activating transcription factor 2-derived peptide sensitizes melanoma cells to apoptosis and inhibits their tumorigenicity. Proc Natl Acad Sci USA. 2004;101:4222–4227. doi: 10.1073/pnas.0400195101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle WJ, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243–247. doi: 10.1016/s1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- Buchner K. The role of protein kinase C in the regulation of cell growth and in signalling to the cell nucleus. J Cancer Res Clin Oncol. 2000;126:1–11. doi: 10.1007/PL00008458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BY, Chiang M, Cartwright CA. The interaction of Src and RACK1 is enhanced by activation of protein kinase C and tyrosine phosphorylation of RACK1. J Biol Chem. 2001;276:20346–20356. doi: 10.1074/jbc.M101375200. [DOI] [PubMed] [Google Scholar]
- Chang BY, Harte RA, Cartwright CA. RACK1: a novel substrate for the Src protein-tyrosine kinase. Oncogene. 2002;21:7619–7629. doi: 10.1038/sj.onc.1206002. [DOI] [PubMed] [Google Scholar]
- Cox EA, Bennin D, Doan AT, O’Toole T, Huttenlocher A. RACK1 regulates integrin-mediated adhesion, protrusion, and chemotactic cell migration via its Src-binding site. Mol Biol Cell. 2003;2:658–669. doi: 10.1091/mbc.E02-03-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;13:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Devary Y, Gottlieb RA, Smeal T, Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992;71:1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- Fleming Y, Armstrong CG, Morrice N, Paterson A, Goedert M, Cohen P. Synergistic activation of stress-activated protein kinase 1/c-Jun N-terminal kinase (SAPK1/JNK) isoforms by mitogen-activated protein kinase kinase 4 (MKK4) and MKK7. Biochem J. 2000;352:145–154. [PMC free article] [PubMed] [Google Scholar]
- Fuchs SY, Ronai Z. Ubiquitination and degradation of ATF2 are dimerization dependent. Mol Cell Biol. 1999;19:3289–3298. doi: 10.1128/mcb.19.5.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M, Oka M, Ichihashi M, Yamamoto T, Matsuzaki H, Kikkawa U. UV-induced tyrosine phosphorylation of PKC delta and promotion of apoptosis in the HaCaT cell line. Biochem Biophys Res Commun. 2001;289:573–579. doi: 10.1006/bbrc.2001.6025. [DOI] [PubMed] [Google Scholar]
- Heo YS, Kim SK, Seo CI, Kim YK, Sung BJ, Lee HS, Lee JI, Park SY, Kim JH, Hwang KY, et al. Structural basis for the selective inhibition of JNK1 by the scaffolding protein JIP1 and SP600125. EMBO J. 2004;23:2185–2195. doi: 10.1038/sj.emboj.7600212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanto U, Zong CS, Li W, Wang LH. RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling and promotes cell spreading and contact with extracellular matrix. Mol Cell Biol. 2002;22:2345–2365. doi: 10.1128/MCB.22.7.2345-2365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–223. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Bhoumik A, Krasilnikov M, Raz R, Owen-Schaub LB, Levy D, Horvath CM, Ronai Z. Cooperation between STAT3 and c-jun suppresses Fas transcription. Mol Cell. 2001;7:517–528. doi: 10.1016/s1097-2765(01)00199-x. [DOI] [PubMed] [Google Scholar]
- Kaneki M, Kharbanda S, Pandey P, Yoshida K, Takekawa M, Liou JR, Stone R, Kufe D. Functional role for protein kinase C beta as a regulator of stress-activated protein kinase activation and monocytic differentiation of myeloid leukemia cells. Mol Cell Biol. 1999;19:461–470. doi: 10.1128/mcb.19.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Hartman SE, Holland PM, Cooper JA, Kawakami T. Multiple signaling pathways for the activation of JNK in mast cells: involvement of Bruton’s tyrosine kinase, protein kinase C, and JNK kinases, SEK1 and MKK7. J Immunol. 1998;161:1795–1802. [PubMed] [Google Scholar]
- Kazanietz MG. Eyes wide shut: protein kinase C isozymes are not the only receptors for the phorbol ester tumor promoters. Mol Carcinog. 2000;28:5–11. doi: 10.1002/(sici)1098-2744(200005)28:1<5::aid-mc2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Lang W, Wang H, Ding L, Xiao L. Cooperation between PKC-alpha and PKC-epsilon in the regulation of JNK activation in human lung cancer cells. Cell Signal. 2004;16:457–467. doi: 10.1016/j.cellsig.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Laouar A, Glesne D, Huberman EJ. Involvement of protein kinase C-beta and ceramide in tumor necrosis factor-alpha-induced but not Fas-induced apoptosis of human myeloid leukemia cells. J Biol Chem. 1999;274:23526–23534. doi: 10.1074/jbc.274.33.23526. [DOI] [PubMed] [Google Scholar]
- Mauro A, Ciccarelli C, De Cesaris P, Scoglio A, Bouche M, Molinaro M, Aquino A, Zani BM. PKC alpha-mediated ERK, JNK and p38 activation regulates the myogenic program in human rhabdomyosarcoma cells. J Cell Sci. 2002;115:3587–3599. doi: 10.1242/jcs.00037. [DOI] [PubMed] [Google Scholar]
- Mitsutake N, Namba H, Shklyaev SS, Tsukazaki T, Ohtsuru A, Ohba M, Kuroki T, Ayabe H, Yamashita S. PKC delta mediates ionizing radiation-induced activation of c-Jun NH(2)-terminal kinase through MKK7 in human thyroid cells. Oncogene. 2001;20:989–996. doi: 10.1038/sj.onc.1204179. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D, Gordon AS. Anchoring proteins for protein kinase C: a means for isozyme selectivity. FASEB J. 1998;12:35–42. [PubMed] [Google Scholar]
- Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- Park HS, Kim MS, Huh SH, Park J, Chung J, Kang SS, Choi EJ. Akt (protein kinase B) negatively regulates SEK1 by means of protein phosphorylation. J Biol Chem. 2002;277:2573–2578. doi: 10.1074/jbc.M110299200. [DOI] [PubMed] [Google Scholar]
- Ping P. Identification of novel signaling complexes by functional proteomics. Circ Res. 2003;93:595–603. doi: 10.1161/01.RES.0000093221.98213.E0. [DOI] [PubMed] [Google Scholar]
- Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc Natl Acad Sci USA. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Jiang Z, Yao L, Vagts A, Diamond I, Gordon A. Coordinated movement of RACK1 with activated betaIIPKC. J Biol Chem. 1999;274:27039–27046. doi: 10.1074/jbc.274.38.27039. [DOI] [PubMed] [Google Scholar]
- Saito A, Fujii G, Sato Y, Gotoh M, Sakamoto M, Toda G, Hirohashi S. Detection of genes expressed in primary colon cancers by in situ hybridisation: overexpression of RACK 1. Mol Pathol. 2002;55:34–39. doi: 10.1136/mp.55.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyamoorthy K, DeJesus E, Linnenbach AJ, Kraj B, Kornreich DL, Rendle S, Elder DE, Herlyn M. Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res. 1997;7:S35–S42. [PubMed] [Google Scholar]
- Schechtman D, Mochly-Rosen D. Adaptor proteins in protein kinase C-mediated signal transduction. Oncogene. 2001;20:6339–6347. doi: 10.1038/sj.onc.1204778. [DOI] [PubMed] [Google Scholar]
- Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh JW, Weinstein IB. Roles of specific isoforms of protein kinase C in the transcriptional control of cyclin D1 and related genes. J Biol Chem. 2003;278:34709–34716. doi: 10.1074/jbc.M302016200. [DOI] [PubMed] [Google Scholar]
- Theodosiou A, Smith A, Gillieron C, Arkinstall S, Ashworth A. MKP5, a new member of the MAP kinase phosphatase family, which selectively dephosphorylates stress-activated kinases. Oncogene. 1999;18:6981–6988. doi: 10.1038/sj.onc.1203185. [DOI] [PubMed] [Google Scholar]
- Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 2001;15:1419–1426. doi: 10.1101/gad.888501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werlen G, Jacinto E, Xia Y, Karin M. Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. EMBO J. 1998;17:3101–3111. doi: 10.1093/emboj/17.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Gu Y, Fox T, Coll JT, Fleming MA, Markland W, Caron PR, Wilson KP, Su MS. Crystal structure of JNK3: a kinase implicated in neuronal apoptosis. Structure. 1998;6:983–991. doi: 10.1016/s0969-2126(98)00100-2. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Yin G, Yan C, Cavet M, Berk BC. 14-3-3beta binds to big mitogen-activated protein kinase 1 (BMK1/ERK5) and regulates BMK1 function. J Biol Chem. 2004;279:8787–8791. doi: 10.1074/jbc.M310212200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.