Abstract
Background
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency and the leading surgical cause of death in premature infants. We have shown that administration of exogenous heparin-binding EGF-like growth factor (HB-EGF) in protects the intestines from experimental NEC. The aim of the current study was to evaluate the effect of gain-of-function of endogenous HB-EGF on susceptibility to NEC.
Methods
Neonatal HB-EGF transgenic (TG) mice and their wild type (WT) counterparts were exposed to experimental NEC. An additional group of HB-EGF TG pups were also exposed to NEC, but received the HB-EGF antagonist cross-reacting material 197 (CRM 197) injected subcutaneously immediately after birth. To examine gut barrier function, HB-EGF TG and WT pups received intragastric fluorescein isothiocyanate-labeled dextran (FITC-dextran) under basal and stressed conditions, and serum FITC-dextran levels were measured.
Results
WT mice had an incidence of NEC of 54.2% whereas HB-EGF TG mice had a significantly decreased incidence of NEC of 22.7% (p=0.03). Importantly, administration of CRM 197 to HB-EGF TG pups significantly increased the incidence of NEC to 65% (p=0.004). HB-EGF TG mice had significantly decreased intestinal permeability compared to WT mice both under basal and stressed conditions.
Conclusions
Our results provide evidence that over expression of the HB-EGF gene decreases susceptibility to NEC, and that administration of the HB-EGF antagonist CRM 197 reverses this protective effect.
Keywords: HB-EGF, CRM 197, intestine, permeability, injury, necrotizing enterocoliitis
Introduction
Necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in preterm infants, and remains a major cause of long-term disability in those who survive the disease.[1,2] Although several lines of evidence suggest that neonatal risk factors, including prematurity, asphyxia, intestinal ischemia, and formula feeding, all contribute to the occurrence of NEC, the pathogenesis of this disease remains unclear. [3] Several potential preventive strategies have aimed at induction of gastrointestinal maturation in premature infants, however, none of them have led to consistently positive results. [4]
Heparin-binding EGF-like growth factor (HB-EGF) was initially identified in the conditioned medium of cultured human macrophages [5] and later found to be a member of the EGF family of growth factors. [6] HB-EGF exerts its mitogenic effects by binding to and activation of EGF receptor subtypes ErbB-1 and ErbB-4. [7] In addition, HB-EGF exerts chemotactic effects when binding to the HB-EGF specific receptor N-arginine dibasic convertase (NrdC). [8] The ability of HB-EGF to evoke a mitogenic response in a variety of cell types, and its expression in a large number of tissues, suggests multiple potential roles for HB-EGF in vivo.
Our previous studies have shown that administration of exogenous HB-EGF protects the intestines from injury in animal models of intestinal ischemia/reperfusion (I/R) injury[9], hemorrhagic shock and resuscitation (HS/R) [10], and neonatal NEC. [11] Examination of the role of endogenous HB-EGF in protection of the intestines from injury has been obtained by examining HB-EGF knockout (KO) mice. Using a model of intestinal I/R injury based on superior mesenteric artery occlusion (SMAO), we have shown that HB-EGF KO mice have increased intestinal histologic injury, decreased intestinal restitution, and decreased survival. [12] In addition, we have used a model of HS/R to show that HB-EGF KO mice have increased intestinal epithelial cell apoptosis and increased gut barrier failure. [13] More recently we have used a newborn mouse model of experimental NEC to show that loss of HB-EGF gene expression results in increased susceptibility to NEC and increased gut barrier failure, and that administration of enteral exogenous HB-EGF to HB-EGF KO mice reverses these effects. [14] These experiments using HB-EGF KO mice support our premise that endogenous HB-EGF is an important intestinal cytoprotective agent.
HB-EGF is initially produced as a membrane-anchored precursor molecule (pro-HB-EGF) that undergoes extracellular proteolytic cleavage to produce a 22-kDa soluble, secreted, mature glycoprotein (sHB-EGF). In order to further explore the roles of endogenous HB-EGF in the intestine, we produced HB-EGF TG mice in our laboratory. These mice were designed to specifically overexpress the human HB-EGF precursor (proHB-EGF) in the intestine using a 12.4 kb villin regulatory and promoter sequence to drive human proHB-EGF gene expression. [15] The villin promoter ensures the constant expression of HB-EGF throughout the entire intestine from the duodenum to the colon, throughout the entire crypt-villous axis.
It has been shown that the membrane anchored form of HB-EGF also acts as a diphtheria toxin receptor (DTR) [16,17]. HB-EGF/DTR is expressed in multiple tissues of many species including primates and rodents. [18] Cross-reacting material 197 (CRM197) includes a group of mutant diphtheria toxins obtained in the early 1970s from strains of Corynebacterium diphtheriae lysogenized with β-phages carrying a mutated tox gene. CRM197 is immunologically indistinguishable from diphtheria toxin (DT) and shares the ability of the native counterpart to bind to its specific cell-membrane receptor. [19] CRM specifically inhibits the activity of human HB-EGF without affecting other EGF family members. [20]
The goals of the current study were to utilize HB-EGF TG mice to examine the effects of HB-EGF over expression on susceptibility to experimental NEC and to determine whether we could use CRM197 in vivo to reverse the effects of HB-EGF overexpression.
Materials and Methods
Production of HB-EGF transgenic mice
HB-EGF TG mice were produced in our laboratory and were designed to specifically overexpress the human HB-EGF precursor (proHB-EGF) in the intestine using a 12.4 kb villin regulatory and promoter sequence to drive human proHB-EGF gene expression. [15] We confirmed expression of HB-EGF throughout the entire intestine from the duodenum to the colon, but not in other organs. Prolonged HB-EGF over-expression was confirmed by RT-PCR using Vill-HB-EGF specific primers at 1 and 5 months of life. The pBSII-12.4kbVill plasmid containing the 12.4kb promoter fragment from the villin gene was a generous gift from Dr. Deborah Gumucio (University of Michigan, Ann Arbor, MI). [21]
Neonatal mouse model of experimental necrotizing enterocolitis
The experimental protocol was performed according to the guidelines for the ethical treatment of experimental animals and approved by our Institutional Animal Care and Use Committee (Protocol #02205AR). NEC was induced using a modification of the model initially described by Barlow et al. [22], and modified for mice by Jilling et al. [23] Pregnant time-dated mice were delivered by C-section under inhaled 2% Isoflurane (Butler Animal Health, Dublin, OH) anesthesia on day 18.5 of gestation. Newborn mouse pups were placed in an incubator (37°C) and fed via gastric gavage with formula containing 15 g Similac 60/40 (Ross Pediatrics, Columbus, OH) in 75 mL Esbilac (Pet-Ag, New Hampshire, IL), providing 836.8 kJ/kg per day. Feeds were started at 0.03 mL every 3h beginning 2h after birth and advanced as tolerated up to a maximum of 0.05 mL per feeding by the fourth day of life. Animals were exposed to a single dose of intragastric lipopolysaccharide (LPS; 2 mg/kg), (Sigma-Aldrich, St. Louis, MO), 8h after birth, and were stressed by exposure to hypoxia (100% nitrogen for 1 min) followed by hypothermia (4°C for 10 min) twice a day beginning immediately after birth until the end of the experiment. Exposure of pups to hypoxia, hypothermia and hypertonic feeds will subsequently be referred to as exposure to “stress”.
To investigate the effects of HB-EGF gain-of-function on NEC incidence and severity, HB-EGF TG mouse pups (n=22) and their WT counterparts (n=24) were exposed to experimental NEC. An additional group of HB-EGF TG pups (n=20) were exposed to experimental NEC as described, but received a single dose of CRM197 (5 mg/kg) (List Biological Laboratories, Inc., Campbell, CA) injected subcutaneously immediately after birth. The dose of CRM197 administered was based on previous studies in mice that showed dose-dependent suppression of tumor growth with doses ranging from 5 to 50 mg/kg delivered by intraperitoneal injections.[24] In all experiments, pups were euthanized upon development of clinical signs of NEC (abdominal distention, bloody bowel movements, respiratory distress, lethargy) or by 96 h after birth.
Mucosal Permeability
To investigate mucosal permeability, we used fluorescein isothiocyanate (FITC)-labeled dextran molecules (molecular weight, 73 kDa) (Sigma-Aldrich Inc, St Louis, MO) as a probe. Previous studies by others have shown that use of 73-kDa dextran molecules results in a reliable assessment of mucosal perturbations 4 h after enteral administration. [25] In these experiments, FITC-labeled dextran molecules (750 mg/kg) were administered via orogastric tube to rat pups, and 4h later blood was collected for measurement of plasma FITC-dextran levels by spectrophotofluorometry (Molecular Devices, SpectraMax M2, Sunnyvale, Ca). Blood from mouse pups was collected by means of decapitation and use of heparinized microhematocrit capillary tubes (Fisher Scientific, Pittsburgh, PA). The amount of blood obtained (20μl) was then diluted using PBS to a total volume of 200 μl. The amount of dextran in the plasma was calculated based on standard dilution curves of known dextran concentrations. The resulting concentration was then corrected to compensate for the 1:10 dilution by multiplying it by 10. Mouse pups were divided into 7 groups as follows: 1) WT mice that received intragastric FITC-dextran immediately after birth with no exposure to stress (n=13); 2) HB-EGF TG mice that received intragastric FITC-dextran immediately after birth with no exposure to stress (n=11); 3) WT control pups that were delivered by C-section, were breast fed by surrogate mothers (since their natural mothers were sacrificed after the C-section), and that received intragastric FITC dextran after 24h with no exposure to stress (n=7); 4) WT control pups that received CRM197 immediately after delivery by C-section, that were breast fed by surrogate mothers, and that received intragastric FITC dextran after 24h with no exposure to stress (n=5); 5) WT mice that received intragastric FITC dextran after 24 h of stress (n=14); 6) HB-EGF TG mice that received intragastric FITC dextran after 24 h of stress (n=9); 7) HB-EGF TG mice that received CRM197 immediately after birth and intragastric FITC dextran after 24 h of stress (n=9).
Histologic Injury Score
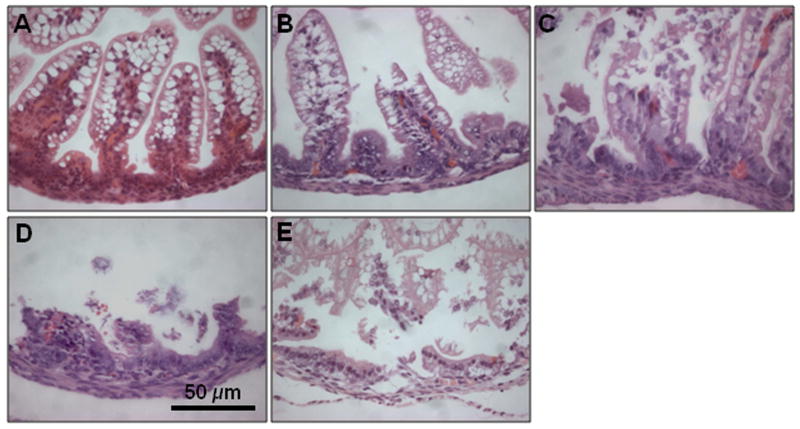
Upon sacrifice, the gastrointestinal tract was carefully removed and visually evaluated for signs of NEC (areas of bowel necrosis, intestinal hemorrhage, perforation). Three pieces of duodenum, jejunum, ileum, and colon from every animal were fixed in 10% formalin for 24 h, paraffin-embedded, sectioned at 5 μm thickness, and stained with hematoxylin and eosin for histological evaluation of the presence and/or degree of NEC using the NEC histologic injury scoring system described by Caplan et al. [26] Histological changes were graded as follows: grade 0, no damage; grade 1, epithelial cell lifting or separation; grade 2, sloughing of epithelial cells to the mid villus level; grade 3, necrosis of the entire villus; and grade 4, transmural necrosis (Figure 1). Tissues with histological scores of 2 or higher were considered positive for NEC. Tissues were graded blindly by two independent observers.
Figure 1.
Histologic injury score in mouse pups subjected to experimental NEC. Shown are representative H&E stained sections showing: A) normal intestine; B) Grade 1, epithelial cell lifting or separation; C) Grade 2, sloughing of epithelial cells to the mid villous level; D) Grade 3, necrosis of the entire villus; and E) Grade 4, transmural necrosis. Magnification × 40.
Statistical Analyses
The Chi-square test was used for comparing the incidence of NEC between groups. Serum concentrations of FITC-dextran were compared using the Student's t test. p-values less then 0.05 were considered statistically significant. All statistical analyses were performed using SAS software (Version 9.1, SAS Institute, Cary, NC).
Results
Histologic Injury
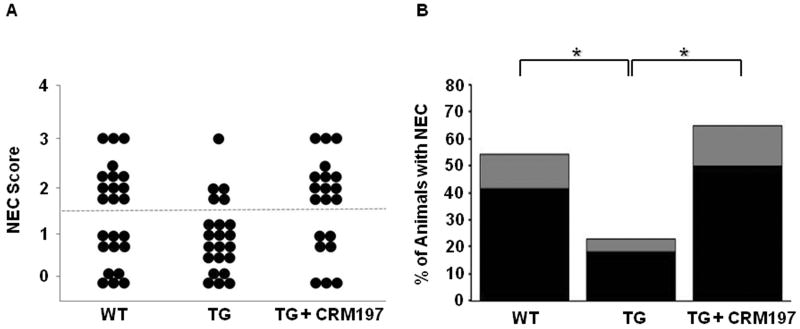
WT mice had an incidence of NEC of 54.2%, with histopathologic changes ranging from moderate, mid-level villous necrosis (grade 2) to severe necrosis of the entire villous (grade 3) (Figure 2). Of the 54.2% of WT pups that developed NEC, 41.7% had grade 2 injury and 12.5% had grade 3 injury. On the other hand, HB-EGF TG mouse pups had a significantly decreased incidence of NEC of 22.7%, with grade 2 injury seen in 18.2% and grade 3 injury seen in 4.5% of the animals that did develop NEC (p=0.03). Furthermore, HB-EGF TG pups that were exposed to stress but that received the HB-EGF antagonist CRM197 after birth showed a significant increase in the incidence of NEC to 65% compared with non-CRM197-treated HB-EGF TG pups (p=0.004). In addition to an increased incidence of NEC, administration of CRM197 to HB-EGF TG pups resulted in increased severity of NEC. Of the 65% of HB-EGF TG CRM treated pups that developed NEC, 50% had grade 2 injury and 15% had grade 3 injury.
Figure 2.
Incidence and severity of NEC in WT and HB-EGF TG mice. A) NEC score. Each dot represents a single rat pup exposed to stress, and the NEC score for each pup is shown. HB-EGF TG pups that were treated with CRM197 received 5 mg/kg injected subcutaneously immediately after birth. Dots located above the dotted line represent histologic injury > Grade 2, and are considered positive for NEC. WT (n=24); TG (n=22); TG+CRM197 (n=20) B) Incidence and severity of NEC. The percent of animals with NEC is shown. Black bars, grade 2 injury; grey bars, grade 3 injury; *p<0.05
Gut Barrier Function
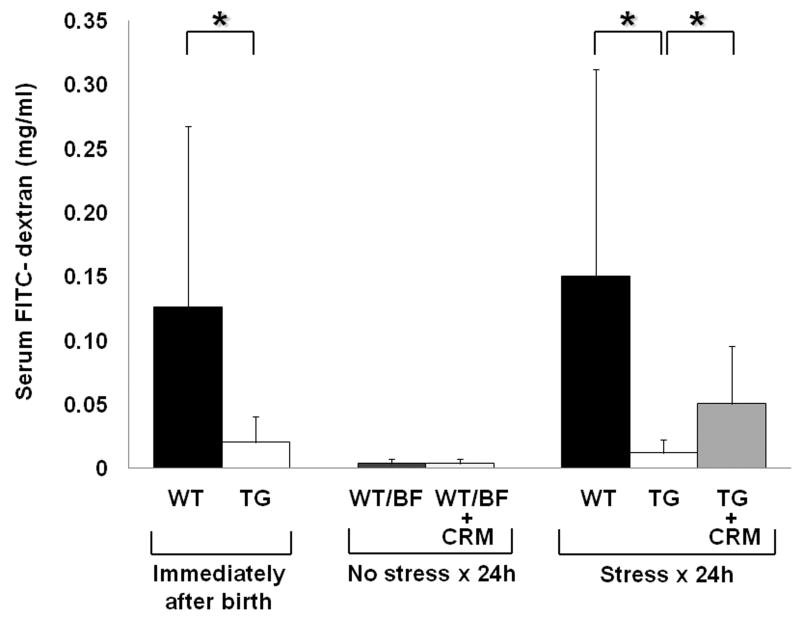
We next examined intestinal permeability to determine gut barrier function in WT, HB-EGF TG and HB-EGF TG + CRM197-treated pups exposed to experimental NEC. Under basal, non-stressed conditions immediately after birth, HB-EGF TG pups had significantly decreased serum FITC-dextran levels compared to WT pups (0.020 ± 0.019 mg/ml vs. 0.125 ± 0.141 mg/ml; p=0.02) (Figure 3). After 24 h of exposure to stress, HB-EGF TG mice still had significantly decreased serum FITC-dextran levels compared to WT mice under basal conditions (0.012 ± 0.010 mg/ml vs. 0.14 ± 0.16 mg/ml; p=0.01). However, HB-EGF TG pups that received CRM197 immediately after birth and were exposed to stress for 24h had significantly increased serum FITC-dextran levels compared to HB-EGF TG mouse pups that were exposed to the same stress for 24h without CRM197 administration (0.050 ± 0.045 mg/ml vs. 0.012 ± 0.010 mg/ml; p=0.02).
Figure 3.
Gut barrier function in WT and HB-EGF TG mice. Gut barrier function was determined by measuring serum FITC-dextran (mg/ml) levels 4 h after gastric administration of FITC-dextran. HB-EGF TG and WT pups that were treated with CRM197 received 5 mg/kg injected subcutaneously immediately after birth. *p<0.05. Gut barrier function was measured; immediately after birth in WT pups (n=24) and TG pups (n=22); after 24h of surrogate breast feeding with no exposure to stress in WT/BF (n=7) and WT/BF+CRM (n=22) pups; and after exposure to stress for 24h in WT (n=14), TG (n=9) and TG+CRM197 pups (n=9).
WT control pups that were delivered by C-section, treated with CRM197 immediately after birth, and breast fed for 24h by surrogate mothers, had similar serum FITC-dextran levels compared with WT mouse pups that were delivered by C-section and breast fed for 24h by surrogate mothers without CRM197 administration (0.0034 ± 0.0037 mg/ml vs. 0.003429 ± 0.00378 mg/ml; p=0.98). These results demonstrate that administration of CRM197 itself does not increase mucosal permeability.
HB-EGF TG mice have low FITC-dextran serum levels immediately after birth and maintain these low serum levels even after 24h of stress, suggesting that overexpression of endogenous HB-EGF acts to preserve gut barrier function. This may explain, in part, the decreased susceptibility of HB-EGF TG mice to NEC.
Discussion
We have previously examined HB-EGF mRNA expression in intestine resected from patients with NEC, comparing intestinal samples from areas afflicted with acute NEC to the more normal intestine at the resection margins. We found that NEC-afflicted intestine had significantly lower HB-EGF mRNA levels compared to intestine at the resection margins. [4] These findings suggest that decreased endogenous HB-EGF may predispose the intestines to the development of NEC. We have also shown that HB-EGF expression is increased in adult rat intestine exposed to experimental injury in vivo. [27] These seemingly different results may be due to an inability of premature newborns to increase intestinal HB-EGF levels upon exposure of the intestines to injury, due to immaturity of the premature intestinal tract. Previous studies from our laboratory as well as others have shown that expression of endogenous HB-EGF is significantly increased in response to tissue damage, [27,28] hypoxia [29] and oxidative stress, [30] and during wound healing and regeneration. [31] This pattern of expression is consistent with a pivotal role for HB-EGF in tissue regeneration and repair.
We have shown that administration of exogenous HB-EGF under experimental conditions protects the intestines from diverse injuries including intestinal I/R, [32] HS/R [33] and NEC. [11,34,35] Furthermore, we have previously shown that adult HB-EGF KO mice have increased intestinal injury upon exposure to intestinal I/R[12], HS/R [13] and NEC[14], and that the increased susceptibility of HB-EGF KO mice to NEC can be reversed by administration of enteral exogenous HB-EGF. [14] In addition, using a terminal ileal anastomosis model in mice, we have shown that HB-EGF KO mice have delayed healing of the intestinal anastomoses when compared to their WT counterparts, which was also reversed by administration of exogenous enteral HB-EGF. [36]
To investigate the effects of long term over-expression of HB-EGF on the intestine in vivo, mice that specifically over-expressed the human precursor HB-EGF gene in the intestine were produced in our laboratory. Under basal, non-injury conditions, mice over-expressing HB-EGF do not show any significant phenotypic alterations, even up to 1 ½ years of age. [15] However, when HB-EGF TG mice are exposed to HS/R, they are more resistant to intestinal injury. [15] In addition, we have examined HB-EGF TG mice using a terminal ileal anastomosis model, and have demonstrated that HB-EGF TG mice have augmented healing of intestinal anastomoses compared to WT mice. [36] We now show that endogenous HB-EGF over-expression in HB-EGF TG mouse pups protects the intestine from NEC.
Studies in critically ill adults have shown that impairment of mucosal barrier function with overgrowth of pathogenic bacteria in the gastrointestinal tract enhances translocation of bacteria and endotoxin, resulting in a septic inflammatory response and multiorgan failure. [37,38] Pathologic or dysregulated transfer of antigens across the intestinal barrier can lead to various diseases, including inflammatory bowel disease in adults, and NEC in premature infants. [39,40] Current hypotheses regarding the pathogenesis of NEC suggest that immaturity of the intestinal epithelial barrier and neonatal mucosal immune system predispose the premature infant to bacterial invasion and intestinal inflammation. Perinatal insults trigger the disease by inflicting a localized intestinal mucosal injury. [41] The impaired gut barrier function of premature babies under basal conditions may be similar to the impaired intestinal permeability we have previously reported in newborn HB-EGF KO mice under basal conditions. [14] When HB-EGF expression is decreased or absent, as in the intestine of neonates afflicted with NEC or in HB-EGF KO mice, gut barrier function is impaired, which may contribute to bacterial translocation leading to a systemic inflammatory response. [14] The results of the current study show that HB-EGF TG mice have preserved gut barrier function both under basal conditions and after exposure to stress. This may explain, in part, their decreased susceptibility to NEC.
Mitamura et al. have showed that CMR197 inhibits the mitogenic activity of HB-EGF.[20] Moreover, CRM197 does not inhibit other EGFR-binding growth factors. Therefore, although there is great redundancy amongst EGF family members, and although other EGF family members are also produced as membrane-anchored precursors that bind to EGFR in a manner similar to HB-EGF, HB-EGF appears to be the only EGF family member that functions as an efficient receptor for DT. The results of the current study show that administration of CRM197 to HB-EGF TG mice that over-express HB-EGF in the intestine results in impaired gut barrier function and increased incidence and severity of NEC. Furthermore, our CRM197 data suggests that despite HB-EGF over-expression during development, the HB-EGF being over-expressed needs to be functionally active at the time of injury in order to have a protective effect. The findings further support the contention that HB-EGF expression is important in protection of the intestines from NEC. Our long-term goal is the clinical use of HB-EGF in the prevention or treatment of NEC. Given the evidence to date demonstrating the protective effects of HB-EGF against intestinal injury, its use may represent a promising therapeutic strategy for necrotizing enterocolitis.
Acknowledgments
This work was funded by NIH R01 DK074611 (GEB). The authors thank Dr. Deborah Gumucio (Ann Arbor, MI) for providing us with the pBSII-12.4kbVill plasmid containing the 12.4kb promoter fragment from the villin gene and William Gardner Ph.D. (Biostatistics Core, The Research Institute at Nationwide Children's Hospital) for assistance with statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gribar SC, Anand RJ, Sodhi CP, Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J Leukoc Biol. 2008;83:493–498. doi: 10.1189/jlb.0607358. [DOI] [PubMed] [Google Scholar]
- 2.Anand RJ, Leaphart CL, Mollen KP, Hackam DJ. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock. 2007;27:124–133. doi: 10.1097/01.shk.0000239774.02904.65. [DOI] [PubMed] [Google Scholar]
- 3.Henry MC, Moss RL. Current issues in the management of necrotizing enterocolitis. Seminars in perinatology. 2004;28:221–233. doi: 10.1053/j.semperi.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Feng J, El-Assal ON, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:167–174. doi: 10.1053/j.sempedsurg.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Besner G, Higashiyama S, Klagsbrun M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990;1:811–819. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 7.Junttila TT, Sundvall M, Maatta JA, Elenius K. Erbb4 and its isoforms: selective regulation of growth factor responses by naturally occurring receptor variants. Trends in cardiovascular medicine. 2000;10:304–310. doi: 10.1016/s1050-1738(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 8.Nishi E, Prat A, Hospital V, Elenius K, Klagsbrun M. N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration. The EMBO journal. 2001;20:3342–3350. doi: 10.1093/emboj/20.13.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin AE, Luquette MH, Besner GE. Timing, route, and dose of administration of heparin-binding epidermal growth factor-like growth factor in protection against intestinal ischemia-reperfusion injury. J Pediatr Surg. 2005;40:1741–1747. doi: 10.1016/j.jpedsurg.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 10.El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142:234–242. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Radulescu A, Zorko NA, Yu X, Besner GE. Preclinical neonatal rat studies of heparin-binding EGF-like growth factor in protection of the intestines from necrotizing enterocolitis. Pediatr Res. 2009;65:437–442. doi: 10.1203/PDR.0b013e3181994fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Assal ON, Paddock H, Marquez A, Besner GE. Heparin-binding epidermal growth factor-like growth factor gene disruption is associated with delayed intestinal restitution, impaired angiogenesis, and poor survival after intestinal ischemia in mice. J Pediatr Surg. 2008;43:1182–1190. doi: 10.1016/j.jpedsurg.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang HY, Radulescu A, Besner GE. Heparin-binding epidermal growth factor-like growth factor is essential for preservation of gut barrier function after hemorrhagic shock and resuscitation in mice. Surgery. 2009;146:334–339. doi: 10.1016/j.surg.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radulescu A, Yu X, Orvets ND, et al. Deletion of the Heparin -binding EGF-like Growth Factor Gene Increases Susceptibility to Necrotizing Enterocolitis. J Pediatr Surg. 2010 doi: 10.1016/j.jpedsurg.2009.06.035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CL, Mehta VB, Zhang HY, et al. Intestinal phenotype in mice overexpressing a heparin-binding EGF-like growth factor transgene in enterocytes. Growth Factors. 2010;28:82–97. doi: 10.3109/08977190903407365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naglich JG, Metherall JE, Russell DW, Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992;69:1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto R, Higashiyama S, Mitamura T, et al. Heparin-binding EGF-like growth factor, which acts as the diphtheria toxin receptor, forms a complex with membrane protein DRAP27/CD9, which up-regulates functional receptors and diphtheria toxin sensitivity. The EMBO journal. 1994;13:2322–2330. doi: 10.1002/j.1460-2075.1994.tb06516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraham JA, Damm D, Bajardi A, et al. Heparin-binding EGF-like growth factor: characterization of rat and mouse cDNA clones, protein domain conservation across species, and transcript expression in tissues. Biochem Biophys Res Commun. 1993;190:125–133. doi: 10.1006/bbrc.1993.1020. [DOI] [PubMed] [Google Scholar]
- 19.Pappenheimer AM., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- 20.Mitamura T, Higashiyama S, Taniguchi N, Klagsbrun M, Mekada E. Diptheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. JBiolChem. 1995;270:1015–1019. doi: 10.1074/jbc.270.3.1015. [DOI] [PubMed] [Google Scholar]
- 21.Madison BB, Dunbar L, Qiao XT, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 22.Barlow B, Santulli TV, Heird WC, et al. An experimental study of acute neonatal enterocolitis--the importance of breast milk. J Pediatr Surg. 1974;9:587–595. doi: 10.1016/0022-3468(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 23.Jilling T, Simon D, Lu J, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177:3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yagi H, Yotsumoto F, Sonoda K, et al. Synergistic anti-tumor effect of paclitaxel with CRM197, an inhibitor of HB-EGF, in ovarian cancer. Int J Cancer. 2009;124:1429–1439. doi: 10.1002/ijc.24031. [DOI] [PubMed] [Google Scholar]
- 25.Caplan MS, Miller-Catchpole R, Kaup S, et al. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 26.Caplan MS, Hedlund E, Adler L, Hsueh W. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol. 1994;14:1017–1028. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 27.Xia G, Rachfal AW, Martin AE, Besner GE. Upregulation of endogenous heparin-binding EGF-like growth factor (HB-EGF) expression after intestinal ischemia/reperfusion injury. J Invest Surg. 2003;16:57–63. [PubMed] [Google Scholar]
- 28.Cribbs RK, Harding PA, Luquette MH, Besner GE. Endogenous production of heparin-binding EGF-like growth factor during murine partial-thickness burn wound healing. J Burn Care Rehabil. 2002;23:116–125. doi: 10.1097/00004630-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Jin K, Mao XO, Sun Y, et al. Heparin-binding epidermal growth factor-like growth factor: hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci. 2002;22:5365–5373. doi: 10.1523/JNEUROSCI.22-13-05365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank GD, Mifune M, Inagami T, et al. Distinct mechanisms of receptor and nonreceptor tyrosine kinase activation by reactive oxygen species in vascular smooth muscle cells: role of metalloprotease and protein kinase C-delta. Mol Cell Biol. 2003;23:1581–1589. doi: 10.1128/MCB.23.5.1581-1589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy DW, Downing MT, Brigstock DR, et al. Production of heparin-binding epidermal growth factor-like growth factor (HB-EGF) at sites of thermal injury in pediatric patients. J Invest Dermatol. 1996;106:49–56. doi: 10.1111/1523-1747.ep12327214. [DOI] [PubMed] [Google Scholar]
- 32.El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609–625. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 33.El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142:234–242. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2006;41:144–149. doi: 10.1016/j.jpedsurg.2005.10.018. discussion 144-149. [DOI] [PubMed] [Google Scholar]
- 35.Yu X, Radulescu A, Zorko N, Besner GE. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology. 2009;137:221–230. doi: 10.1053/j.gastro.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radulescu A, Zhang H, Chen CL, et al. Expression levels of Heparin -binding EGF-like Growth Factor affect Intestinal Anastomotic Wound Healing. J Surg Res. 2010 doi: 10.1016/j.jss.2010.06.036. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deitch EA. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch Surg. 1990;125:403–404. doi: 10.1001/archsurg.1990.01410150125024. [DOI] [PubMed] [Google Scholar]
- 38.Hadfield RJ, Sinclair DG, Houldsworth PE, Evans TW. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. American journal of respiratory and critical care medicine. 1995;152:1545–1548. doi: 10.1164/ajrccm.152.5.7582291. [DOI] [PubMed] [Google Scholar]
- 39.Sharma R, Tepas JJ, 3rd, Hudak ML, et al. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007;42:454–461. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 40.Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- 41.Petrosyan M, Guner YS, Williams M, Grishin A, Ford HR. Current concepts regarding the pathogenesis of necrotizing enterocolitis. Pediatr Surg Int. 2009;25:309–318. doi: 10.1007/s00383-009-2344-8. [DOI] [PubMed] [Google Scholar]