Abstract
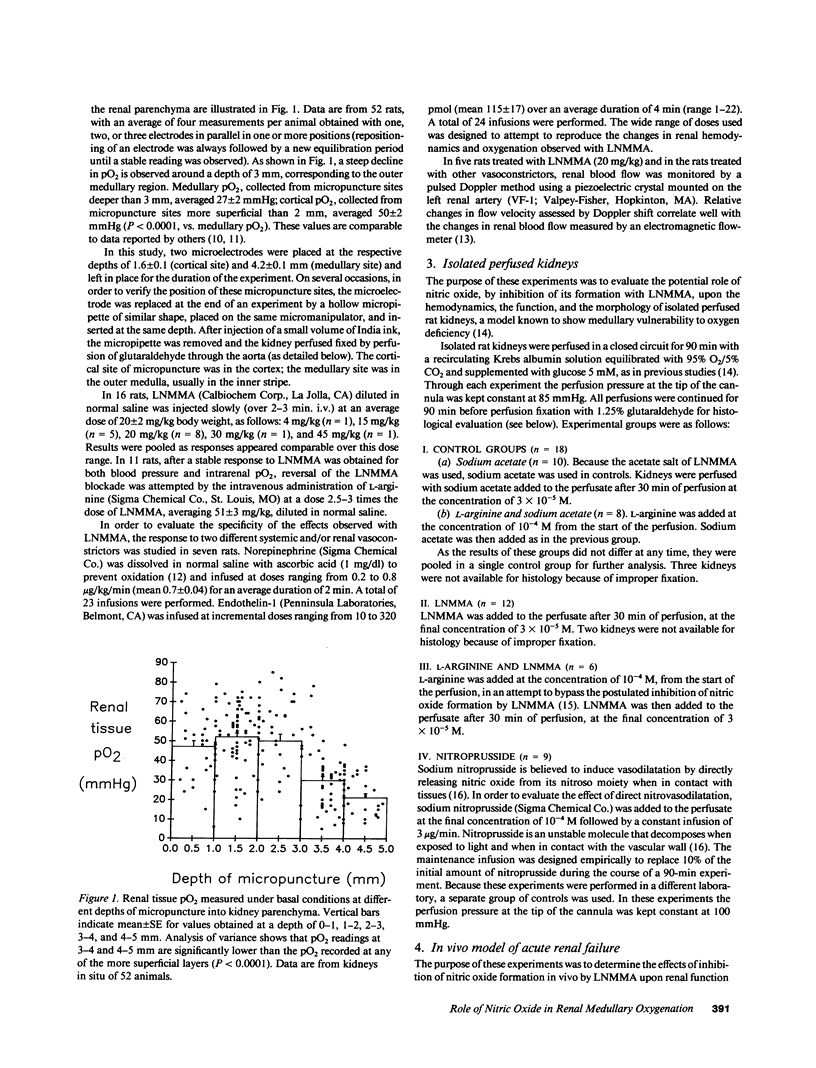
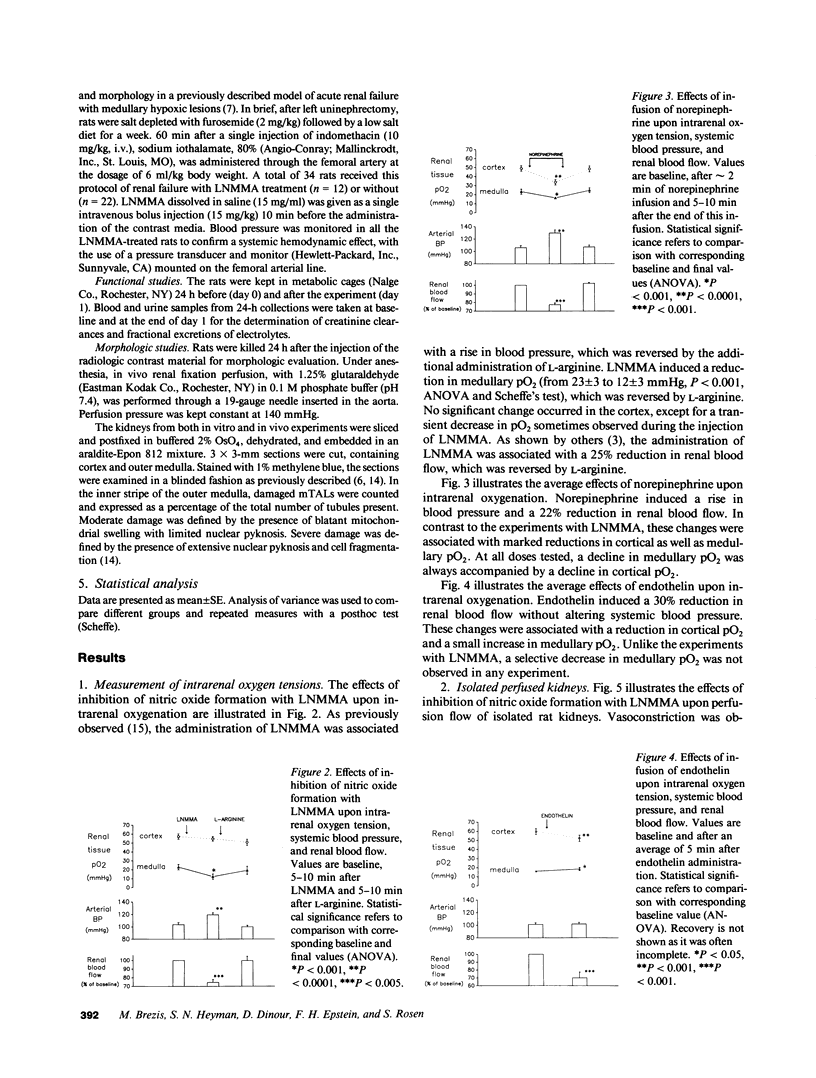
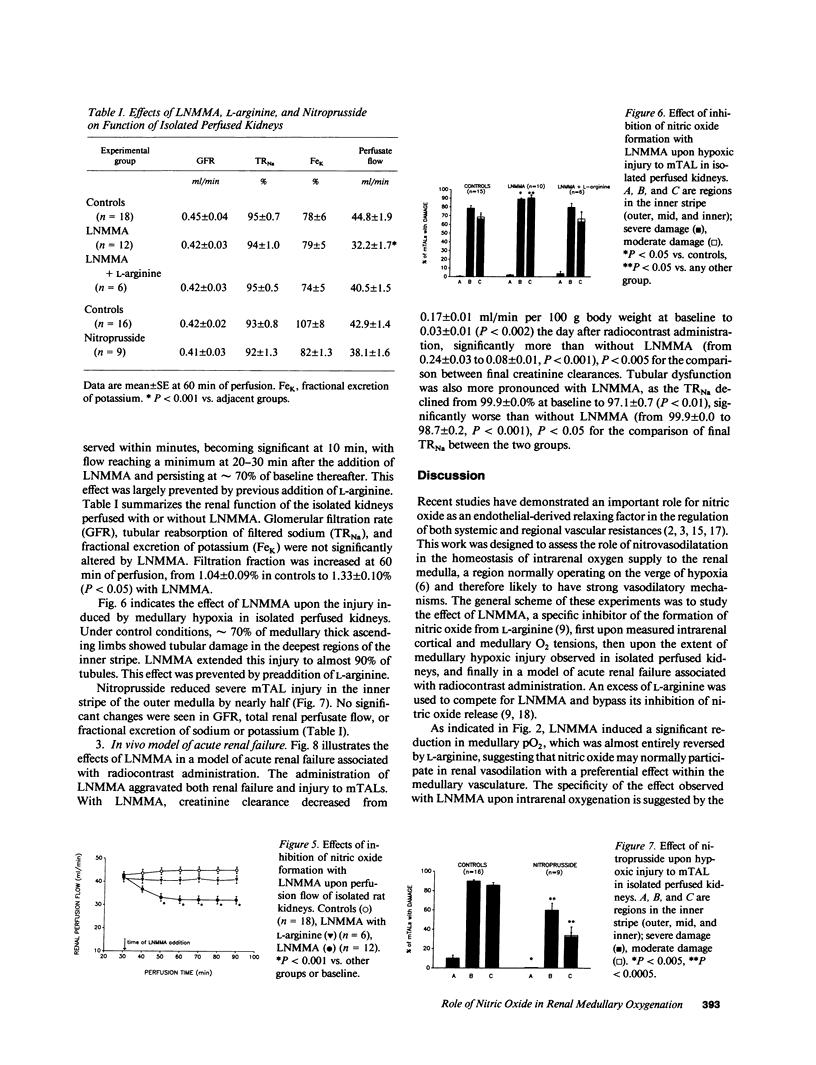
We investigated the role of the endothelial-derived relaxing factor nitric oxide (NO) in the homeostasis of O2 supply to the renal medulla, a region normally operating on the verge of hypoxia. Sensitive Clark-type O2 microelectrodes were inserted into renal cortex and medulla of anesthetized rats. The inhibitor of NO formation, L-NG-monomethylarginine (LNMMA), while increasing blood pressure and reducing renal blood flow, decreased medullary pO2 from 23 +/- 3 mmHg to 12 +/- 3 (P less than 0.001), with no change in the cortex. These responses were promptly reversed by L-arginine, which bypasses the LNMMA blockade. In isolated rat kidneys, LNMMA reduced perfusion flow without altering glomerular filtration rate, and augmented deep medullary hypoxic injury to thick ascending limbs from 68 to 90% of the tubules (P less than 0.02). These changes were prevented by L-arginine. Nitroprusside had a protective effect upon thick limb injury. Finally, in a previously reported model of radiocontrast nephropathy (1988. J. Clin. Invest. 82:401), LNMMA increased the severity of renal failure (final plasma creatinine from 2.3 +/- 2 mg% to 3.4 +/- 3, P less than 0.005) and the proportion of damaged thick limbs (from 24 +/- 6% to 53 +/- 9, P less than 0.01). Nitrovasodilatation may participate in the balance of renal medullary oxygenation and play an important role in the prevention of medullary hypoxic injury.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badr K. F., Murray J. J., Breyer M. D., Takahashi K., Inagami T., Harris R. C. Mesangial cell, glomerular and renal vascular responses to endothelin in the rat kidney. Elucidation of signal transduction pathways. J Clin Invest. 1989 Jan;83(1):336–342. doi: 10.1172/JCI113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach R. E., Schwab S. J., Brazy P. C., Dennis V. W. Norepinephrine increases Na+-K+-ATPase and solute transport in rabbit proximal tubules. Am J Physiol. 1987 Feb;252(2 Pt 2):F215–F220. doi: 10.1152/ajprenal.1987.252.2.F215. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Troy J. L., Ballermann B. J. Endothelium-dependent vascular responses. Mediators and mechanisms. J Clin Invest. 1989 Nov;84(5):1373–1378. doi: 10.1172/JCI114309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezis M., Greenfeld Z., Shina A., Rosen S. Angiotensin II augments medullary hypoxia and predisposes to acute renal failure. Eur J Clin Invest. 1990 Apr;20(2):199–207. doi: 10.1111/j.1365-2362.1990.tb02269.x. [DOI] [PubMed] [Google Scholar]
- Brezis M., Rosen S. N., Epstein F. H. The pathophysiological implications of medullary hypoxia. Am J Kidney Dis. 1989 Mar;13(3):253–258. doi: 10.1016/s0272-6386(89)80062-9. [DOI] [PubMed] [Google Scholar]
- Brezis M., Rosen S., Silva P., Epstein F. H. Renal ischemia: a new perspective. Kidney Int. 1984 Oct;26(4):375–383. doi: 10.1038/ki.1984.185. [DOI] [PubMed] [Google Scholar]
- Brezis M., Rosen S., Silva P., Epstein F. H. Selective vulnerability of the medullary thick ascending limb to anoxia in the isolated perfused rat kidney. J Clin Invest. 1984 Jan;73(1):182–190. doi: 10.1172/JCI111189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. Y., Porush J. G., Faubert P. F. Renal medullary circulation: hormonal control. Kidney Int. 1990 Jan;37(1):1–13. doi: 10.1038/ki.1990.1. [DOI] [PubMed] [Google Scholar]
- Dantzler W. H., Silbernagl S. Amino acid transport by juxtamedullary nephrons: distal reabsorption and recycling. Am J Physiol. 1988 Sep;255(3 Pt 2):F397–F407. doi: 10.1152/ajprenal.1988.255.3.F397. [DOI] [PubMed] [Google Scholar]
- Eriksen E. F., Richelsen B., Gesser B. P., Jacobsen N. O., Stengaard-Pedersen K. Prostaglandin-E2 receptors in the rat kidney: biochemical characterization and localization. Kidney Int. 1987 Aug;32(2):181–186. doi: 10.1038/ki.1987.190. [DOI] [PubMed] [Google Scholar]
- Fadem S. Z., Hernandez-Llamas G., Patak R. V., Rosenblatt S. G., Lifschitz M. D., Stein J. H. Studies on the mechanism of sodium excretion during drug-induced vasodilatation in the dog. J Clin Invest. 1982 Mar;69(3):604–610. doi: 10.1172/JCI110487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990 May;15(5):486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- Haywood J. R., Shaffer R. A., Fastenow C., Fink G. D., Brody M. J. Regional blood flow measurement with pulsed Doppler flowmeter in conscious rat. Am J Physiol. 1981 Aug;241(2):H273–H278. doi: 10.1152/ajpheart.1981.241.2.H273. [DOI] [PubMed] [Google Scholar]
- Heyman S. N., Brezis M., Reubinoff C. A., Greenfeld Z., Lechene C., Epstein F. H., Rosen S. Acute renal failure with selective medullary injury in the rat. J Clin Invest. 1988 Aug;82(2):401–412. doi: 10.1172/JCI113612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987 Dec;61(6):866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Kleinjans J. C., Smits J. F., van Essen H., Kasbergen C. M., Struyker Boudier H. A. Hemodynamic characterization of hypertension induced by chronic intrarenal or intravenous infusion of norepinephrine in conscious rats. Hypertension. 1984 Sep-Oct;6(5):689–699. doi: 10.1161/01.hyp.6.5.689. [DOI] [PubMed] [Google Scholar]
- Lahera V., Salom M. G., Fiksen-Olsen M. J., Raij L., Romero J. C. Effects of NG-monomethyl-L-arginine and L-arginine on acetylcholine renal response. Hypertension. 1990 Jun;15(6 Pt 1):659–663. doi: 10.1161/01.hyp.15.6.659. [DOI] [PubMed] [Google Scholar]
- Leichtweiss H. P., Lübbers D. W., Weiss C., Baumgärtl H., Reschke W. The oxygen supply of the rat kidney: measurements of int4arenal pO2. Pflugers Arch. 1969 Jun 19;309(4):328–349. doi: 10.1007/BF00587756. [DOI] [PubMed] [Google Scholar]
- Levillain O., Hus-Citharel A., Morel F., Bankir L. Localization of arginine synthesis along rat nephron. Am J Physiol. 1990 Dec;259(6 Pt 2):F916–F923. doi: 10.1152/ajprenal.1990.259.6.F916. [DOI] [PubMed] [Google Scholar]
- MacCumber M. W., Ross C. A., Glaser B. M., Snyder S. H. Endothelin: visualization of mRNAs by in situ hybridization provides evidence for local action. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7285–7289. doi: 10.1073/pnas.86.18.7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn F. A., Millan M., Quirion R., Aguilera G., Chou S. T., Catt K. J. Localization of angiotensin II receptors in rat and monkey kidney by in vitro autoradiography. Kidney Int Suppl. 1987 May;20:S40–S44. [PubMed] [Google Scholar]
- Pallone T. L., Robertson C. R., Jamison R. L. Renal medullary microcirculation. Physiol Rev. 1990 Jul;70(3):885–920. doi: 10.1152/physrev.1990.70.3.885. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Radermacher J., Förstermann U., Frölich J. C. Endothelium-derived relaxing factor influences renal vascular resistance. Am J Physiol. 1990 Jul;259(1 Pt 2):F9–17. doi: 10.1152/ajprenal.1990.259.1.F9. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma I., Stuehr D. J., Gross S. S., Nathan C., Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurek H. J., Jost U., Baumgärtl H., Bertram H., Heckmann U. Evidence for a preglomerular oxygen diffusion shunt in rat renal cortex. Am J Physiol. 1990 Dec;259(6 Pt 2):F910–F915. doi: 10.1152/ajprenal.1990.259.6.F910. [DOI] [PubMed] [Google Scholar]
- Tolins J. P., Palmer R. M., Moncada S., Raij L. Role of endothelium-derived relaxing factor in regulation of renal hemodynamic responses. Am J Physiol. 1990 Mar;258(3 Pt 2):H655–H662. doi: 10.1152/ajpheart.1990.258.3.H655. [DOI] [PubMed] [Google Scholar]
- Vane J. R., Anggård E. E., Botting R. M. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]



