Mutations in otoferlin are linked to human hearing loss. New research defines a function for this C2 domain–containing protein in synaptic vesicle exocytosis in cochlear hair cells.
Abstract
Otoferlin is a large multi–C2 domain protein proposed to act as a calcium sensor that regulates synaptic vesicle exocytosis in cochlear hair cells. Although mutations in otoferlin have been associated with deafness, its contribution to neurotransmitter release is unresolved. Using recombinant proteins, we demonstrate that five of the six C2 domains of otoferlin sense calcium with apparent dissociation constants that ranged from 13–25 µM; in the presence of membranes, these apparent affinities increase by up to sevenfold. Using a reconstituted membrane fusion assay, we found that five of the six C2 domains of otoferlin stimulate membrane fusion in a calcium-dependent manner. We also demonstrate that a calcium binding–deficient form of the C2C domain is incapable of stimulating membrane fusion, further underscoring the importance of calcium for the protein’s function. These results demonstrate for the first time that otoferlin is a calcium sensor that can directly regulate soluble N-ethyl-maleimide sensitive fusion protein attachment protein receptor–mediated membrane fusion reactions.
Introduction
The cochlea is a fluid-filled coiled tube found within the inner ear, where it plays a central role in mammalian hearing. In response to sound, hair bundles located on hair cells within the cochlea are deflected, resulting in the opening of mechanosensitive ion channels that alter the voltage of the cell membrane. This change in membrane potential results in the activation of L-type voltage-gated calcium channels to allow calcium influx, which triggers synaptic vesicle exocytosis and neurotransmitter release (Glowatzki and Fuchs, 2002). This chain of events must occur quickly to distinguish temporally between sounds (Rose et al., 1967). Synapses formed by inner hair cells (IHCs) also contain specialized structures called synaptic ribbons that are capable of tethering numerous synaptic vesicles at release sites to facilitate high rates of sustained synaptic transmission (Liberman, 1980; Glowatzki and Fuchs, 2002).
The vesicle fusion step is believed to be mediated by the SNARE proteins synapbtobrevin 2, syntaxin 1A, and SNAP-25 on the vesicle and plasma membranes (Safieddine and Wenthold, 1999). However, SNAREs are not sensitive to calcium, and in reconstituted fusion assays, they mediate fusion on time scales that are orders of magnitude slower than observed in vivo (Weber et al., 1998). In neurons, calcium sensitivity and fast kinetics are typically conferred by SNARE-binding proteins, including synaptotagmin I/II (Chapman, 2008). However, mature hair cells have little or no synaptotagmin I/II (Safieddine and Wenthold, 1999), and it has been proposed that the transmembrane protein otoferlin might serve as the calcium sensor that regulates hair cell neurotransmitter release by accelerating SNARE-mediated membrane fusion (Roux et al., 2006).
Otoferlin occurs in multiple splice variants, including a 1,997–amino acid form, consisting of a transmembrane segment and six cytoplasmic C2 domains, and a shorter three–C2 domain isoform found in humans but not mice (Yasunaga et al., 2000). A role for otoferlin in IHC neurotransmitter release is based on several lines of evidence, including the finding that mice lacking otoferlin are profoundly deaf but have intact and functional mechanoelectrical transduction processes (Roux et al., 2006). These mice also have functional afferent neurons, suggesting that otoferlin’s role in the auditory pathway lies within the hair cell between the mechanoelectrical transduction and afferent neuron activation steps. In support of this idea, hair cells in otoferlin knockout mice do not release neurotransmitter in response to membrane depolarization and calcium influx, despite having apparently normal synapse morphology (Roux et al., 2006). In addition, >16 different nonsense and missense mutations within the human gene encoding otoferlin have been linked to a type of nonsyndromic hearing loss known as DFNB9. However, subcellular localization studies have led to conflicting conclusions. An immunogold staining study reports localization to synaptic vesicles (Roux et al., 2006), whereas immunofluorescence indicated that otoferlin is localized predominantly in the Golgi and possibly in early endosomes (Schug et al., 2006; Heidrych et al., 2008). Thus, otoferlin may have alternative or additional functions, such as vesicle recycling or membrane trafficking in the Golgi. Recently, it was reported that point mutations within otoferlin resulted in plasma membrane infolding (Heidrych et al., 2008). This suggests a role for otoferlin in the endocytosis and vesicle recycling and could explain why otoferlin IHC knockouts exhibit defects in exocytosis.
From cell-based studies, it is not entirely clear as to whether otoferlin contributes directly to exocytosis or is involved in secondary events up and/or downstream of synaptic vesicle fusion. However, it should be noted that these cellular processes place different functional demands on the protein, and one can thereby gain insight into the role of otoferlin in IHCs through in vitro functional studies. Specifically, if otoferlin is indeed a calcium sensor involved in neurotransmitter release, we would expect recombinant forms of the protein to bind calcium, SNAREs, and membranes and to accelerate SNARE-mediated membrane fusion in a calcium-dependent manner. In this study, we screened the individual otoferlin C2 domains for these properties to determine whether otoferlin operates as a calcium sensor. In addition, a larger three–C2 domain construct, C2DEF, was created to ascertain the properties of the reported smaller three–C2 domain splice variant. A C2ABC construct was analyzed in parallel to determine what the additional N-terminal three–C2 domains, found in the larger isoform, contribute to the function of the molecule.
Results
Otoferlin is a calcium sensor
It has been suggested that otoferlin might function as a calcium sensor in cochlear IHCs, outer hair cells, and vestibular cells, analogous to the role that synaptotagmin I/II play during SNARE-mediated neurotransmitter release in neurons (Roux et al., 2006). Both C2 domains of synaptotagmin I bind calcium ions through coordination with aspartate residues located on one end of each C2 domain (Shao et al., 1998; Fernandez et al., 2001). Alignment of the primary sequence of the C2 domains of otoferlin with the calcium-binding aspartate residues of synaptotagmin I reveals a lack of conservation among the first three N-terminal C2 domains of the protein (Fig. 1, A and B). However, C2 domains from several other proteins do not possess all five aspartate residues yet, in some cases, are still able to bind calcium (Therrien et al., 2009). Therefore, it is unclear from inspection of the sequence as to which domains of otoferlin, if any, bind calcium. To address this question, we examined the intrinsic fluorescence of recombinant otoferlin constructs as a function of calcium concentration. In several cases, the fluorescence spectra of aromatic amino acids within C2 domains have been shown to be sensitive to the binding of calcium (Nalefski et al., 2001).
Figure 1.
Schematic diagram of full-length otoferlin and the otoferlin fragments used in this study. (A) Otoferlin is composed of six tandem C2 domains followed by a C-terminal transmembrane domain (TMD). Recombinant proteins composed of either isolated C2 domains or three–C2 domain fragments were generated according to the amino acid designations listed on the right. (B) Conservation of putative calcium-binding ligands of the C2 domains of otoferlin (oto). Synaptotagmin I (syt) C2A and C2B are listed for comparison. The numbers correspond to the order of the five aspartate residues that function as ligands in the C2A (D172, 178, 230, 232, and 238) and C2B domains (D303, 309, 363, 365, 371) of synaptotagmin I (Sutton et al., 1995; Shao et al., 1998; Fernandez et al., 2001).
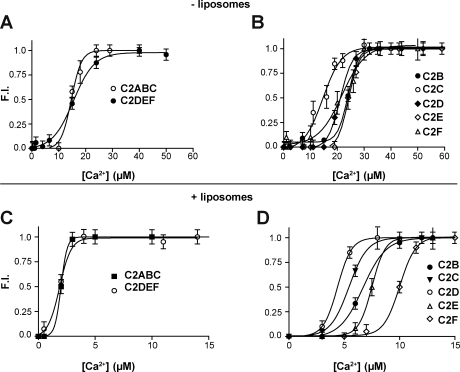
As an initial screen for calcium binding, the fluorescence spectra of recombinant otoferlin constructs were measured in the presence either 0.1 mM EGTA or 1 mM calcium. As shown in Fig. 2, addition of calcium resulted in significant increases in the fluorescence intensity of isolated C2B, C2C, C2D, C2E, and C2F, but not C2A. In addition, the recombinant fragments C2ABC and C2DEF also exhibited significant increases in fluorescence in response to calcium. For several otoferlin fragments, we also detected a shift in the emission wavelength maxima (λmax). These shifts were most prominent in the larger three–C2 domain fragments, with the N-terminal C2ABC fragment displaying a noticeable blue shift and the C2DEF a slight red shift. Subsequent calcium titrations resulted in sigmoidal dose-response curves for both the larger constructs and the isolated C2 domains (Fig. 3, A and B). From these plots, [Ca2+]1/2 values were determined (Table I). To test the specificity of calcium binding, the titration experiments were repeated in the presence of 0.75 mM magnesium. Magnesium had no effect on the calcium dose-responses, indicating that binding was specific for calcium (unpublished data).
Figure 2.
Normalized fluorescence emission spectra of native aromatic side chains of otoferlin in the presence of 0.1 mM EGTA or 1 mM calcium. 1 µM purified otoferlin fragments were excited at 280 nm and the emission spectra collected from 290 to 380 nm. Representative spectra for each domain under EGTA or calcium conditions are shown. The traces are representative data from four independent trials. FI, fluorescence intensity.
Figure 3.
Calcium-dependent changes in otoferlin fluorescence. (A and B) Normalized plots of the emission intensities of 1 µM of the three–C2 domain fragments C2ABC and C2DEF (A) and 1 µM of the isolated C2 domains C2B, C2C, C2D, C2E, and C2F (B) as a function of increasing calcium concentration in the absence of liposomes. (C and D) Calcium titrations of C2ABC, C2DEF (C), and each isolated C2 domain of otoferlin (D) in the presence of liposomes composed of 15% PS, 55% PC, and 30% PE. Error bars indicate mean ± SD (n = 4).
Table I.
[Ca2+]1/2 values for each C2 domain of otoferlin
| C2 domain | − Liposomes [Ca2+]1/2 | + Liposomes [Ca2+]1/2 |
| µM | µM | |
| C2A | ND | ND |
| C2B | 21.1 ± 1.1 | 6.7 ± 0.3 |
| C2C | 15.1 ± 2.0 | 5.4 ± 0.1 |
| C2D | 17.0 ± 1.1 | 4.4 ± 0.1 |
| C2E | 25.0 ± 1.50 | 7.5 ± 0.8 |
| C2F | 21.1 ± 2.0 | 10 ± 0.2 |
| C2ABC | 15.4 ± 0.60 | 2.0 ± 0.1 |
| C2DEF | 14.7 ± 0.60 | 1.9 ± 0.8 |
Many C2 domains bind to membranes, with varying affinity and lipid headgroup specificity, as an integral part of their function (Nalefski et al., 2001). Binding is often calcium dependent, with the cation, protein, and lipid forming a complex with an enhanced protein–calcium-binding affinity. To probe for potential shifts in calcium affinity induced by membranes, calcium titration curves were generated in the presence of liposomes composed of 15% DOPS, 30% POPE, and 55% POPC. Analogous to the liposome-free samples, all of the otoferlin constructs except C2A displayed increases in fluorescence in the presence of calcium as compared with measurements made in EGTA. The resultant calcium titration curves (Fig. 3, C and D) for the otoferlin–liposome samples show a leftward shift in [Ca2+]1/2, with values ≤10 µM for all constructs tested (Table I). We conclude that otoferlin binds calcium via at least five of its six domains and that membranes increase the apparent calcium-binding affinity of these domains.
The C2 domains of otoferlin bind SNARE proteins
As described in the Introduction, it has been reported that otoferlin associates with the t-SNARE proteins syntaxin 1 and SNAP-25. However, neither the specific domains of otoferlin that bind t-SNAREs nor the effects of calcium on binding have been examined. To address these issues, an immunoprecipitation assay was performed using the recombinant t-SNARE heterodimer syntaxin1A/SNAP-25B and an anti-syntaxin antibody (Fig. 4, A and B). 20 µM of each otoferlin C2 domain was tested for coimmunoprecipitation in the presence of 0.1 mM EGTA or 1 mM calcium. Although all six domains immunoprecipitated with the t-SNARE heterodimer, four of the isolated domains, C2C, C2D, C2E, and C2F, displayed calcium-promoted binding activity (Fig. 4, A and B). Binding of the other two domains, C2A and C2B, was not enhanced by calcium. These results are consistent with a previous GST pull-down study, which concluded that C2A bound in a calcium-independent manner to syntaxin, whereas the SNARE-binding activity of C2F was regulated by calcium (Ramakrishnan et al., 2009).
Figure 4.
Coimmunoprecipitation of t-SNARE heterodimers with the isolated C2 domains of otoferlin. (A) The assays were performed using 20 µM of each C2 domain using an anti-syntaxin monoclonal antibody. T denotes the total C2 domain loaded into the sample, and Ca and E denote the presence of 1 mM calcium or 0.1 mM EGTA, respectively. H-chain denotes the heavy chain of the anti-syntaxin antibody (HPC-1). (B) Quantitation of the coimmunoprecipitation data using densitometry. (C) Representative gel in which each isolated C2 domain was assayed for its ability to cofloat with vesicles in an Accudenz gradient in the presence of 0.1 mM EGTA or 1 mM free calcium. (D) Calcium-triggered C2 domain–induced liposome aggregation as measured by OD400 is shown. Turbidity of samples containing liposomes was monitored in either 1 mM free calcium or 0.1 mM EGTA. Error bars indicate mean ± SD (n = 3).
We also probed for interactions between native otoferlin and ternary SNARE complexes. Coimmunoprecipitation assays using whole brain detergent extracts and GST pull-down experiments using recombinant synaptobrevin 2 revealed that otoferlin binds ternary syntaxin 1/SNAP-25/synaptobrevin 2 complexes but does not directly interact with synaptobrevin (Fig. S1, A and B), which is analogous to synaptotagmin 1 (Earles et al., 2001).
The C2 domains of otoferlin bind to lipid membranes
The ability of otoferlin to bind membranes has not been addressed. Because synaptotagmin I operates at least in part by binding to membranes, we tested each otoferlin C2 domain for membrane-binding activity in the presence of EGTA or calcium using a liposome coflotation assay. In this assay, 15% DOPS, 55% POPC, and 30% POPE liposomes in a solution containing the C2 domain of interest are floated through a density gradient, carrying along with them any bound protein. SDS-PAGE is performed on the collected sample to assay for protein bound to the liposomes. Unlike the aforementioned fluorescence measurements, this assay directly measures the binding of protein to liposomes instead of the shift in calcium affinity. Although all six C2 domains bound to liposomes in the presence of 1 mM free calcium (Fig. 4 C), the C2A, C2B, and C2C domains also bound to some extent under calcium-free conditions. We also note that the larger three–C2 domain fragments of otoferlin directly associated with liposomes in EGTA, and binding was enhanced by addition of calcium (Fig. S1 C).
Otoferlin aggregates liposomes in a calcium-dependent manner
A previous study of synaptotagmin demonstrated its ability to aggregate membranes in a calcium-dependent manner (Popoli and Paternò, 1992), and this activity has been proposed to facilitate membrane fusion. Given the hypothesized role of otoferlin in exocytosis, we sought to determine whether otoferlin can also aggregate membranes. Membrane aggregation was assayed by monitoring changes in the OD400 of liposomes over time (Fig. 4 D). Although the addition of isolated or multi–C2 domain forms of otoferlin to a liposome sample in EGTA did not result in an appreciable increase in the OD400 of the sample over the course of 5 min, addition of 1 mM free calcium resulted in a significant increase in the OD400 for five of the six isolated C2 domains tested and for both larger three–C2 domain fragments (Fig. 4 D). This increased OD400 value remained stable over the monitoring period (∼15 min) and was reversed by addition of excess EGTA. Among the C2 domains tested, only C2A failed to induce significant changes in the OD400. Addition of calcium to protein-free liposome solutions did not result in any significant degree of aggregation.
Calcium/otoferlin accelerates SNARE-mediated membrane fusion
Using an in vitro reconstituted system, the in vivo effects of synaptotagmin on SNARE-mediated membrane fusion have been recapitulated (Tucker et al., 2004; Bhalla et al., 2006; Stein et al., 2007; Chicka et al., 2008; Gaffaney et al., 2008; Xue et al., 2008). In this system, v-SNARE proteoliposomes containing membrane-bound NBD and rhodamine fluorescence resonance energy transfer pairs are mixed with t-SNARE proteoliposomes that do not contain fluorophores. Fusion between v- and t-SNARE proteoliposomes results in lipid mixing of the two proteoliposomes and dilution of the fluorescence resonance energy transfer pair. This dilution increases the distance between the donor and acceptor, resulting in dequenching of the NBD. Using this system, we asked whether otoferlin can accelerate SNARE-mediated membrane fusion and whether calcium modulates otoferlin’s putative function during fusion.
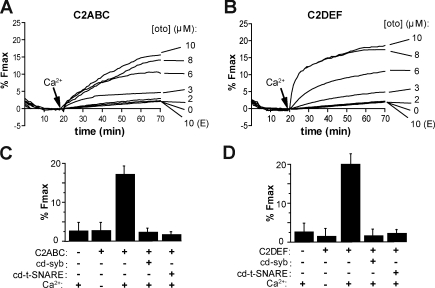
We first screened the three–C2 domain constructs (C2ABC and C2DEF) for their ability to regulate fusion between v-SNARE and t-SNARE proteoliposomes (Fig. 5, A and B). The otoferlin fragments were incubated with a 1:2 ratio of the v- and t-SNARE proteoliposomes for 20 min at 37°C in 0.1 mM EGTA followed by the injection of calcium to a final concentration of 1 mM (Fig. 5, A and B, arrows). Upon addition of calcium, both fragments of otoferlin induced a marked increase in the rate and final extent of fusion, as indicated by dequenching of NBD fluorescence. In the absence of calcium, neither otoferlin fragment enhanced SNARE-mediated fusion to any appreciable extent. In the absence of otoferlin, only a slow SNARE-dependent linear increase in fusion was observed, which is consistent with a previous study of reconstituted SNARE-mediated membrane fusion (Chicka et al., 2008). As a final control, and to distinguish between full and hemi fusion, we quenched the fluorescence of the outer leaflet with dithionite and monitored the fate of the inner leaflet during reconstituted fusion reactions. After dithionite treatment, dequenching of the inner leaflet was observed, demonstrating that full fusion occurred for all of the otoferlin constructs reported in this study (unpublished data).
Figure 5.
Otoferlin stimulates SNARE-mediated proteoliposome fusion in a calcium-dependent manner. Fusion experiments were performed using donor v-SNARE proteoliposomes and t-SNARE acceptor proteoliposomes with varying concentrations of recombinant otoferlin fragments. The components were incubated together for 20 min at 37°C in the presence of 0.1 mM EGTA. At t = 20 min, calcium was added to a final concentration of 1 mM. (A and B) Both C2ABC (A) and C2DEF (B) stimulated SNARE-mediated proteoliposome fusion in response to calcium. (A and B) E indicates that the experiment was performed under EGTA conditions. (C and D) Mean value for the extent of fusion expressed as a percentage of maximum fluorescence intensity after addition of detergent for 10 µM C2ABC (C) and C2DEF (D) under the indicated conditions. Fusion was attenuated by addition of cd-syb or cd–t-SNARE. Error bars represent SD (n = 3).
Titration of the otoferlin fragments led to an increase in the rate and extent of fusion until saturation was achieved (Fig. 5, A and B), and calcium dose-response experiments revealed [Ca2+]1/2 values of 109 µM and 131 µM for C2ABC and C2DEF, respectively (Fig. S2). To ensure that fusion proceeded through a SNARE-dependent pathway, assays were also conducted in the presence of either the soluble cytoplasmic domain of synaptobrevin (cd-syb) or a soluble form of the t-SNARE heterodimer (cytoplasmic domain of the t-SNARE heterodimer [cd–t-SNARE]), which will prevent proper trans-SNARE pairing of the v- and t-SNARE proteoliposomes. Both cd-syb and cd–t-SNARE reduced the final extent of fusion (Fig. 5, C and D), indicating that otoferlin-accelerated fusion proceeds through a SNARE-dependent pathway and that otoferlin alone cannot induce liposome fusion. We also note that otoferlin could not promote membrane fusion of liposomes that lacked v- or t-SNARE proteins (Fig. 5 D). Together, these data demonstrate for the first time that both the N- and C-terminal halves of otoferlin can stimulate SNARE-mediated membrane fusion in a calcium-promoted manner.
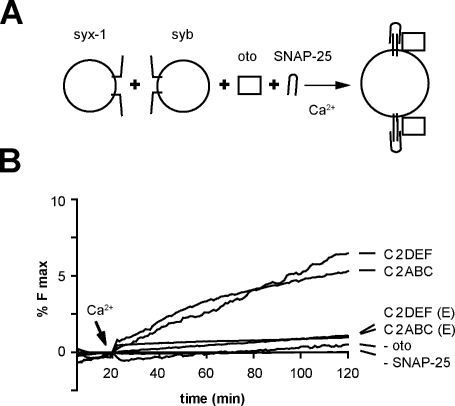
We next tested whether otoferlin can do “work” on SNARE proteins. A previous study has shown that syntaxin 1 and SNAP-25 must be preassembled before reconstitution to mediate fusion with v-SNARE proteoliposomes (Bhalla et al., 2006). However, calcium and synaptotagmin I can drive folding of soluble SNAP-25 onto reconstituted syntaxin, resulting in fusion activity (Bhalla et al., 2006). We repeated these experiments and, as shown in Fig. 6, appreciable membrane fusion was not observed when syntaxin was reconstituted but SNAP-25 was added in a soluble form. However, in the presence of either half of otoferlin, C2ABC or C2DEF, fusion was observed upon addition of calcium; in the absence of calcium, fusion was not observed under any conditions (Fig. 6). These findings provide functional evidence that calcium/otoferlin regulates the folding and activity of SNARE proteins.
Figure 6.
Calcium/otoferlin enables t-SNAREs, which have not been preassembled into heterodimers, to drive membrane fusion. (A) Fusion assays were conducted using reconstituted syntaxin 1 and synaptobrevin 2 vesicles in the presence of 15 µM soluble SNAP-25 and 4 µM otoferlin. (B) Either half of otoferlin, C2ABC, or C2DEF was sufficient to accelerate membrane fusion using soluble SNAP-25. In the absence of calcium or otoferlin, fusion was not observed. E indicates that the experiment was performed under EGTA conditions.
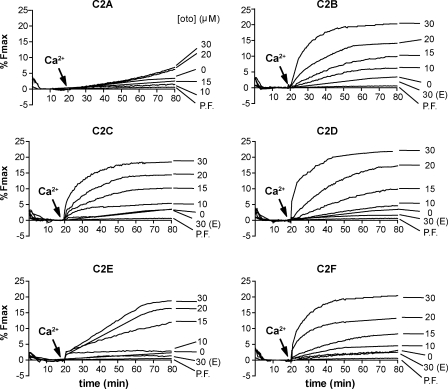
As a final survey of otoferlin’s functional properties, we conducted membrane fusion assays with isolated C2 domains to determine which ones were responsible for the observed stimulatory effect observed in the larger fragments (Fig. 7 and Fig. S3). C2 domain concentrations ranging from 0 to 30 µM were titrated into the fusion assay, as detailed for the aforementioned three–C2 domain fragments. Upon the addition of calcium at t = 20 min, membrane fusion was observed for reactions that contained C2B, C2C, C2D, C2E, and C2F to yield a final extent of fusion of ∼20% at the maximum concentrations tested. Interestingly, examination of the shape of the fusion curves reveals differences in the kinetics of fusion, with C2E showing slower kinetics relative to isolated domains C2B, C2C, C2D, and C2F (Fig. 7). As shown in Fig. 7, the use of SNARE-free liposomes resulted in the total absence of fusion for all five C2 domains, again demonstrating the requirement for SNAREs (Fig. 7). At up to 30 µM, C2A failed to accelerate SNARE-mediated fusion regardless of the presence of calcium (Fig. 7).
Figure 7.
The isolated C2 domains of otoferlin stimulate membrane fusion. Titrations of each isolated C2 domain, from 0 to 30 µM, demonstrate the ability of the otoferlin C2 domains C2B, C2C, C2D, C2E, and C2F to stimulate fusion between v- and t-SNARE proteoliposomes. The isolated C2 domains failed to stimulate membrane fusion between protein-free (PF) liposomes. E indicates that the experiment was performed under EGTA conditions.
Point mutations disrupt the function of the C2 domains of otoferlin
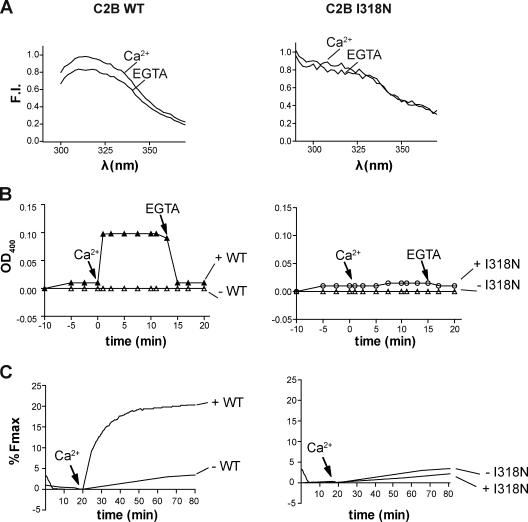
A previously reported isoleucine to asparagine mutation in the C2B domain of otoferlin was found to induce severe hearing loss in mouse models (Longo-Guess et al., 2007). Although the structure of this domain is not currently known, sequence alignment with other C2 domains suggests that this mutation lies within one of the β strands of the domain. A previous study suggested that this mutation may disrupt folding, resulting in decreased stability and increased turnover rates within the cell (Longo-Guess et al., 2007). To investigate the functional properties of this mutant, we introduced the I318N mutation into the isolated C2B domain. Fluorescence spectra of the wild-type (WT) and mutant domain differed markedly (Fig. 8 A), suggesting a perturbation of the tertiary structure, and the fluorescence spectrum of the I318N mutant was unaffected by calcium (Fig. 8 A), signifying a loss of calcium-sensing activity. As a second test of functionality, the mutant C2B domain was assayed for its ability to aggregate membranes; in contrast to WT, the mutant failed to aggregate membranes in the presence of 1 mM calcium (Fig. 8 B). Lastly, at equivalent 30 µM C2 domain concentrations, the WT C2B domain accelerated SNARE-mediated fusion in response to added calcium, whereas the I318N C2B mutant failed to have any effect on fusion in the presence of EGTA or 1 mM calcium (Fig. 8 C).
Figure 8.
A pathological isoleucine to asparagine missense mutation disrupts the structure and function of the C2B domain of otoferlin. (A) The fluorescence spectra of endogenous aromatic residues of the isolated WT (left) and I318N (right) mutant C2B domain differ in their spectral profiles. Although a notable difference in the fluorescence intensity (FI) of WT C2B is observed in 0.1 mM EGTA versus 1 mM calcium conditions, the spectra of the I318N mutant shows no discernable change in fluorescence in response to calcium. (B) The membrane aggregation activity of C2B is disrupted by the I318N mutation. After injection of protein at t = −5 min, calcium was added at t = 0 min to a final concentration of 1 mM. OD400 measurements for the WT C2B (left) and I318N (right) reveal that the point mutation abrogates calcium-triggered C2B-mediated liposome aggregation. The OD400 values after addition of calcium were 0.11 ± 0.02 for WT and 0.02 ± 0.01 for the mutant C2B (n = 3). (C) Reconstituted fusion assays performed in the presence of WT or I318N mutant C2B. After the addition of 1 mM free calcium, WT C2B accelerated SNARE-mediated fusion, whereas the I318N failed to enhance the rate or extent of fusion.
Finally, we studied the effects of mutating two of the putative calcium-binding residues in the C2C domain of otoferlin (Fig. S4). These mutations should abrogate the calcium-binding activity of the C2 domain and therefore eliminate calcium-triggered stimulation of SNARE-mediated membrane fusion. Indeed, although circular dichroism measurements indicated that the mutant protein was properly folded (unpublished data), we found that this mutant did not accelerate membrane fusion in the presence or absence of calcium (Fig. S4).
Discussion
The goal of this study was to ascertain whether otoferlin is indeed a calcium sensor capable of directly controlling SNARE-mediated membrane fusion reactions. Although a previous study of IHCs found otoferlin to be essential for calcium-evoked vesicle release (Roux et al., 2006), a recent immunofluorescence study failed to detect colocalization between otoferlin and hair cell synaptic ribbons, and Schug et al. (2006) suggested alternative roles for the protein, including possible contributions to Golgi and endosomal trafficking. In this study, we have conducted direct measurements using reduced in vitro approaches, including a reconstituted SNARE-mediated membrane fusion system, to test otoferlin’s functional properties. These experiments are advantageous in that they are free from the ambiguities that can arise because of the complexity of cellular systems and are thus able to directly address otoferlin’s ability to act as a calcium-activated regulator of membrane fusion.
Otoferlin senses micromolar concentrations of calcium
By monitoring the fluorescence spectra of the endogenous aromatic residues of otoferlin, we determined that at least five of the six C2 domains of the protein bind calcium with [Ca2+]1/2 values lower than those reported for synaptotagmin I (Davis et al., 1999; Fernandez et al., 2001; Nalefski et al., 2001). Only C2A failed to exhibit any change in fluorescence in the presence of calcium. Interestingly, we observed significant calcium-induced shifts in the emission maxima for several of the C2 domains and both three–C2 domain fragments. Because it has been well established that the emission spectra of tryptophan is highly sensitive to the polarity of its local environment (Vivian and Callis, 2001), we suspect that the shifts are caused by changes in the degree of exposure of the aromatic amino acid side chains to solvent. Finally, calcium titrations in the presence of membranes resulted in left-shifted [Ca2+]1/2 values as compared with samples lacking membranes. Therefore, the presence of membranes enhances the affinity of the otoferlin C2 domains for calcium. We note that the apparent affinities, measured in the presence of membranes, are also greater than those previously reported for synaptotagmin I (Brose et al., 1992; Davis et al., 1999; Nalefski et al., 2001).
All six C2 domains of otoferlin bind SNARE proteins
Using an immunoprecipitation procedure, we found that all six otoferlin C2 domains bound to syntaxin1A/SNAP-25 heterodimers. This finding marks the first time all six domains have been assayed for SNARE-binding activity and suggests that each of these domains may function to modulate SNARE activity. Interestingly, the C-terminal C2 domains C2C, C2D, C2E, and C2F were more responsive to calcium in terms of their SNARE-binding properties than the N-terminal C2A and C2B domains. Given that a splice variant containing only the three C-terminal domains has been reported, the stoichiometry, binding affinity, and calcium sensitivity of otoferlin may be tuned via preferential expression of long or short forms of the protein.
The otoferlin C2 domains bind and aggregate lipid membranes
A hallmark characteristic of many C2 domains concerns their ability to bind to lipid bilayers. In addition, it has been shown that synaptotagmin I operates at least in part by penetrating and bending membranes (Martens et al., 2007; Hui et al., 2009). However the question of whether otoferlin can bind lipid membranes had not been addressed in any previous study. Using a coflotation assay, we found that all six C2 domains bound to liposomes composed of POPC/DOPS/POPE. Membrane-binding activity was promoted by calcium for the C2C, C2D, CD2E, and C2F domains. C2A and C2B exhibited a strong calcium-independent component of binding. Surprisingly, despite its ability to bind membranes in the absence of calcium, C2B still required calcium to induce liposome aggregation, as did C2C, C2D, C2E, and C2F. In contrast, the N-terminal C2A domain failed to aggregate membranes under any of the conditions tested. Interestingly, synaptotagmin I can also aggregate membranes through its C2 domains (Popoli and Paternò, 1992; Bhalla et al., 2006; Gaffaney et al., 2008). It is possible that all membrane fusion–accelerating C2 domains operate in part through a membrane aggregation mechanism.
Otoferlin stimulates SNARE-mediated membrane fusion in response to calcium
Using a reconstituted membrane fusion assay, we found that five of the six C2 domains accelerate membrane fusion in the presence of calcium but not under EGTA conditions. Fusion was SNARE dependent in that otoferlin failed to induce lipid mixing in the presence of cd-syb, cd–t-SNARE, or when protein-free liposomes were used in the fusion assay. These results are what one would expect from a calcium sensor for membrane fusion and qualitatively agree with previous work focused on synaptotagmin I (Tucker et al., 2004; Chicka et al., 2008; Gaffaney et al., 2008). However, otoferlin is distinct from synaptotagmin I in that it contains multiple fusion-active C2 domains linked in series and possesses longer linkers between each C2 domain that may endow otoferlin with flexibility and reach. When combined with the findings that otoferlin binds myosin VI and calcium channels (Heidrych et al., 2009; Ramakrishnan et al., 2009), it is tempting to speculate that the protein links vesicles to SNAREs and calcium channels in a docked and primed state. Given our finding that most of the C2 domains of otoferlin can directly regulate membrane fusion and the recent proposal that synaptic vesicles bound to ribbons are capable of compound fusion (Matthews and Sterling, 2008), the scenarios by which otoferlin might operate become numerous.
Point mutations disrupt the function of otoferlin C2 domains
Pathological missense mutations in the C2C (Mirghomizadeh et al., 2002), C2D (Tekin et al., 2005), and C2F domains (Migliosi et al., 2002) of otoferlin have been linked to profound deafness in several human patients. Interestingly, in each case, a single amino acid change in a single C2 domain results in deafness; in some cases, this appears to be caused by degradation of the entire protein in response to misfolding of single domains. For example, a recently reported missense mutation in the C2B domain resulted in the loss of otoferlin and deafness in mice (Longo-Guess et al., 2007). We found that this I318N mutation gave rise to intrinsic fluorescence that was altered as compared with the WT C2 domain. The emission maxima and intensity of fluorescence spectra are highly sensitive to the environment of aromatic side chains of the protein, and the fact that the mutant protein spectrum differs from WT suggests alteration in the tertiary structure of the domain. Furthermore, no change in fluorescence was observed upon the addition of calcium to the I318N mutant nor was the mutant capable of accelerating SNARE-mediated membrane fusion in the presence of calcium. Thus, the conserved isoleucine is essential for proper tertiary structure, and substitution with an asparagine results in the loss of activity of the C2B domain. As a further control, two aspartate residues were substituted with alanines in the C2C domain, and this mutant protein failed to stimulate SNARE-mediated membrane fusion reactions in response to calcium, thus confirming the finding that C2 domains from otoferlin are bona fide functional calcium-sensing modules.
In summary, we have demonstrated that otoferlin possesses the ability to bind calcium, membranes, and SNAREs and is able to directly accelerate SNARE-mediated membrane fusion. However, several questions related to otoferlin and its function have yet to be addressed. One critical issue concerns the lipid-binding specificities of each C2 domain. This question is of particular interest because the lipid specificities of many C2 domains are often tailored to their target membranes, and thus, insight into this specificity could shed light as to where, within cells, otoferlin functions.
Materials and methods
Materials and reagents
Synthetic DOPS (1,2-dioleoyl-sn-glycero-3-phospho-l-serine), DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine), POPE (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine), dansyl-PE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(5-dimethylamino-1-naphthalenesulfonyl)), NBD-PE (1,2-dipalmitoyl-sn-glycero-3-phospho-ethanolamine-N-(7-nitro-2-1,3-benzoxadiazol-4-yl)), and rhodamine-PE (N-(lissamine rhodamine B sulfonyl)-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine) were purchased from Avanti Polar Lipids, Inc. Affinity media Ni2+-Sepharose high performance beads were obtained from GE Healthcare. Accudenz was obtained from Accurate Chemical & Scientific Corporation.
Plasmids and protein purification
cDNA-encoding mouse otoferlin was provided by C. Petit (Institut Pasteur, Paris, France). All otoferlin fragments were subcloned into pTrcHisA or PGEX expression vectors (Invitrogen) via BamHI and EcoRI and expressed in Escherichia coli as fusion proteins. Purification was performed essentially as described previously (Gaffaney et al., 2008). Point mutations were prepared using a site-directed mutagenesis kit (QuikChange; Agilent Technologies). Plasmids for mouse synaptobrevin 2 and the t-SNARE heterodimer (mouse SNAP-25B and rat syntaxin 1A) were provided by J.E. Rothman (Yale University, New Haven, CT). Synaptobrevin 2 and the t-SNARE complex were expressed as previously described (Gaffaney et al., 2008).
Protein-free and SNARE-bearing liposomes
Reconstitution of SNARE proteoliposomes was performed as previously reported (Gaffaney et al., 2008). Synaptobrevin 2 v-SNARE proteoliposomes were composed of 30 mol% POPE, 52% POPC, 15% DOPS, 1.5% rhodamine-PE (acceptor), and 1.5% NBD-PE (donor). Syntaxin 1A/SNAP-25B t-SNARE heterodimer proteoliposomes were composed of 30 mol% POPE, 55 mol% POPC, and 15 mol% DOPS. Proteoliposomes contained ∼100 copies of SNARE proteins per liposome. Protein-free liposomes were formed via extrusion using a lipid mixture matching that of the t-SNARE proteoliposomes as described previously (Hui et al., 2009).
Flotation assays
Flotation assays using protein-free extruded liposomes were performed using procedures described previously (Bhalla et al., 2006; Gaffaney et al., 2008). All otoferlin–liposome-binding reactions were performed in either 0.1 mM EGTA or 1 mM free calcium in Hepes buffer (10 mM Hepes and 100 mM NaCl, pH 7.5). 15 µM otoferlin was incubated with liposomes before mixing with an Accudenz density medium. The mixture was overlaid with decreasing amounts of Accudenz (35%, 30%, and 0%). After centrifugation, the liposomes floated to the 0/30% Accudenz interface along with any bound fragments of otoferlin. The sample at the interface was collected from the Accudenz interface, resolved by SDS-PAGE, and stained with Coomassie blue. Gels were imaged using a flatbed scanner and processed using Illustrator (Adobe).
Fusion assays
All fusion assays were conducted using a 96-well plate reader (460-nm excitation and 538-nm emission; BioTek Instruments, Inc.) as described previously with minor modifications (Chicka et al., 2008; Gaffaney et al., 2008). Fusion samples contained a total volume of 75 µl with 30 µl purified t-SNARE proteoliposomes, 15 µl purified v-SNARE proteoliposomes, 0.1 mM EGTA, and 0–30 µM otoferlin protein in Hepes buffer. For samples in which calcium was added, the free calcium concentration was 1 mM. At the end of each run, 0.5% wt/vol n-dodecylmaltoside was added to determine the maximal dequenched NBD signal. Raw fluorescence was normalized to obtain the percentage of maximum fluorescence as described previously (Chicka et al., 2008; Gaffaney et al., 2008).
Protein fluorescence measurements
Fluorescence measurements of native aromatic residues in various otoferlin fragments were performed using a fluorometer (QM-1; Photon Technology International) in Hepes buffer plus 0.1 mM EGTA using liposomes with a lipid composition matching that of the t-SNARE liposomes. For the calcium titrations experiments, a Hepes-buffered stock solution of calcium chloride was used, and the concentration of free calcium was determined using WebMaxC (www.stanford.edu/∼cpatton/webmaxcS.htm).
Immunoprecipitation assay
Immunoprecipitation experiments were performed as described previously (Hui et al., 2009) with modifications. 10 µM t-SNARE heterodimer was incubated with 20 µM otoferlin C2 domain in Hepes buffer plus 0.5% Triton X-100 for 1 h on ice in the presence of 0.1 mM EGTA or 1 mM calcium. t-SNAREs were precipitated through the addition of 3 µl of a monoclonal antibody directed against syntaxin (HPC-1) that was provided by R. Jahn (Max Planck Institute, Gottingen, Germany), followed by the addition of 40 µl protein G–Sepharose Fast Flow bead slurry (GE Healthcare). After 1 h, beads were collected by centrifugation and washed four times. The proteins in each sample were resolved via SDS-PAGE and visualized by staining with Coomassie blue. Gels were digitized using a scanner and processed using Illustrator.
Whole brain lysate immunoprecipitation assays were performed using rat brain detergent extracts that were prepared as described previously (Chapman et al., 1995; Lewis et al., 2001). 1% Triton X-100 was used for solubilization. Immunoprecipitation experiments were performed as described previously (Bai et al., 2004). For syntaxin 1A immunoprecipitation, the HPC-1 antibody was used. Subsequent immunoblots against otoferlin used a monoclonal anti-otoferlin provided by S. Safieddine (Université Pierre et Marie Curie, Paris, France). For otoferlin immunoprecipitation, an anti-otoferlin antibody obtained from Abcam was used followed by immunoblotting with the HPC-1 antibody to detect syntaxin 1A. Chemiluminescence film (Research Products International Corp.) was used to visualize the blots. Blots were digitized using a desktop scanner and processed using Illustrator.
Turbidity measurements
Membrane aggregation experiments were made by monitoring OD400 values using a UV-visible spectrophotometer (BioSpec-1601; Shimadzu Corporation). 500 µl samples contained liposomes (1 mM lipid) composed of 55% POPC/15% DOPS/30% POPE plus the indicated otoferlin fragment in Hepes buffer and 100 µM EGTA; calcium was added to a final concentration of 1 mM as indicated in Figs. 1–8.
Pull-down assays
GST-pull down assays were performed as described previously using 80 µg bead-immobilized GST-C2ABC or GST-C2DEF (Lewis et al., 2001; Dong et al., 2003). In brief, SNARE complexes were formed by incubating 50 µM t-SNARE heterodimer and 50 µM synaptobrevin 2 overnight at 4°C. The SNARE complexes were incubated with GST-otoferlin in a binding buffer composed of 10 mM Hepes, 100 mM NaCl, 0.5% Triton X-100, and either 0.1 mM EGTA or 1 mM calcium. After 1 h, the beads were washed three times with binding buffer, and the sample was treated with SDS sample buffer, subjected to SDS-PAGE, and visualized by immunoblotting. For blotting, the HPC-1 anti-syntaxin antibody was used. Chemiluminescence film was used to visualize the blots. Blots were digitized using a desktop scanner and processed using Illustrator.
Online supplemental material
Fig. S1 shows that otoferlin binds ternary SNARE complexes but does not bind to isolated synaptobrevin 2. Fig. S2 shows calcium dose-response for otoferlin-regulated SNARE-mediated membrane fusion. Fig. S3 shows quantitation of 30 µM otoferlin C2 domain–stimulated SNARE-mediated membrane fusion. Fig. S4 shows that neutralization of the putative calcium ligands D515 and 517 in the C2C domain of otoferlin abolishes calcium-stimulated fusion. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201002089/DC1.
Acknowledgments
We thank M. Beurg and R. Fettiplace for constructive discussions.
This work was supported by the National Institutes of Health (grant MH 61876). E.R. Chapman is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Abbreviations used in this paper:
- cd-syb
- cytoplasmic domain of synaptobrevin
- cd–t-SNARE
- cytoplasmic domain of the t-SNARE heterodimer
- IHC
- inner hair cell
- WT
- wild type
References
- Bai J., Wang C.T., Richards D.A., Jackson M.B., Chapman E.R. 2004. Fusion pore dynamics are regulated by synaptotagmin*t-SNARE interactions. Neuron. 41:929–942 10.1016/S0896-6273(04)00117-5 [DOI] [PubMed] [Google Scholar]
- Bhalla A., Chicka M.C., Tucker W.C., Chapman E.R. 2006. Ca(2+)-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 13:323–330 10.1038/nsmb1076 [DOI] [PubMed] [Google Scholar]
- Brose N., Petrenko A.G., Südhof T.C., Jahn R. 1992. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 256:1021–1025 10.1126/science.1589771 [DOI] [PubMed] [Google Scholar]
- Chapman E.R. 2008. How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 77:615–641 10.1146/annurev.biochem.77.062005.101135 [DOI] [PubMed] [Google Scholar]
- Chapman E.R., Hanson P.I., An S., Jahn R. 1995. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J. Biol. Chem. 270:23667–23671 10.1074/jbc.270.40.23667 [DOI] [PubMed] [Google Scholar]
- Chicka M.C., Hui E., Liu H., Chapman E.R. 2008. Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+. Nat. Struct. Mol. Biol. 15:827–835 10.1038/nsmb.1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A.F., Bai J., Fasshauer D., Wolowick M.J., Lewis J.L., Chapman E.R. 1999. Kinetics of synaptotagmin responses to Ca2+ and assembly with the core SNARE complex onto membranes. Neuron. 24:363–376 10.1016/S0896-6273(00)80850-8 [DOI] [PubMed] [Google Scholar]
- Dong M., Richards D.A., Goodnough M.C., Tepp W.H., Johnson E.A., Chapman E.R. 2003. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J. Cell Biol. 162:1293–1303 10.1083/jcb.200305098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earles C.A., Bai J., Wang P., Chapman E.R. 2001. The tandem C2 domains of synaptotagmin contain redundant Ca2+ binding sites that cooperate to engage t-SNAREs and trigger exocytosis. J. Cell Biol. 154:1117–1123 10.1083/jcb.200105020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez I., Araç D., Ubach J., Gerber S.H., Shin O., Gao Y., Anderson R.G., Südhof T.C., Rizo J. 2001. Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron. 32:1057–1069 10.1016/S0896-6273(01)00548-7 [DOI] [PubMed] [Google Scholar]
- Gaffaney J.D., Dunning F.M., Wang Z., Hui E., Chapman E.R. 2008. Synaptotagmin C2B domain regulates Ca2+-triggered fusion in vitro: critical residues revealed by scanning alanine mutagenesis. J. Biol. Chem. 283:31763–31775 10.1074/jbc.M803355200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E., Fuchs P.A. 2002. Transmitter release at the hair cell ribbon synapse. Nat. Neurosci. 5:147–154 10.1038/nn796 [DOI] [PubMed] [Google Scholar]
- Heidrych P., Zimmermann U., Bress A., Pusch C.M., Ruth P., Pfister M., Knipper M., Blin N. 2008. Rab8b GTPase, a protein transport regulator, is an interacting partner of otoferlin, defective in a human autosomal recessive deafness form. Hum. Mol. Genet. 17:3814–3821 10.1093/hmg/ddn279 [DOI] [PubMed] [Google Scholar]
- Heidrych P., Zimmermann U., Kuhn S., Franz C., Engel J., Duncker S.V., Hirt B., Pusch C.M., Ruth P., Pfister M., et al. 2009. Otoferlin interacts with myosin VI: implications for maintenance of the basolateral synaptic structure of the inner hair cell. Hum. Mol. Genet. 18:2779–2790 10.1093/hmg/ddp213 [DOI] [PubMed] [Google Scholar]
- Hui E., Johnson C.P., Yao J., Dunning F.M., Chapman E.R. 2009. Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion. Cell. 138:709–721 10.1016/j.cell.2009.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J.L., Dong M., Earles C.A., Chapman E.R. 2001. The transmembrane domain of syntaxin 1A is critical for cytoplasmic domain protein-protein interactions. J. Biol. Chem. 276:15458–15465 10.1074/jbc.M011687200 [DOI] [PubMed] [Google Scholar]
- Liberman M.C. 1980. Morphological differences among radial afferent fibers in the cat cochlea: an electron-microscopic study of serial sections. Hear. Res. 3:45–63 10.1016/0378-5955(80)90007-6 [DOI] [PubMed] [Google Scholar]
- Longo-Guess C., Gagnon L.H., Bergstrom D.E., Johnson K.R. 2007. A missense mutation in the conserved C2B domain of otoferlin causes deafness in a new mouse model of DFNB9. Hear. Res. 234:21–28 10.1016/j.heares.2007.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens S., Kozlov M.M., McMahon H.T. 2007. How synaptotagmin promotes membrane fusion. Science. 316:1205–1208 10.1126/science.1142614 [DOI] [PubMed] [Google Scholar]
- Matthews G., Sterling P. 2008. Evidence that vesicles undergo compound fusion on the synaptic ribbon. J. Neurosci. 28:5403–5411 10.1523/JNEUROSCI.0935-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliosi V., Modamio-Høybjør S., Moreno-Pelayo M.A., Rodríguez-Ballesteros M., Villamar M., Tellería D., Menéndez I., Moreno F., Del Castillo I. 2002. Q829X, a novel mutation in the gene encoding otoferlin (OTOF), is frequently found in Spanish patients with prelingual non-syndromic hearing loss. J. Med. Genet. 39:502–506 10.1136/jmg.39.7.502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirghomizadeh F., Pfister M., Apaydin F., Petit C., Kupka S., Pusch C.M., Zenner H.P., Blin N. 2002. Substitutions in the conserved C2C domain of otoferlin cause DFNB9, a form of nonsyndromic autosomal recessive deafness. Neurobiol. Dis. 10:157–164 10.1006/nbdi.2002.0488 [DOI] [PubMed] [Google Scholar]
- Nalefski E.A., Wisner M.A., Chen J.Z., Sprang S.R., Fukuda M., Mikoshiba K., Falke J.J. 2001. C2 domains from different Ca2+ signaling pathways display functional and mechanistic diversity. Biochemistry. 40:3089–3100 10.1021/bi001968a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M., Paternò R. 1992. Isolated p65 protein reproduces membrane binding activity of synaptic vesicles. Neuroreport. 3:177–180 10.1097/00001756-199202000-00014 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan N.A., Drescher M.J., Drescher D.G. 2009. Direct interaction of otoferlin with syntaxin 1A, SNAP-25, and the L-type voltage-gated calcium channel Cav1.3. J. Biol. Chem. 284:1364–1372 10.1074/jbc.M803605200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J.E., Brugge J.F., Anderson D.J., Hind J.E. 1967. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. J. Neurophysiol. 30:769–793 [DOI] [PubMed] [Google Scholar]
- Roux I., Safieddine S., Nouvian R., Grati M., Simmler M.C., Bahloul A., Perfettini I., Le Gall M., Rostaing P., Hamard G., et al. 2006. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 127:277–289 10.1016/j.cell.2006.08.040 [DOI] [PubMed] [Google Scholar]
- Safieddine S., Wenthold R.J. 1999. SNARE complex at the ribbon synapses of cochlear hair cells: analysis of synaptic vesicle- and synaptic membrane-associated proteins. Eur. J. Neurosci. 11:803–812 10.1046/j.1460-9568.1999.00487.x [DOI] [PubMed] [Google Scholar]
- Schug N., Braig C., Zimmermann U., Engel J., Winter H., Ruth P., Blin N., Pfister M., Kalbacher H., Knipper M. 2006. Differential expression of otoferlin in brain, vestibular system, immature and mature cochlea of the rat. Eur. J. Neurosci. 24:3372–3380 10.1111/j.1460-9568.2006.05225.x [DOI] [PubMed] [Google Scholar]
- Shao X., Fernandez I., Südhof T.C., Rizo J. 1998. Solution structures of the Ca2+-free and Ca2+-bound C2A domain of synaptotagmin I: does Ca2+ induce a conformational change? Biochemistry. 37:16106–16115 10.1021/bi981789h [DOI] [PubMed] [Google Scholar]
- Stein A., Radhakrishnan A., Riedel D., Fasshauer D., Jahn R. 2007. Synaptotagmin activates membrane fusion through a Ca2+-dependent trans interaction with phospholipids. Nat. Struct. Mol. Biol. 14:904–911 10.1038/nsmb1305 [DOI] [PubMed] [Google Scholar]
- Sutton R.B., Davletov B.A., Berghuis A.M., Südhof T.C., Sprang S.R. 1995. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell. 80:929–938 10.1016/0092-8674(95)90296-1 [DOI] [PubMed] [Google Scholar]
- Tekin M., Akcayoz D., Incesulu A. 2005. A novel missense mutation in a C2 domain of OTOF results in autosomal recessive auditory neuropathy. Am. J. Med. Genet. A. 138:6–10 [DOI] [PubMed] [Google Scholar]
- Therrien C., Di Fulvio S., Pickles S., Sinnreich M. 2009. Characterization of lipid binding specificities of dysferlin C2 domains reveals novel interactions with phosphoinositides. Biochemistry. 48:2377–2384 10.1021/bi802242r [DOI] [PubMed] [Google Scholar]
- Tucker W.C., Weber T., Chapman E.R. 2004. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science. 304:435–438 10.1126/science.1097196 [DOI] [PubMed] [Google Scholar]
- Vivian J.T., Callis P.R. 2001. Mechanisms of tryptophan fluorescence shifts in proteins. Biophys. J. 80:2093–2109 10.1016/S0006-3495(01)76183-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T., Zemelman B.V., McNew J.A., Westermann B., Gmachl M., Parlati F., Söllner T.H., Rothman J.E. 1998. SNAREpins: minimal machinery for membrane fusion. Cell. 92:759–772 10.1016/S0092-8674(00)81404-X [DOI] [PubMed] [Google Scholar]
- Xue M., Ma C., Craig T.K., Rosenmund C., Rizo J. 2008. The Janus-faced nature of the C(2)B domain is fundamental for synaptotagmin-1 function. Nat. Struct. Mol. Biol. 15:1160–1168 10.1038/nsmb.1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga S., Grati M., Chardenoux S., Smith T.N., Friedman T.B., Lalwani A.K., Wilcox E.R., Petit C. 2000. OTOF encodes multiple long and short isoforms: genetic evidence that the long ones underlie recessive deafness DFNB9. Am. J. Hum. Genet. 67:591–600 10.1086/303049 [DOI] [PMC free article] [PubMed] [Google Scholar]