The MAPK kinase kinase Wallenda is regulated by the Highwire E3 ubiquitin ligase and initiates injury signaling in axons.
Abstract
Regenerative responses to axonal injury involve changes in gene expression; however, little is known about how such changes can be induced from a distant site of injury. In this study, we describe a nerve crush assay in Drosophila melanogaster to study injury signaling and regeneration mechanisms. We find that Wallenda (Wnd), a conserved mitogen-activated protein kinase (MAPK) kinase kinase homologous to dual leucine zipper kinase, functions as an upstream mediator of a cell-autonomous injury signaling cascade that involves the c-Jun NH2-terminal kinase MAPK and Fos transcription factor. Wnd is physically transported in axons, and axonal transport is required for the injury signaling mechanism. Wnd is regulated by a conserved E3 ubiquitin ligase, named Highwire (Hiw) in Drosophila. Injury induces a rapid increase in Wnd protein concomitantly with a decrease in Hiw protein. In hiw mutants, injury signaling is constitutively active, and neurons initiate a faster regenerative response. Our data suggest that the regulation of Wnd protein turnover by Hiw can function as a damage surveillance mechanism for responding to axonal injury.
Introduction
Regenerative responses to axonal injury require a transcriptional reprogramming of the injured neuron, and there is great interest in understanding how this reprogramming is activated and controlled (Richardson et al., 2009; Sun and He, 2010). Because axons are long (as long as 1 m for human motoneurons), a key element of an injury response mechanism is the ability to signal to the nucleus that the axon has been damaged. Several studies suggest that signaling molecules are physically transported in axons via microtubule-based motors, such as the minus end–directed motor dynein (for reviews see Hanz and Fainzilber, 2006; Abe and Cavalli, 2008). The transported molecules include transcription factors, intermediate filaments, and activated MAPKs ERK and JNK. How such molecules are orchestrated to detect and respond to axonal damage is poorly understood.
Recent findings have brought attention to a conserved MAPK kinase kinase, dual leucine zipper kinase (DLK), as a candidate regulator of axonal damage signaling. DLK localizes to axons (Hirai et al., 2005; Eto et al., 2010) and is functionally required for regeneration after axotomy in Caenorhabditis elegans and mice (Hammarlund et al., 2009; Itoh et al., 2009; Yan et al., 2009). Interestingly, DLK is also required for Wallerian degeneration of the distal stump after injury (Miller et al., 2009). The dual function in degeneration and regeneration suggests that DLK may be acutely activated by axonal injury to mediate these various downstream injury responses.
In Drosophila melanogaster, we have previously found that the homologue of DLK, Wallenda (Wnd), regulates synaptic bouton growth and morphology at the larval neuromuscular junction (NMJ) via a nuclear signaling pathway that requires JNK and the transcription factor Fos (Collins et al., 2006). Wnd and its homologue in C. elegans are regulated by a conserved E3 ubiquitin ligase, named Highwire (Hiw) in Drosophila (Nakata et al., 2005; Collins et al., 2006). Mutations in hiw lead to increased levels of Wnd protein in axons (Collins et al., 2006), and this misregulation of Wnd leads to increased branching and bouton growth at the motoneuron nerve terminus (Wan et al., 2000).
In this study, we test the hypothesis that Hiw and Wnd function to regulate an injury response pathway. For this, we developed an axon injury and regeneration assay in Drosophila motoneurons. Importantly, a downstream molecular reporter, whose induction coincides with the initiation of regeneration, allows us to dissect the steps required for neurons to mount a transcriptional response to injury. Our findings indicate that Wnd acts as a key upstream mediator of a nuclear signaling pathway, which is activated by axonal damage and promotes axonal sprouting after injury. Furthermore, transport and destruction of Wnd in axons are important elements of a damage surveillance mechanism. By regulating the levels of Wnd in axons, the Hiw ubiquitin ligase plays a key role in regulating a retrograde injury signaling pathway.
Results
A nerve crush injury assay in Drosophila
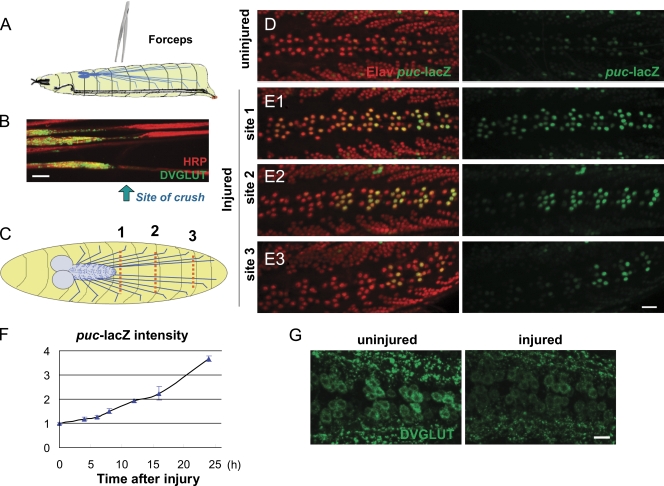
To study axon injury response in the Drosophila system, we established a nerve crush assay for larval segmental nerves (see Materials and methods; Fig. 1 A and Fig. S1 A), which contain motoneuron and sensory neuron axons. Because they run close to the ventral midline, the segmental nerves can be visualized through the cuticle in third instar larvae under a standard dissection stereomicroscope. After anesthetization with CO2, the nerves and surrounding cuticle are pinched tightly with number 5 forceps. The injury leads to paralysis in the posterior segments; however, the animal is still able to feed and, remarkably, survives pupation and ecloses as a fully motile adult. Fig. 1 B shows an example segmental nerve 24 h after nerve crush. The nerve becomes stretched and thin, and vesicular cargoes accumulate at the crush site but do not pass through. Staining for MAP1B (Futsch) indicates a loss of microtubule structure at the crush site (Fig. S1 B), and single-neuron labeling indicates that individual axons are transected by the injury (Fig. S1 C). Distal to the crush site, nerves and synaptic NMJs degenerate within 24 h after the injury (Fig. S1 D).
Figure 1.
Axon injury induces transcriptional changes in the Motoneuron cell body. (A) Schematic of the nerve crush assay. The segmental nerves within a third instar larva are crushed by pinching the ventral cuticle with forceps. (B) Injured segmental nerves 24 h after nerve crush. Synaptic vesicle precursors detected by staining for DVGLUT staining (green) accumulate at the proximal side of the crush site (arrow). (C) Cartoon of neuron cell bodies (blue dots) and segmental nerves (blue lines). Different crush sites (dashed red lines) injure a predictable number of motoneurons. Crush site 1 injures more cells than crush sites 2 and 3. (D) In uninjured animals, puc-lacZ expression is barely detectable. A nuclear localization signal on lacZ (green) localizes the reporter to the nucleus, and neuronal nuclei are detected by staining for the ElaV (red) marker. (E1–3) Injury induces puc-lacZ expression. 24 h (at 25°C) after injury at sites 1–3 induces expression of puc-lacZ in a defined subset of motoneurons as predicted by the anatomy cartooned in C. (F) Time course quantitation of puc-lacZ. The mean intensity of puc-lacZ is measured as described in Materials and methods for the dorsal midline neurons. 24 h after injury, puc-lacZ intensity is increased 3.5-fold compared with uninjured animals (n > 15). (G) Axon injury leads to a decrease of DVGLUT protein in motoneuron cell bodies. Error bars indicate mean ± SEM. Bars, 25 µm.
Nuclear and cell body responses to the nerve crush injury
To study the mechanism of nuclear injury response, we first needed to identify a reporter for cellular changes induced by injury. The JNK phosphatase puckered, whose expression can be reported via a lacZ enhancer trap (Martín-Blanco et al., 1998), is an attractive candidate because it has been previously observed to be induced around sites of traumatic brain injury in Drosophila (Leyssen et al., 2005). Puc-lacZ is expressed at very low levels in uninjured neurons (Fig. 1 D); however, nerve crush induces a dramatic increase in puc-lacZ expression in injured motoneurons (Fig. 1 E). The induction of puc-lacZ by injury is cell autonomous. This is shown by varying the location of injury (Fig. 1 C) to different segments in the animal, which induces puc-lacZ only in the motoneurons whose axons have been injured (Fig. 1, E1–E3).
To quantify the change in puc promoter activity, the mean intensity of lacZ staining was measured in the neurons that lie along the dorsal midline, most of which are motoneurons (Sanyal, 2009). When segmental nerves are crushed at injury site 1, puc-lacZ expression begins to increase within 8 h after injury (at 25°C) and continues to increase linearly until pupation (Fig. 1 F).
A well-documented response to injury in vertebrate neurons is a reduction in synaptic transmission (Brumovsky et al., 2007). In Drosophila motoneurons, the Drosophila vesicular glutamate transporter (DVGLUT) plays a critical role in neurotransmission (Daniels et al., 2006) and is robustly detected by antibody staining in motoneuron cell bodies (Daniels et al., 2008). We found that injury induces a dramatic reduction in the cell body levels of DVGLUT (Fig. 1 G).
Wnd and downstream signaling via JNK and Fos mediate the nuclear response to axonal injury
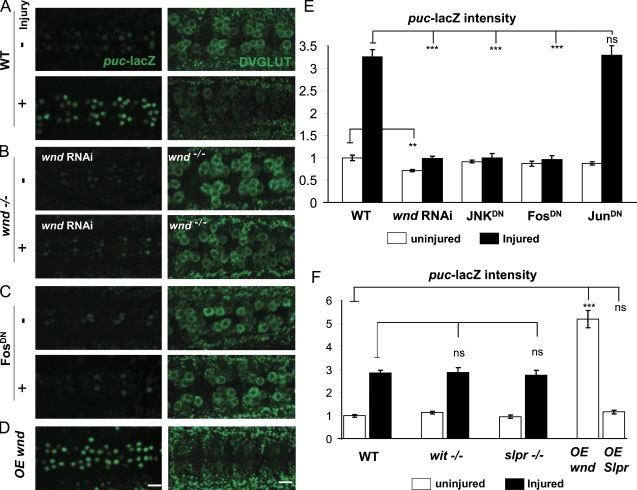
With puc-lacZ and DVGLUT as downstream molecular responses to axonal injury, we could use these markers as reporters to dissect the mechanism of injury signaling. We first tested the role of the Drosophila DLK homologue Wnd in injury response because DLK has recently been implicated in both regenerative and degenerative injury responses in other organisms (Hammarlund et al., 2009; Itoh et al., 2009; Miller et al., 2009; Yan et al., 2009). Because of the chromosomal location of wnd (near the centromere), we were unable to recombine wnd mutations with the puc-lacZ reporter. Therefore, we used a transgenic RNAi knockdown strategy to reduce wnd transcript (Dietzl et al., 2007). An upstream activation sequence (UAS)–wnd-RNAi transgene was coexpressed with Dcr2 using the neuronal BG380-Gal4 driver line, which drives strong expression in many neurons, including motoneurons (Sanyal, 2009). This reduced the total levels of Wnd protein in whole brain extracts (Fig. S2 A) and was sufficient to recapitulate other loss of function phenotypes for wnd in motoneurons, such as disruption of axonal transport (Fig. S2 B; Horiuchi et al., 2007) and suppression of synaptic overgrowth caused by mutation in hiw (Fig. S2 C; Collins et al., 2006). Expression of wnd-RNAi completely inhibited the induction of puc-lacZ by injury (Fig. 2 B). In addition, wnd1/+ heterozygotes partially inhibited the effect of injury on puc-lacZ (Fig. S2 D). In wnd1/wnd3 loss of function mutants, the effect of injury upon DVGLUT was abolished (Fig. 2 B and Fig. S2 E). Conversely, overexpression of wnd in uninjured neurons causes a dramatic increase in puc-lacZ and decrease in DVGLUT (Fig. 2, D and F), mimicking the effect of the nerve crush. We conclude that Wnd is a critical upstream regulator of the cellular responses to injury.
Figure 2.
The nuclear and cell body response to axonal injury specifically requires Wnd and downstream signaling components. (A–D) puc-lacZ expression (left) and staining for DVGLUT (right) in VNCs uninjured and 24 h after injury. (A) The nerve crush injury induces an increase in puc-lacZ expression (left) and decrease in staining for DVGLUT (right) in motoneuron cell bodies. (B) The response to injury requires Wnd function. No obvious change in puc-lacZ (left) and DVGLUT (right) is observed after injury when Wnd is disrupted. (C) The response to injury is inhibited by FosDN. (D) Overexpression (OE) of wnd in neurons is sufficient to activate the injury response, including induction of puc-lacZ and reduction in DVGLUT staining. (E and F) Quantification of the puc-lacZ expression level before (white bars) or 24 h after (black bars) injury in different genotypes. The BG380-Gal4 driver is used to drive expression of all UAS lines (wnd RNAi, JNKDN, FosDN, JunDN, and Slpr). P > 0.05 was not significant. **, P < 0.001; ***, P < 0.0001. Error bars indicate mean ± SEM. Bars, 25 µm.
Because Wnd was previously found to promote synaptic overgrowth via JNK and Fos (Collins et al., 2006), we tested the requirement of JNK and Fos in the injury signaling mechanism. Expression of dominant-negative (DN) transgenes JNKDN and FosDN inhibited the injury-induced effects upon both puc-lacZ and DVGLUT (Fig. 2, C and E). In contrast, JunDN had no effect (Fig. 2 E), which parallels previous observations that FosDN but not JunDN inhibits synaptic overgrowth (Collins et al., 2006).
Two controls further address the specificity of Wnd’s role and effect. First, the other mixed lineage kinase family member in Drosophila, Slpr, has no effect on puc-lacZ in neurons either when it is mutant or overexpressed (Fig. 2 F) despite the fact that Slpr regulates puckered in epithelial cells during dorsal closure (Stronach and Perrimon, 2002). Second, mutations in bone morphogenetic protein signaling, which also affect NMJ morphology and development via a nuclear signaling pathway (Keshishian and Kim, 2004; Marqués and Zhang, 2006), do not affect the puc-lacZ induction by injury (Fig. 2 F). Furthermore, injury has no effect upon levels of nuclear phospho-MAD (unpublished data).
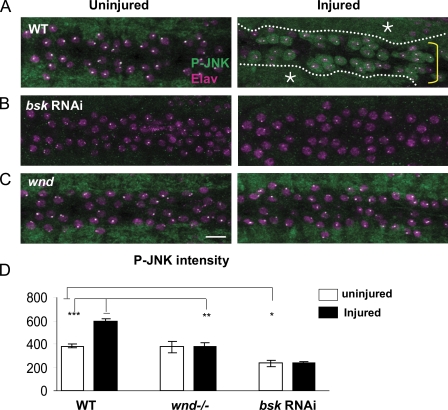
The puc-lacZ reporter is regulated by JNK signaling in other developmental contexts (Dobens et al., 2001; Stronach and Perrimon, 2002; Galko and Krasnow, 2004; Bosch et al., 2005; McEwen and Peifer, 2005). To address directly whether JNK is activated by axon injury and whether this activation requires Wnd, we probed larvae with an antibody against phosphorylated JNK (p-JNK). In uninjured animals, p-JNK is prominent in the neuropil of the larval ventral nerve cord (VNC; Fig. 3 A). This staining is specific for JNK because transgenic RNAi knockdown of the Drosophila JNK homologue bsk in neurons abolishes the p-JNK staining (Fig. 3 B). Dramatically, nerve crush induces the appearance of p-JNK in injured motoneuron cell bodies (Fig. 3 A). Similarly to puc-lacZ, p-JNK appears only in the cell bodies of injured neurons. However, its accumulation is more transient, beginning around 6 h (for injury site 1), peaking around 12 h, and decreasing thereafter, concomitant with the induction of the puckered phosphatase (Fig. 1 F). Importantly, p-JNK did not appear in the cell body in wnd mutants after injury (Fig. 3, C and D), which indicates that Wnd is required for JNK activation in injured neurons. Because wnd mutants did not have reduced p-JNK staining in the neuropil (Fig. 3 C) or segmental nerves (not depicted), we infer that Wnd is only one of the regulators of JNK in axons. However, Wnd is a critical upstream regulator of JNK in response to axonal injury.
Figure 3.
Injury induces p-JNK accumulation in the cell bodies and nuclei of injured neurons. (A–C) VNCs are costained for p-JNK (green) and the nuclear marker Elav (magenta). In both uninjured and injured animals, p-JNK stains the neuropil (A, asterisks and dotted lines), which can be seen surrounding the cell bodies (A, yellow bracket). (A) p-JNK appears in motoneuron cell bodies within 12 h after injury. Bar, 25 µm. (B) This p-JNK staining is abolished by expression of bsk-RNAi (Dietzl et al., 2007) in neurons. (C) Mutations in wnd (wnd1/wnd3) inhibit the cell body accumulation of p-JNK after injury. The bright dots in the nuclei are fixation artifacts. (D) Quantification of p-JNK intensity in the cell body before (white bars) and 12 h after (black bars) injury. *, P < 0.05; **, P < 0.001; ***, P < 0.0001. Error bars indicate mean ± SEM.
The Wnd pathway is required for axon regeneration after injury
What is the physiological role of the nuclear and cell body response to injury? An attractive model is that this injury signaling pathway induces a regenerative response in the injured neuron. To test whether larval motoneuron axons regenerate after injury, we used the Gal4/UAS system to label single-motoneuron axons within each segmental nerve by expression of UAS–mCD8-GFP. Two different driver lines were used for this purpose. m12-Gal4 (Ritzenthaler et al., 2000) drives strong expression in only two axons per segmental nerve, which are tightly fasciculated with one another. RRa(eve)-Gal4 (Fujioka et al., 2003) drives specific expression, albeit more weakly, in RP2 and aCC motoneurons in third instar larvae.
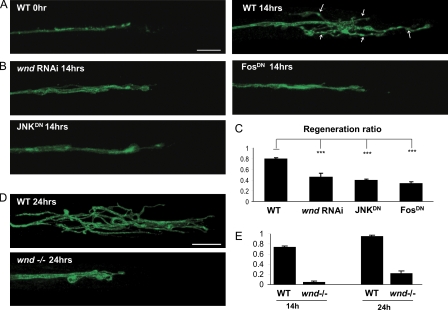
With either subset of labeled motoneurons, extensive branching is observed from the proximal stump within 14 h of injury (Fig. 4 A). Within 24 h, branches can be longer than 100 µm (Fig. 4 D). Because the lesion is a great distance (>1 mm) from the target muscles and the time before pupation is limited (<3 d), we do not observe functional reconnection of the injured axons to their targets. Likewise, reconnection to the distal stump also does not occur because the distal stump degenerates. Nonetheless, the axonal branching represents new axonal growth in response to injury, which can be considered as an attempted regenerative response. To quantify this response, we counted the fraction of injured nerves that showed more than five branches of at least 10 µm in length at the injury site (Fig. 4 A, arrows) while blinded to genotype. Because only two axons per nerve are labeled by each Gal4 driver, the new branches must have arisen from new remodeling/growth of these injured axons. By this criteria, 70–80% of the injured axons (in a wild-type [WT] genetic background) show signs of regenerative growth within 14 h after injury (Fig. 4, C and E).
Figure 4.
Wnd/JNK/Fos injury signaling is required for axonal sprouting after injury. (A–E) Axons are labeled by driving expression of UAS-mCD8-GFP by m12-Gal4 (A–C) and RRa(eve)-Gal4 (D and E). (A) In WT animals, the proximal stump forms extensive new branches (arrows) by 14 h after injury. (B) Sprouting after injury is inhibited by expression of wnd-RNAi, JNKDN, or FosDN in the GFP-labeled neurons. (C) Quantification of regeneration ratio 14 h after injury. The fraction of injured axons that exhibited sprouting was measured for at least 100 axons per genotype while blinded to the genotype. (D) At 24 h after injury, branches from the proximal stump are even longer in the WT background; however, this sprouting remains strongly inhibited in the wnd1/wnd2 mutant background. Bar, 25 µm. (E) Quantification of the regeneration ratio for WT and wnd1/2 at 14 and 24 h after injury, conducted similarly as in C. ***, P < 0.0001. Error bars indicate mean ± SEM.
To test whether the Wnd/JNK/Fos signaling pathway is required for this regenerative response, we used the strong m12-Gal4 driver to express wnd-RNAi, JNKDN, or FosDN in the labeled motoneurons (Fig. 4 B). We find that disruption of each component of the pathway significantly inhibits the formation of new axonal branches at the injury site (Fig. 4, B and C). Because we were unable to isolate recombinants between m12-Gal4 and wnd, we used the RRa-Gal4 driver to label single axons in segmental nerves in a wnd1/wnd2 mutant background. We find that regeneration is dramatically inhibited when wnd is mutant (Fig. 4, D and E). These observations indicate that the Wnd pathway plays an important role in the regenerative response to injury. We note that the formation of branches at the injury site bears similarities to the induction of puc-lacZ in the requirement for JNK and Fos. The timing of the branch formation, which begins to appear ∼8 h after injury, is also similar to the timing of puc-lacZ induction (Fig. 1 F). We conclude that the nuclear response to injury, which is mediated by Wnd signaling, induces new axonal growth/branching of the injured neuron.
Wnd protein is transported in axons
Because Wnd is an upstream mediator of an injury response pathway, an attractive hypothesis is that Wnd is locally activated in axons. Consistent with this hypothesis, the vertebrate homologue of Wnd, DLK, can be detected in axons, growth cones, and synapses (Mata et al., 1996; Hirai et al., 2005; Eto et al., 2010). Therefore, we tested whether Wnd localizes in axons in Drosophila.
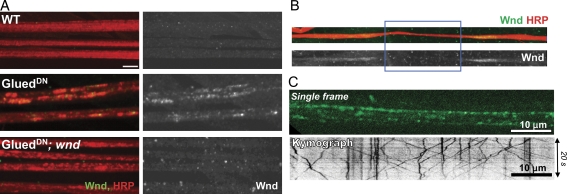
Endogenous Wnd is barely detectable by immunocytochemistry in a WT background (Fig. 5 A). However, when axonal transport was inhibited by overexpressing a truncated form of the dynactin subunit p150/Glued (GluedDN) in motoneurons (Allen et al., 1999), cargo for axonal transport accumulate in axonal swellings (Martin et al., 1999), and Wnd protein can be detected in these swellings (Fig. 5 A). No such axonal accumulations are observed when transport is disrupted in a wnd3 mutant (Fig. 5 A) when the Wnd antibody epitope is absent (Collins et al., 2006). Also consistent with transport in axons, Wnd protein accumulates at both sides of the site of nerve crush within 2 h of axonal injury (Fig. 5 B), similarly to other cargo for axonal transport (Horiuchi et al., 2005; Barkus et al., 2008). To directly assay the transport of Wnd protein, we used the Gal4/UAS system to drive expression of a UAS–GFP-wndKD transgene exclusively in motoneurons using OK6-Gal4 (Sanyal, 2009). The GFP-wndKD transgene contains a mutation in the kinase domain (kinase dead [KD]), which allows the protein to be expressed at detectable levels for live imaging without causing lethality. Importantly, the GFP-wndKD transgenic protein can be visualized in live dissected third instar larvae by rapid time-lapse imaging. Fig. 5 C shows a single frame from a representative video (Video 1) and the kymograph generated from a single-axon tract. The GFP-WndKD protein localizes to discrete puncta, many of which move anterogradely and/or retrogradely with mean segment velocities of 0.83 ± 0.02 µm/s (n = 342) and 0.62 ± 0.02 µm/s (n = 271), respectively. These velocities are comparable with other cargo for axonal transport machinery in Drosophila axons (Miller et al., 2005; Haghnia et al., 2007; Barkus et al., 2008).
Figure 5.
Wnd protein is transported in axons. (A) Wnd protein accumulates in segmental nerves when axonal transport is disrupted. Segmental nerves from third instar larvae are stained for axonal membrane (anti-HRP) in red and Wnd (anti-Wnd) in green. (right) Wnd staining alone is shown. (B) Wnd protein (top, green; bottom, alone) accumulates at the ligation (injury) site (blue rectangle). (C) Live imaging indicates that Wnd particles rapidly translocate both anterogradely and retrogradely in axons (Video 1). The GFP-wndKD transgene was expressed in motoneurons using the OK6-Gal4 driver. (top) A single 0.5-s exposure is shown. (bottom) A representative kymograph from a single axon is shown. Bars, 10 µm.
The localization of Wnd to discrete motile particles suggests that Wnd associates with vesicles. Wnd and its homologues in vertebrates have no transmembrane domains and are predicted to be cytoplasmic proteins. However, biochemical characterization of DLK from mouse brain homogenates indicates that a significant fraction of the protein cofractionates with membranes (Mata et al., 1996). Differential centrifugation and sucrose floatation assays from Drosophila head extracts indicate that Wnd behaves biochemically like a membrane-associated protein (Fig. S3). We conclude that Wnd is transported in axons while associated with vesicles.
Injury signaling and regenerative response via Wnd requires axonal transport machinery
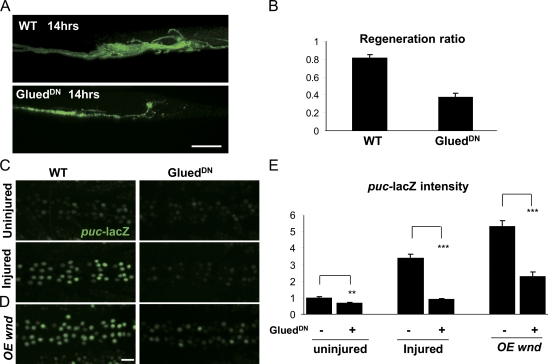
If the axonal localization of Wnd is functionally relevant for the injury signaling mechanism, a prediction is that mutations that disrupt axonal transport machinery would inhibit both the nuclear and downstream regenerative responses to injury. We disrupted axonal transport using mutations in either the minus end–directed motor dynein (Fig. S4) or the dynactin complex (Fig. 6), which plays a critical role in dynein cargo binding (Schroer, 2004). Dynactin was disrupted by expressing a DN-truncated subunit, p150/Glued (GluedDN; Allen et al., 1999), in larval motoneurons. This resulted in a strong inhibition to the regenerative response (Fig. 6, A and B). However, this was not particularly informative alone because axonal transport may also be required for steps downstream of the nuclear injury signal, such as the transport of new material into the regenerating axon. The puc-lacZ reporter allowed us to directly test the requirement for dynactin and dynein in injury signaling independent of downstream events. Both GluedDN (Fig. 6, C and E) and dynein (sw1; Fig. S4) mutations cause a near-complete block to the induction of puc-lacZ by injury. The cell body/nuclear accumulation of p-JNK is also inhibited (unpublished data). We conclude that dynein and dynactin are required for the transduction of the injury signal to the nucleus.
Figure 6.
Axonal transport is required for regeneration and injury signaling. (A) Inhibition of axonal transport inhibits regeneration after injury. Expression of the p150-Glued dynactin subunit, UAS-GluedDN, inhibits the formation of new branches at the injury site. (B) Quantitation of the regeneration ratio similar to that in Fig. 4 C. (C) Inhibition of axonal transport inhibits the effect of axon injury on puc-lacZ expression. (D) Ectopic Wnd signaling also requires axonal transport. The induction of puc-lacZ by overexpression (OE) of wnd is suppressed by coexpression of GluedDN. (E) Quantification of puc-lacZ intensities for genotypes described in C and D. **, P < 0.001; ***, P < 0.0001. Error bars indicate mean ± SEM. Bars, 25 µm.
Interestingly, the requirement for dynactin in injury signaling cannot be simply bypassed by overexpressing wnd in neurons. That is, although overexpression of wnd is sufficient to activate puc-lacZ and down-regulate DVGLUT (Fig. 2, D and E), it is not sufficient when axonal transport is inhibited by GluedDN (Fig. 6, D and E). We interpret that localization of Wnd alone to the cell body is not enough to induce the signaling pathway. Rather, Wnd may need to be transported into axons to become activated or to encounter a necessary cofactor or substrate. It is difficult to distinguish between these possibilities because mutations in components of axonal transport machinery inhibit both anterograde and retrograde transport in Drosophila motoneurons (Allen et al., 1999; Haghnia et al., 2007; Barkus et al., 2008; unpublished data). Furthermore, additional cellular processes may be disrupted by the GluedDN and sw1 mutations (Levy and Holzbaur, 2006). Nonetheless, these finding imply that axonal localization and transport is an important element of Wnd’s function in regulating an axon to nucleus signaling cascade.
Injury regulates Wnd protein turnover
The aforementioned observations suggest that injury signaling involves local activation of Wnd in axons. A previous study in vertebrate cells suggests that DLK protein is activated via dimerization (Nihalani et al., 2000). Because overexpression of wnd alone can activate downstream signaling (Fig. 2, D and F), a potential mechanism of activation is to increase local levels of Wnd so that it can dimerize and self-activate. Previous studies suggest that Wnd protein levels are tightly regulated by protein turnover (Nakata et al., 2005; Collins et al., 2006; Wu et al., 2007). The Hiw E3 ubiquitin ligase plays an important role in this regulation because mutations in hiw lead to increased levels of Wnd protein in axons (Collins et al., 2006). Therefore, we tested whether axonal injury induces changes in Wnd protein levels.
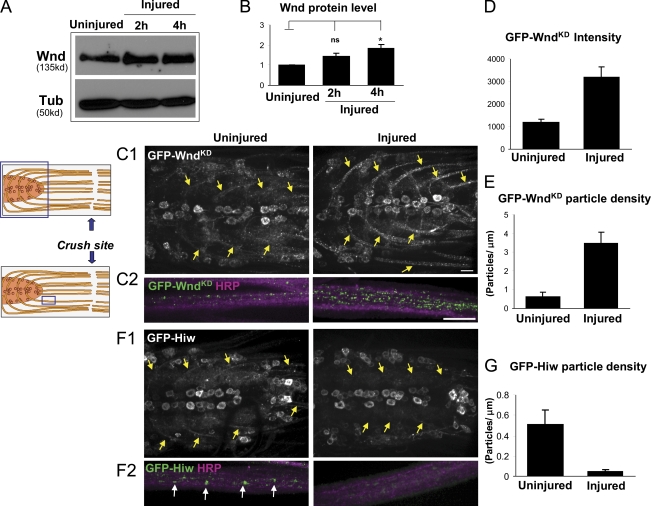
VNCs and connected segmental nerves from uninjured or injured larvae were microdissected and processed for Western blotting at different time points after injury. Intriguingly, we observed an 80% increase in Wnd protein within 4 h of injury (Fig. 7, A and B). This short time frame (much shorter than the time required for puc-lacZ induction) suggests that increased Wnd levels may be an earlier event in the injury signaling mechanism.
Figure 7.
Injury induces an increase in Wnd protein in axons concomitant with a decrease in Hiw. (A) Western blots from nerve cords with attached segmental nerves to detect endogenous Wnd protein levels (20 VNCs per lane) before and after injury. (B) Quantification of changes in Wnd protein level based on Wnd/tubulin ratio from five independent experiments. (C1 and C2) GFP-WndKD particles in nerve cords and segmental nerves before and 4 h after injury. UAS–GFP-wndKD is expressed in motoneurons by OK6-Gal4. The cartoon shows the anatomy of nerve cord and segmental nerves, with the blue rectangles indicating the sites shown. (C1) Nerve cords of uninjured and injured animals. Injury induces dramatic increase of GFP-WndKD intensity in the axons projecting from motoneuron cell bodies (yellow arrows). (C2) Segmental nerves proximal to injury site are shown in higher magnification costained with HRP to label the nerve membrane. (D and E) The injured segmental nerves contain a higher mean GFP intensity (D) and a higher density of GFP-WndKD particles (E). Quantification is described in Materials and methods. (F1 and F2) GFP-Hiw (driven by OK6-Gal4) in nerve cords and segmental nerves in uninjured and injured (4 h) animals. (F1) Injury induces overall decrease of GFP-Hiw in indicated axons (yellow arrows). (F2) GFP-Hiw localizes to particles (white arrows) in uninjured axons, which are dramatically decreased in number within 4 h after injury. (G) Measurement of GFP-Hiw particle density in segmental nerves. P > 0.05 was not significant. *, P < 0.05. Error bars indicate mean ± SEM. Bars, 25 µm.
To test whether the effect of injury upon Wnd protein level is posttranscriptional, we used the Gal4/UAS system drive expression of GFP-wndKD ectopically in motoneurons. Because expression from the OK6-Gal4 driver is not affected by injury (unpublished data), any changes to GFP-WndKD protein levels should be posttranscriptional, reflecting altered localization, translation, or protein turnover. We find that injury induces a dramatic increase in the amount of GFP-WndKD in axons. This can be detected both in axonal segments within the VNC (Fig. 7 C1, arrows) and segmental nerve (Fig. 7 C2), where a greater than threefold increase in mean intensity (Fig. 7 D) and particle density (Fig. 7 E) was observed 4 h after injury. In contrast, we measured no significant change in the intensity of GFP-WndKD in cell bodies after injury (P > 0.45; Fig. 7 C1 and Fig. S5 A). Because the total levels of Wnd increase after injury (Fig. 7 A) and transgenic Wnd levels also increase in axons, we hypothesize that Wnd levels increase via an alteration in stability or protein turnover.
An attractive model is that injury induces Wnd by inhibiting its regulation by Hiw. Therefore, we investigated whether injury altered the levels or localization of Hiw protein. UAS–GFP-hiw was expressed in motoneurons using the OK6-Gal4 driver. This transgenic protein, which is capable of rescuing the hiw mutant phenotype of synaptic overgrowth (Wu et al., 2005), localizes to both axons and cell bodies (Fig. 7, F1 and F2). Although injury does not induce significant changes in the levels of GFP-Hiw in cell bodies (P > 0.2; Fig. 7 F1 and Fig. S5 B), it induces a clear reduction in axonal GFP-Hiw (Fig. 7, F1, F2, and G). Although the localization of endogenous Hiw is not known, we suspect that the axonal Hiw is a functionally relevant pool because mutations in hiw lead to increased levels of Wnd in axons and because the vertebrate homologue Phr1 localizes in axons of cultured neurons (Lewcock et al., 2007). Because injury leads to a reduction in this axonal GFP-Hiw, we propose that injury induces activation of Wnd by down-regulating Hiw, which allows Wnd protein levels to increase in axons.
Hiw negatively regulates a retrograde injury response pathway
Because Hiw negatively regulates Wnd protein level (Collins et al., 2006), we hypothesized that the injury signaling pathway would be constitutively activated when hiw is absent. Two observations support this model.
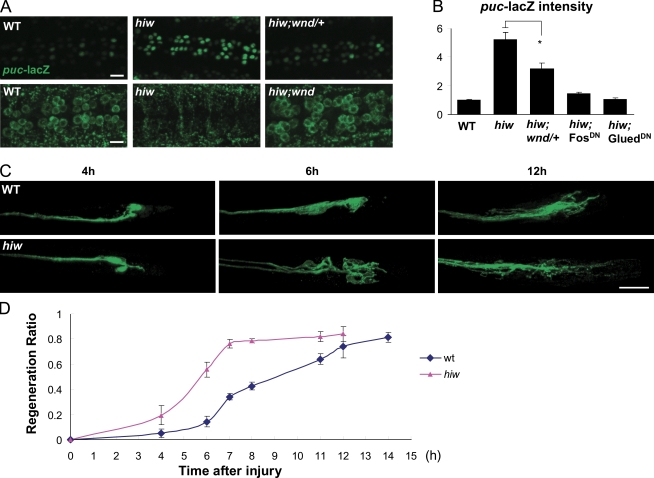
First, when hiw is mutant, puc-lacZ is increased more than fivefold above basal levels, whereas DVGLUT expression is dramatically decreased in motoneuron cell bodies (Fig. 8, A and B), resembling neurons that have been injured (Fig. 1). These effects can be suppressed by removing a single copy of wnd (Fig. 8, A and B). The induction of puc-lacZ by mutation of hiw also requires Fos and Glued (Fig. 8 B). We conclude that when hiw is mutant, the retrograde injury signaling pathway is ectopically active.
Figure 8.
The Hiw E3 ubiquitin ligase negatively regulates injury signaling and regeneration. (A) The hiwΔN mutant shows a dramatic increase of puc-lacZ and decrease of DVGLUT intensity in motoneuron cell bodies, and these changes require wnd function. (B) Quantification of puc-lacZ intensity for genotypes in A and when Fos and Glued are inhibited by expressing UAS-FosDN or UAS-GluedDN in the hiwΔN;puc-lacZ/+ mutant background. These results suggest that hiw regulates a retrograde signaling pathway. (C) Examples of the proximal stump morphology at different time points after injury. In hiwΔN mutants, many injured axons form long new branches within 6 h of injury. In contrast, sprouting is not readily apparent in WT axons until 12 h after injury. (D) Time course of regeneration ratio comparing WT and hiwΔN mutants. *, P < 0.05. Error bars indicate mean ± SEM. Bars, 25 µm.
Second, when hiw is mutant, injured neurons require less time to initiate a regenerative response (Fig. 8, C and D). Most neurons exhibit new branching within 7 h of injury in hiw mutants, whereas it takes >12 h for WT neurons to exhibit the equivalent amount of branching (Fig. 8, C and D). This acceleration of ∼5 h resembles the time required for p-JNK to appear in cell bodies after injury. This time could be bypassed in the hiw mutant because the injury signaling pathway is constitutively active. Notably, mutants in the homologue of hiw in C. elegans, rpm-1, also cause enhanced regeneration (Hammarlund et al., 2009). Thus, this role in regulating an injury response pathway is likely an evolutionarily conserved function for the Hiw/Rpm-1/Phr1 E3 ubiquitin ligase.
Discussion
In this study, we describe an axonal injury and regeneration assay in Drosophila larval motoneurons, which creates a powerful paradigm for studying the molecular events and requirements for axonal regeneration of defined neurons within an intact living animal. Importantly, a molecular reporter (the JNK phosphatase puckered) whose expression is induced by axonal injury in a cell-autonomous fashion allows us to dissect the steps required for neurons to mount a transcriptional response to injury. We use this new assay to reach several insights into the function of a conserved MAPK kinase kinase, Wnd/DLK, and its mechanism of activation during injury signaling and regeneration.
First, we show that Wnd functions as an upstream regulator of a nuclear signaling pathway that is activated by axonal injury. Second, we show that activation of this signaling pathway promotes axonal growth and branching of the injured neuron. Third, we demonstrate that Wnd is transported in axons, peripherally associated with membrane-bound vesicles, and the injury signaling mechanism requires functional axonal transport machinery. Fourth, we find that injury signaling is regulated by the Hiw E3 ubiquitin ligase and that protein turnover of Wnd in axons may be a surveillance mechanism for detecting axonal damage. Injury induces a rapid increase in Wnd protein, concomitant with a decrease in Hiw protein. In hiw mutants, the nuclear response to injury is constitutively active, and neurons require less time to initiate a regenerative response to injury. We conclude that the regulation of Wnd by Hiw in axons comprises an important mechanism for detecting and responding to axonal injury.
The Wnd/DLK kinase regulates a regenerative response to axonal injury
Our findings indicate that an important component of Wnd/DLK’s role in regeneration is to regulate a retrograde signaling pathway, linking injury, which activates the Wnd pathway in axons, to a downstream transcriptional response in the nucleus. In the vertebrate nervous system, not all neurons are capable of regenerating after axonal injury. Conditioning lesion studies in dorsal root ganglion neurons suggest that the capacity to regenerate in the central nervous system is linked to the ability to mount a cellular and transcriptional response to injury (Hannila and Filbin, 2008; Hoffman, 2010). Therefore, DLK is an attractive candidate regulator of injury signaling in vertebrate axons. It will be interesting to determine the function of DLK in different neuronal types.
The role for Wnd in activating a nuclear injury response does not rule out additional functions for Wnd in the axon. Recent studies in C. elegans suggest that DLK regulates translation in axons (Yan et al., 2009), which is important for both injury signaling and regeneration (Hanz et al., 2003; Yudin et al., 2008; Gumy et al., 2010). Wnd/DLK may also regulate local cytoskeletal changes because JNK signaling is known to regulate microtubules in axons (Gelderblom et al., 2004; Bogoyevitch and Kobe, 2006; Barnat et al., 2010; Stone et al., 2010). An attractive hypothesis, which is consistent with growth cone phenotypes for vertebrate homologues of hiw (Lewcock et al., 2007; Hendricks and Jesuthasan, 2009), is that Wnd signaling alters cytoskeleton to form or modify growth cones after injury. In addition to these roles in regeneration, we should also consider the involvement of JNK in cell death and degeneration (Johnson and Nakamura, 2007). It is possible that that the activation of Wnd/DLK by injury could have negative consequences in some scenarios.
Mechanism of activation of axonal injury signaling
Wnd is transported in axons.
Wnd is transported in axons, in the form of particles that move at a speed similar to other cargoes for fast axonal transport. Because endogenous Wnd protein cofractionates with membranes, this cytoplasmic kinase may associate peripherally with vesicles. An interesting future pursuit is to determine the molecular nature of these vesicles. Studies in the vertebrate sciatic nerve demonstrate that the JNK scaffolding protein JIP3/Sunday Driver associates with large, multivesicular organelles that travel retrogradely in response to injury (Cavalli et al., 2005; Abe et al., 2009). Because vertebrate DLK also associates with a membrane compartment (Mata et al., 1996), it is possible that DLK associates with a retrograde signaling cargo or a precursor to such cargo. Future tools are needed to follow the transport and associations of kinase-active and endogenous Wnd after injury.
Wnd is activated in axons.
Previous studies of the role of DLK in regeneration in C. elegans (Hammarlund et al., 2009; Yan et al., 2009) and degeneration in cultured dorsal root ganglion neurons (Miller et al., 2009) suggest that DLK functions acutely at the time of injury. The most attractive model for both observations is that DLK/Wnd is locally activated in axons by injury to mediate different responses in different contexts.
Our findings strongly support this model. Both endogenous and GFP-tagged Wnd localize to axons, and functional axonal transport machinery is required for transduction of the injury signaling to the nucleus. Even if Wnd is ectopically overexpressed, when presumably some protein could localize to the cell body, the downstream response requires functional axonal transport machinery. We interpret that the axonal localization and transport of Wnd is functionally relevant for its signal transduction mechanism. It may need to be localized in axons to become activated or to encounter a necessary cofactor or substrate.
Injury regulates protein turnover of Wnd.
Injury leads to an increase in the total levels of endogenous Wnd protein. Furthermore, the levels of transgenically expressed GFP-WndKD are also increased, particularly in axons. Because the OK6-Gal4 driver is not affected by injury, the increase in GFP-WndKD must take place posttranscriptionally either as increased protein synthesis or decreased protein turnover. Several observations favor the model that injury activates Wnd by inhibiting its turnover in axons. First, the GFP-WndKD transgene lacks 5′ and 3′ untranslated regions, which usually function in regulation of translation. Second, Wnd and its DLK homologue in C. elegans are down-regulated by the Hiw E3 ubiquitin ligase (Nakata et al., 2005; Collins et al., 2006), which decreases in axons after injury. Third, regulation of the levels of Wnd is a viable mechanism for regulating its activation because overexpression of Wnd is sufficient to activate this signaling pathway.
Our data suggest that down-regulation of Hiw could be part of the injury signaling mechanism. However, further studies are required to understand the mechanism of Hiw regulation after axon injury. Intriguingly, a recent study suggests that Hiw is regulated by autophagy (Shen and Ganetzky, 2009), which could potentially be induced by axonal injury.
Rescue experiments indicate that Hiw function is required throughout the larval stage to down-regulate Wnd (Wu et al., 2005); thus, Wnd is constantly made, transported, and destroyed in axons. It has previously been perplexing that despite the amount of energy required to regulate this signaling pathway, there was not an obvious function of Wnd in synaptic development or function. We propose that the regulation of Wnd by Hiw in axons could be part of a damage surveillance mechanism for the cell to detect and respond to axonal injury.
Connections between injury response, synaptic growth, and synaptic maintenance
A previous study in Drosophila suggested that Hiw functions to regulate nerve terminal growth at the NMJ (Wan et al., 2000). The fivefold increase in number of presynaptic boutons in hiw mutants is one of the most dramatic phenotypes described at the larval NMJ and is caused by the misregulation of Wnd protein. This synaptic overgrowth may simply be the outcome of a misregulated regenerative response within an intact, uninjured circuit.
However, it is also interesting to consider the possibility that the Wnd signaling pathway, which is regulated by Hiw in larval axons, normally performs other functions. An injury, which disrupts an axon’s connections with its target, may share some similarity with other insults that affect the functional connections at the synapse. Along this line, a recent study found that Hiw-regulated signaling can counteract synaptic retraction caused by loss of the spectrin cytoskeleton and that loss of spectrin induces expression of puckered (Massaro et al., 2009). Although the role of Wnd in this remains to be addressed, an intriguing possibility is that loss of synaptic adhesion induces the injury signaling/regeneration pathway. This could counteract the loss of synaptic contacts by promoting more growth. It is of great interest to understand in more detail the mechanism by which this molecular pathway is regulated and the downstream consequences of its activation. Our data, combined with others’ results in Drosophila and other model organisms, point to Hiw and Wnd as important upstream regulators of a therapeutically interesting cellular response in neurons.
Materials and methods
Genetics
The following strains were used in this study: Canton-S (WT), puc-lacZE69 (Martín-Blanco et al., 1998), BG380-Gal4 (Budnik et al., 1996), OK6-Gal4 (Aberle et al., 2002), m12-Gal4 (P(GAL4)5053A) (Ritzenthaler et al., 2000), RRa(eve)-Gal4 (Fujioka et al., 2003), hiwΔN (Wu et al., 2005), hiwND8 (Wan et al., 2000), wnd1, wnd3, and UAS-wnd (Collins et al., 2006), UAS-FosDN (Fbz) and UAS-JunDN (Jbz; Eresh et al., 1997), slprBS06 and UAS-slpr(WT) (Polaski et al., 2006), UAS-Bsk(JNK)DN (Weber et al., 2000), UAS-p150(Glued)DN, 96B (Allen et al., 1999), UAS–GFP-Hiw (Wu et al., 2005), sw1 (Boylan and Hays, 2002), khc8 (Hurd and Saxton, 1996), khc27 (Brendza et al., 1999), witHA3 (Aberle et al., 2002), and witHA4 (Aberle et al., 2002), which was recombined with puc-lacZE69. UAS–GFP-wndKD was generated directly from UAS-wndKD (Collins et al., 2006), which contains the K188A mutation in kinase domain. UAS–wnd-RNAi and UAS–bsk-RNAi were acquired from the Vienna RNAi center (Dietzl et al., 2007).
Immunocytochemistry
Wandering third instar larvae were dissected in PBS and fixed in either 4% paraformaldehyde in PBS or Bouin’s fixative for 15–30 min, depending on the antibodies used. Antibodies were used at the following dilutions in PBS with 5% normal goat serum: rabbit anti–Wnd A1, 1:100 (Collins et al., 2006); rabbit anti-DVGLUT, 1:5,000 (Daniels et al., 2004); rat anti-elaV, 1:50 (7E8A10; Developmental Studies Hybridoma Bank); mouse anti-lacZ, 1:100 (40-1a; Developmental Studies Hybridoma Bank); Cy3 goat anti-HRP, 1:1,000 (Jackson ImmunoResearch Laboratories, Inc.); A488 rabbit anti-GFP, 1:1,000 (Invitrogen); and mouse anti–p-JNK, 1:1,000 (Cell Signaling Technology). For secondary antibodies, Cy3- and A488-conjugated goat anti–rabbit and –mouse (Invitrogen) were used at 1:1,000. All samples were mounted and imaged in 70% glycerol.
Imaging and quantification
Confocal images were collected at room temperature on a spinning-disk confocal system (PerkinElmer) consisting of a scanner (Nipkow CSU10; Yokogawa) and an electron microscopy charge-coupled device camera (C9100-50; Hamamatsu Photonics) mounted on an inverted microscope (Axio Observer; Carl Zeiss, Inc.) with 25× 0.8 NA multi and 40× 1.3 NA, 63× 1.5 NA, and 100× 1.46 NA oil objectives. Similar settings were used to collect all compared genotypes. All imaging and quantification were conducted with Volocity software (PerkinElmer).
To quantify the mean intensity of puc-lacZ expression, the neuronal nuclei that lie along the dorsal midline of the nerve cord in segments A3–A7 were selected based on staining for elaV. Because of an NLS sequence fused to lacZ, the reporter protein also localized to nuclei. The mean lacZ intensity per nucleus was measured for at least eight animals for each genotype and normalized against analogous measurements in the control (WT) genetic background. A similar strategy was used to quantify the intensity of staining for phosphorylated JNK in motoneuron nuclei.
The total intensity GFP-WndKD (Fig. 7 D) was measured in 100 µm of each segmental nerve adjacent to the site of exit from the VNC. To measure the density of GFP-WndKD (Fig. 7 E) and GFP-Hiw (Fig. 7 G) particles in this area, we used Volocity software to select and count the number of particles above a threshold pixel intensity and size (GFP-WndKD: intensity, >2,000; size, >1 µm2; GFP-Hiw: intensity, >2,000; size, >2.5 µm2).
Live imaging
Adapted from Barkus et al. (2008), third instar larvae were dissected quickly in PBS and laid on microscope slides with the cuticle side against the glass, containing coverslip fragments as spacers. A 22 × 40–mm coverslip was placed over the specimen and anchored at the corners with nail polish. The remaining space around the specimen was filled with PBS. Dissection and imaging was performed at room temperature. Imaging was initiated within 10 min after dissection.
GFP-WndKD particles in segmental nerves were imaged continuously with a frame collected every 500 ms. To generate kymographs, the collection of single frames spanning 1 min of imaging time was processed using the Multiple Kymograph plug-in for ImageJ (National Institutes of Health), submitted by J. Rietdorf and A. Steitz (The European Molecular Biology Laboratory, Heidelberg, Germany). A total of 342 anterograde and 270 retrograde segments were measured from 16 1-min-long kymographs.
Nerve crush assay
The segmental nerves of third instar larvae were visualized through the cuticle under a standard dissection stereomicroscope. Larvae were anesthetized with CO2 gas, then segmental nerves were pinched tightly through the cuticle for 5 s with Dumostar number 5 forceps (Fig. S1 A). The segment injured was varied as described in Fig. 1 B, with injury site 1 at segment A2, injury site 2 at segment A4, and site 3 at A6. After successful injury, the posterior half of the larva was paralyzed. Larvae were transferred to a grape plate and kept alive for varying periods of time at 25°C.
Biochemistry
Adult flies of hiwΔN genotype were frozen in liquid nitrogen and heads were separated from the body through a brass sieve as described previously (van de Goor et al., 1995). Extracts were generated by dounce homogenization in acetate buffer (10 mM Hepes, pH 7.4, 5 mM EGTA, 100 mm potassium acetate, 3 mM magnesium acetate, and 1 mM DTT) with protease inhibitor cocktail (Roche) and proteosome inhibitor (0.05 M MG115; Sigma-Aldrich). Debris was removed by centrifugation two times at 3,000 g for 10 min at 4°C to generate the postnuclear supernatant.
The differential centrifugation steps, adapted from Littleton et al. (1999), are shown in Fig. S3 B. For the sucrose flotation assay, S2 (supernatant from 10,000 g spin), prepared as described in the previous paragraph from ∼4.5 mg of fly heads, was brought to a total volume of 500 µl in 1.375 M (40%) sucrose in acetate buffer. This comprised the bottom of sucrose step gradient, which was layered with 500 µl 1.77 M (35%) and 300 µl 0.241 M (8%) sucrose and centrifuged in a rotor (TLS55; Beckman Coulter) for 1.5 h at 50,000 rpm at 4°C. Equal amounts of each fraction were compared by Western blotting. This protocol is similar to that described previously (Haghnia et al., 2007).
For detection of Wnd protein levels in injured verses uninjured nerve cords (Fig. 7, A and B), the whole brain and connected segmental nerves were carefully dissected from third instar larvae. The two brain lobes were removed, and the remaining VNC and connected segmental nerves were processed for Western blotting (20 VNCs per lane).
For Western blotting, rabbit anti–Wnd A3-1,2 (Collins et al., 2006) was used at 1:700, rabbit anti-APPL (#952; provided by K. White, Brandeis University, Waltham, MA; Luo et al., 1990) was used at 1:500, mouse anti–β-tubulin (E7; Developmental Studies Hybridoma Bank) at 1:10,000, and mouse anti–Fas II (1D4; Developmental Studies Hybridoma Bank) at 1:500.
Online supplemental material
Fig. S1 shows further characterization of injured axons. Fig. S2 shows further evidence that Wnd is required for the injury response. Fig. S3 shows that Wnd protein associates with membrane. Fig. S4 shows that inhibition of axonal transport by sw1 also inhibits injury signaling. Fig. S5 shows that the intensity of GFP-WndKD and GFP-Hiw in cell bodies is not affected by injury. Video 1 shows movement of GFP-WndKD vesicles in motoneurons. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201006039/DC1.
Acknowledgments
We thank Mary Sprader, Sylvia Johnson, and Xiaolu Sun for technical assistance and Brad Miller for helpful experimental suggestions. We thank Carole Weaver and Larry Goldstein for advice on sucrose floatation experiments. For fly lines, we thank Miki Fujioka, Akira Chiba, Beth Stronach, Brian McCabe, Rod Murphey, the Bloomington Stock Center (Indiana University), and the Vienna Drosophila RNAi Center. We thank the Developmental Studies Hybridoma Bank (University of Iowa) and Kalpana White for APPL antibodies.
This work was supported by the National Science Foundation (grant IOS-0842701 to C.A. Collins) and the National Institutes of Health (grants NS069844 to C.A. Collins and DA020812 to A. DiAntonio).
Footnotes
Abbreviations used in this paper:
- DLK
- dual leucine zipper kinase
- DN
- dominant negative
- DVGLUT
- Drosophila vesicular glutamate transporter
- Hiw
- Highwire
- KD
- kinase dead
- NMJ
- neuromuscular junction
- p-JNK
- phosphorylated JNK
- UAS
- upstream activation sequence
- VNC
- ventral nerve cord
- Wnd
- Wallenda
- WT
- wild type
References
- Abe N., Cavalli V. 2008. Nerve injury signaling. Curr. Opin. Neurobiol. 18:276–283 10.1016/j.conb.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe N., Almenar-Queralt A., Lillo C., Shen Z., Lozach J., Briggs S.P., Williams D.S., Goldstein L.S., Cavalli V. 2009. Sunday driver interacts with two distinct classes of axonal organelles. J. Biol. Chem. 284:34628–34639 10.1074/jbc.M109.035022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle H., Haghighi A.P., Fetter R.D., McCabe B.D., Magalhães T.R., Goodman C.S. 2002. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 33:545–558 10.1016/S0896-6273(02)00589-5 [DOI] [PubMed] [Google Scholar]
- Allen M.J., Shan X., Caruccio P., Froggett S.J., Moffat K.G., Murphey R.K. 1999. Targeted expression of truncated glued disrupts giant fiber synapse formation in Drosophila. J. Neurosci. 19:9374–9384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus R.V., Klyachko O., Horiuchi D., Dickson B.J., Saxton W.M. 2008. Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol. Biol. Cell. 19:274–283 10.1091/mbc.E07-03-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnat M., Enslen H., Propst F., Davis R.J., Soares S., Nothias F. 2010. Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. J. Neurosci. 30:7804–7816 10.1523/JNEUROSCI.0372-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoyevitch M.A., Kobe B. 2006. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 70:1061–1095 10.1128/MMBR.00025-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M., Serras F., Martín-Blanco E., Baguñà J. 2005. JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev. Biol. 280:73–86 10.1016/j.ydbio.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Boylan K.L., Hays T.S. 2002. The gene for the intermediate chain subunit of cytoplasmic dynein is essential in Drosophila. Genetics. 162:1211–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendza K.M., Rose D.J., Gilbert S.P., Saxton W.M. 1999. Lethal kinesin mutations reveal amino acids important for ATPase activation and structural coupling. J. Biol. Chem. 274:31506–31514 10.1074/jbc.274.44.31506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumovsky P., Watanabe M., Hökfelt T. 2007. Expression of the vesicular glutamate transporters-1 and -2 in adult mouse dorsal root ganglia and spinal cord and their regulation by nerve injury. Neuroscience. 147:469–490 10.1016/j.neuroscience.2007.02.068 [DOI] [PubMed] [Google Scholar]
- Budnik V., Koh Y.H., Guan B., Hartmann B., Hough C., Woods D., Gorczyca M. 1996. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 17:627–640 10.1016/S0896-6273(00)80196-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli V., Kujala P., Klumperman J., Goldstein L.S. 2005. Sunday Driver links axonal transport to damage signaling. J. Cell Biol. 168:775–787 10.1083/jcb.200410136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C.A., Wairkar Y.P., Johnson S.L., DiAntonio A. 2006. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron. 51:57–69 10.1016/j.neuron.2006.05.026 [DOI] [PubMed] [Google Scholar]
- Daniels R.W., Collins C.A., Gelfand M.V., Dant J., Brooks E.S., Krantz D.E., DiAntonio A. 2004. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J. Neurosci. 24:10466–10474 10.1523/JNEUROSCI.3001-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R.W., Collins C.A., Chen K., Gelfand M.V., Featherstone D.E., DiAntonio A. 2006. A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle. Neuron. 49:11–16 10.1016/j.neuron.2005.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R.W., Gelfand M.V., Collins C.A., DiAntonio A. 2008. Visualizing glutamatergic cell bodies and synapses in Drosophila larval and adult CNS. J. Comp. Neurol. 508:131–152 10.1002/cne.21670 [DOI] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K.C., Barinova Y., Fellner M., Gasser B., Kinsey K., Oppel S., Scheiblauer S., et al. 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 448:151–156 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- Dobens L.L., Martín-Blanco E., Martínez-Arias A., Kafatos F.C., Raftery L.A. 2001. Drosophila puckered regulates Fos/Jun levels during follicle cell morphogenesis. Development. 128:1845–1856 [DOI] [PubMed] [Google Scholar]
- Eresh S., Riese J., Jackson D.B., Bohmann D., Bienz M. 1997. A CREB-binding site as a target for decapentaplegic signalling during Drosophila endoderm induction. EMBO J. 16:2014–2022 10.1093/emboj/16.8.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto K., Kawauchi T., Osawa M., Tabata H., Nakajima K. 2010. Role of dual leucine zipper-bearing kinase (DLK/MUK/ZPK) in axonal growth. Neurosci. Res. 66:37–45 10.1016/j.neures.2009.09.1708 [DOI] [PubMed] [Google Scholar]
- Fujioka M., Lear B.C., Landgraf M., Yusibova G.L., Zhou J., Riley K.M., Patel N.H., Jaynes J.B. 2003. Even-skipped, acting as a repressor, regulates axonal projections in Drosophila. Development. 130:5385–5400 10.1242/dev.00770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galko M.J., Krasnow M.A. 2004. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2:E239 10.1371/journal.pbio.0020239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M., Eminel S., Herdegen T., Waetzig V. 2004. c-Jun N-terminal kinases (JNKs) and the cytoskeleton—functions beyond neurodegeneration. Int. J. Dev. Neurosci. 22:559–564 10.1016/j.ijdevneu.2004.07.014 [DOI] [PubMed] [Google Scholar]
- Gumy L.F., Tan C.L., Fawcett J.W. 2010. The role of local protein synthesis and degradation in axon regeneration. Exp. Neurol. 223:28–37 10.1016/j.expneurol.2009.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghnia M., Cavalli V., Shah S.B., Schimmelpfeng K., Brusch R., Yang G., Herrera C., Pilling A., Goldstein L.S. 2007. Dynactin is required for coordinated bidirectional motility, but not for dynein membrane attachment. Mol. Biol. Cell. 18:2081–2089 10.1091/mbc.E06-08-0695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M., Nix P., Hauth L., Jorgensen E.M., Bastiani M. 2009. Axon regeneration requires a conserved MAP kinase pathway. Science. 323:802–806 10.1126/science.1165527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannila S.S., Filbin M.T. 2008. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp. Neurol. 209:321–332 10.1016/j.expneurol.2007.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanz S., Fainzilber M. 2006. Retrograde signaling in injured nerve—the axon reaction revisited. J. Neurochem. 99:13–19 10.1111/j.1471-4159.2006.04089.x [DOI] [PubMed] [Google Scholar]
- Hanz S., Perlson E., Willis D., Zheng J.Q., Massarwa R., Huerta J.J., Koltzenburg M., Kohler M., van-Minnen J., Twiss J.L., Fainzilber M. 2003. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 40:1095–1104 10.1016/S0896-6273(03)00770-0 [DOI] [PubMed] [Google Scholar]
- Hendricks M., Jesuthasan S. 2009. PHR regulates growth cone pausing at intermediate targets through microtubule disassembly. J. Neurosci. 29:6593–6598 10.1523/JNEUROSCI.1115-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S., Kawaguchi A., Suenaga J., Ono M., Cui D.F., Ohno S. 2005. Expression of MUK/DLK/ZPK, an activator of the JNK pathway, in the nervous systems of the developing mouse embryo. Gene Expr. Patterns. 5:517–523 10.1016/j.modgep.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Hoffman P.N. 2010. A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp. Neurol. 223:11–18 10.1016/j.expneurol.2009.09.006 [DOI] [PubMed] [Google Scholar]
- Horiuchi D., Barkus R.V., Pilling A.D., Gassman A., Saxton W.M. 2005. APLIP1, a kinesin binding JIP-1/JNK scaffold protein, influences the axonal transport of both vesicles and mitochondria in Drosophila. Curr. Biol. 15:2137–2141 10.1016/j.cub.2005.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi D., Collins C.A., Bhat P., Barkus R.V., Diantonio A., Saxton W.M. 2007. Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr. Biol. 17:1313–1317 10.1016/j.cub.2007.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd D.D., Saxton W.M. 1996. Kinesin mutations cause motor neuron disease phenotypes by disrupting fast axonal transport in Drosophila. Genetics. 144:1075–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh A., Horiuchi M., Bannerman P., Pleasure D., Itoh T. 2009. Impaired regenerative response of primary sensory neurons in ZPK/DLK gene-trap mice. Biochem. Biophys. Res. Commun. 383:258–262 10.1016/j.bbrc.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Johnson G.L., Nakamura K. 2007. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim. Biophys. Acta. 1773:1341–1348 10.1016/j.bbamcr.2006.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshishian H., Kim Y.S. 2004. Orchestrating development and function: retrograde BMP signaling in the Drosophila nervous system. Trends Neurosci. 27:143–147 10.1016/j.tins.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Levy J.R., Holzbaur E.L. 2006. Cytoplasmic dynein/dynactin function and dysfunction in motor neurons. Int. J. Dev. Neurosci. 24:103–111 10.1016/j.ijdevneu.2005.11.013 [DOI] [PubMed] [Google Scholar]
- Lewcock J.W., Genoud N., Lettieri K., Pfaff S.L. 2007. The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron. 56:604–620 10.1016/j.neuron.2007.09.009 [DOI] [PubMed] [Google Scholar]
- Leyssen M., Ayaz D., Hébert S.S., Reeve S., De Strooper B., Hassan B.A. 2005. Amyloid precursor protein promotes post-developmental neurite arborization in the Drosophila brain. EMBO J. 24:2944–2955 10.1038/sj.emboj.7600757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton J.T., Serano T.L., Rubin G.M., Ganetzky B., Chapman E.R. 1999. Synaptic function modulated by changes in the ratio of synaptotagmin I and IV. Nature. 400:757–760 10.1038/23462 [DOI] [PubMed] [Google Scholar]
- Luo L.Q., Martin-Morris L.E., White K. 1990. Identification, secretion, and neural expression of APPL, a Drosophila protein similar to human amyloid protein precursor. J. Neurosci. 10:3849–3861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués G., Zhang B. 2006. Retrograde signaling that regulates synaptic development and function at the Drosophila neuromuscular junction. Int. Rev. Neurobiol. 75:267–285 10.1016/S0074-7742(06)75012-7 [DOI] [PubMed] [Google Scholar]
- Martin M., Iyadurai S.J., Gassman A., Gindhart J.G., Jr., Hays T.S., Saxton W.M. 1999. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol. Biol. Cell. 10:3717–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Blanco E., Gampel A., Ring J., Virdee K., Kirov N., Tolkovsky A.M., Martinez-Arias A. 1998. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 12:557–570 10.1101/gad.12.4.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro C.M., Pielage J., Davis G.W. 2009. Molecular mechanisms that enhance synapse stability despite persistent disruption of the spectrin/ankyrin/microtubule cytoskeleton. J. Cell Biol. 187:101–117 10.1083/jcb.200903166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata M., Merritt S.E., Fan G., Yu G.G., Holzman L.B. 1996. Characterization of dual leucine zipper-bearing kinase, a mixed lineage kinase present in synaptic terminals whose phosphorylation state is regulated by membrane depolarization via calcineurin. J. Biol. Chem. 271:16888–16896 10.1074/jbc.271.28.16888 [DOI] [PubMed] [Google Scholar]
- McEwen D.G., Peifer M. 2005. Puckered, a Drosophila MAPK phosphatase, ensures cell viability by antagonizing JNK-induced apoptosis. Development. 132:3935–3946 10.1242/dev.01949 [DOI] [PubMed] [Google Scholar]
- Miller K.E., DeProto J., Kaufmann N., Patel B.N., Duckworth A., Van Vactor D. 2005. Direct observation demonstrates that Liprin-alpha is required for trafficking of synaptic vesicles. Curr. Biol. 15:684–689 10.1016/j.cub.2005.02.061 [DOI] [PubMed] [Google Scholar]
- Miller B.R., Press C., Daniels R.W., Sasaki Y., Milbrandt J., DiAntonio A. 2009. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat. Neurosci. 12:387–389 10.1038/nn.2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K., Abrams B., Grill B., Goncharov A., Huang X., Chisholm A.D., Jin Y. 2005. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 120:407–420 10.1016/j.cell.2004.12.017 [DOI] [PubMed] [Google Scholar]
- Nihalani D., Merritt S., Holzman L.B. 2000. Identification of structural and functional domains in mixed lineage kinase dual leucine zipper-bearing kinase required for complex formation and stress-activated protein kinase activation. J. Biol. Chem. 275:7273–7279 10.1074/jbc.275.10.7273 [DOI] [PubMed] [Google Scholar]
- Polaski S., Whitney L., Barker B.W., Stronach B. 2006. Genetic analysis of slipper/mixed lineage kinase reveals requirements in multiple Jun-N-terminal kinase-dependent morphogenetic events during Drosophila development. Genetics. 174:719–733 10.1534/genetics.106.056564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P.M., Miao T., Wu D., Zhang Y., Yeh J., Bo X. 2009. Responses of the nerve cell body to axotomy. Neurosurgery. 65:A74–A79 10.1227/01.NEU.0000352378.26755.C3 [DOI] [PubMed] [Google Scholar]
- Ritzenthaler S., Suzuki E., Chiba A. 2000. Postsynaptic filopodia in muscle cells interact with innervating motoneuron axons. Nat. Neurosci. 3:1012–1017 10.1038/79833 [DOI] [PubMed] [Google Scholar]
- Sanyal S. 2009. Genomic mapping and expression patterns of C380, OK6 and D42 enhancer trap lines in the larval nervous system of Drosophila. Gene Expr. Patterns. 9:371–380 10.1016/j.gep.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Schroer T.A. 2004. Dynactin. Annu. Rev. Cell Dev. Biol. 20:759–779 10.1146/annurev.cellbio.20.012103.094623 [DOI] [PubMed] [Google Scholar]
- Shen W., Ganetzky B. 2009. Autophagy promotes synapse development in Drosophila. J. Cell Biol. 187:71–79 10.1083/jcb.200907109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M.C., Nguyen M.M., Tao J., Allender D.L., Rolls M.M. 2010. Global up-regulation of microtubule dynamics and polarity reversal during regeneration of an axon from a dendrite. Mol. Biol. Cell. 21:767–777 10.1091/mbc.E09-11-0967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronach B., Perrimon N. 2002. Activation of the JNK pathway during dorsal closure in Drosophila requires the mixed lineage kinase, slipper. Genes Dev. 16:377–387 10.1101/gad.953002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F., He Z. 2010. Neuronal intrinsic barriers for axon regeneration in the adult CNS. Curr. Opin. Neurobiol. 10.1016/j.conb.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Goor J., Ramaswami M., Kelly R. 1995. Redistribution of synaptic vesicles and their proteins in temperature-sensitive shibire(ts1) mutant Drosophila. Proc. Natl. Acad. Sci. USA. 92:5739–5743 10.1073/pnas.92.12.5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H.I., DiAntonio A., Fetter R.D., Bergstrom K., Strauss R., Goodman C.S. 2000. Highwire regulates synaptic growth in Drosophila. Neuron. 26:313–329 10.1016/S0896-6273(00)81166-6 [DOI] [PubMed] [Google Scholar]
- Weber U., Paricio N., Mlodzik M. 2000. Jun mediates Frizzled-induced R3/R4 cell fate distinction and planar polarity determination in the Drosophila eye. Development. 127:3619–3629 [DOI] [PubMed] [Google Scholar]
- Wu C., Wairkar Y.P., Collins C.A., DiAntonio A. 2005. Highwire function at the Drosophila neuromuscular junction: spatial, structural, and temporal requirements. J. Neurosci. 25:9557–9566 10.1523/JNEUROSCI.2532-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Daniels R.W., DiAntonio A. 2007. DFsn collaborates with Highwire to down-regulate the Wallenda/DLK kinase and restrain synaptic terminal growth. Neural Dev. 2:16 10.1186/1749-8104-2-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D., Wu Z., Chisholm A.D., Jin Y. 2009. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 138:1005–1018 10.1016/j.cell.2009.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin D., Hanz S., Yoo S., Iavnilovitch E., Willis D., Gradus T., Vuppalanchi D., Segal-Ruder Y., Ben-Yaakov K., Hieda M., et al. 2008. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron. 59:241–252 10.1016/j.neuron.2008.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]