Abstract
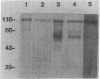
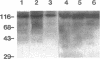
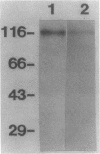
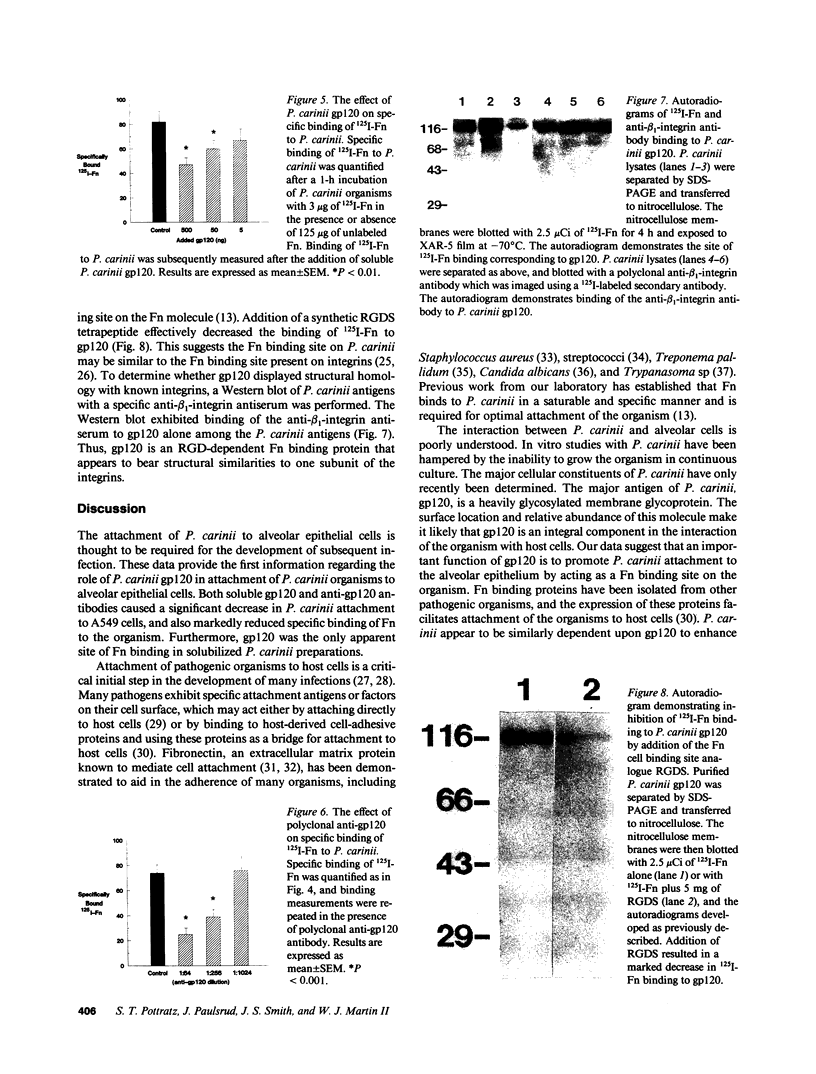
Pneumocystis carinii is an extracellular organism which is thought to require attachment to alveolar epithelial cells for its growth and replication in humans. Fibronectin (Fn) binding to P. carinii is essential for optimal P. carinii attachment. This study demonstrates that gp120, a 110-120-kD membrane glycoprotein on P. carinii, mediates attachment of the organism to cultured lung cells and is the site of Fn binding to P. carinii. A 51Cr-labeled P. carinii binding assay was used to quantify attachment of the organism to the alveolar epithelial cell line A549. Addition of free gp120, purified from whole P. carinii organisms, caused a significant decrease in attachment of P. carinii to A549 cells from 44.2 +/- 5.5% to 22.4 +/- 4.2% (P less than 0.01). Preincubation of the P. carinii organisms with a polyclonal antibody to gp120 also resulted in a marked decrease in P. carinii attachment to A549 cells from 46.8% +/- 5.2% to 21.3 +/- 4.8% (P less than 0.01). Furthermore, addition of free gp120 to P. carinii organisms caused a significant reduction in specific binding of 125I-Fn to P. carinii (from 83.3 +/- 8.5 ng to 47.1 +/- 5.9 ng, P less than 0.01). Similarly, anti-gp 120 antibody decreased specific Fn binding to P. carinii from 74.3 +/- 8.4 ng to 25.5 +/- 5.3 ng (P less than 0.001). Solubilized P. carinii organisms separated by gel electrophoresis and blotted with 125I-Fn demonstrated specific binding of the 125I-Fn to gp120. In addition, a specific anti-beta 1-integrin antiserum reacted with gp120 by Western blot, suggesting structural homology between gp120 and the beta-subunit of integrins. Thus, the data suggest that the P. carinii membrane glycoprotein gp120 functions as a Fn binding protein and is required for optimal P. carinii attachment to alveolar epithelial cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett M. S., Fishman J. A., Queener S. F., Durkin M. M., Jay M. A., Smith J. W. New rat model of Pneumocystis carinii infection. J Clin Microbiol. 1988 Jun;26(6):1100–1102. doi: 10.1128/jcm.26.6.1100-1102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett M. S., Verbanac P. A., Smith J. W. Cultivation of Pneumocystis carinii with WI-38 cells. J Clin Microbiol. 1979 Dec;10(6):796–799. doi: 10.1128/jcm.10.6.796-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Speziale P., Hök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987 May 15;262(14):6564–6571. [PubMed] [Google Scholar]
- Graves D. C., McNabb S. J., Worley M. A., Downs T. D., Ivey M. H. Analyses of rat Pneumocystis carinii antigens recognized by human and rat antibodies by using western immunoblotting. Infect Immun. 1986 Oct;54(1):96–103. doi: 10.1128/iai.54.1.96-103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshaw N. G., Carson J. L., Collier A. M. Ultrastructural observations of Pneumocystis carinii attachment to rat lung. J Infect Dis. 1985 Jan;151(1):181–186. doi: 10.1093/infdis/151.1.181. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Marcantonio E. E., Stepp M. A., Urry L. A., Yee G. H. Integrin heterodimer and receptor complexity in avian and mammalian cells. J Cell Biol. 1989 Jul;109(1):409–420. doi: 10.1083/jcb.109.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. Molecular biology of fibronectin. Annu Rev Cell Biol. 1985;1:67–90. doi: 10.1146/annurev.cb.01.110185.000435. [DOI] [PubMed] [Google Scholar]
- Kalo A., Segal E., Sahar E., Dayan D. Interaction of Candida albicans with genital mucosal surfaces: involvement of fibronectin in adherence. J Infect Dis. 1988 Jun;157(6):1253–1256. doi: 10.1093/infdis/157.6.1253. [DOI] [PubMed] [Google Scholar]
- Keusch G. T. The role of bacterial adherence in infection. Monogr Pathol. 1982;(23):94–113. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leptin M., Aebersold R., Wilcox M. Drosophila position-specific antigens resemble the vertebrate fibronectin-receptor family. EMBO J. 1987 Apr;6(4):1037–1043. doi: 10.1002/j.1460-2075.1987.tb04856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limper A. H., Martin W. J., 2nd Pneumocystis carinii: inhibition of lung cell growth mediated by parasite attachment. J Clin Invest. 1990 Feb;85(2):391–396. doi: 10.1172/JCI114451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke M. J., Cushion M. T., Walzer P. D. Properties of the major antigens of rat and human Pneumocystis carinii. Infect Immun. 1989 May;57(5):1547–1555. doi: 10.1128/iai.57.5.1547-1555.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long E. G., Smith J. S., Meier J. L. Attachment of Pneumocystis carinii to rat pneumocytes. Lab Invest. 1986 Jun;54(6):609–615. [PubMed] [Google Scholar]
- Marcantonio E. E., Hynes R. O. Antibodies to the conserved cytoplasmic domain of the integrin beta 1 subunit react with proteins in vertebrates, invertebrates, and fungi. J Cell Biol. 1988 May;106(5):1765–1772. doi: 10.1083/jcb.106.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., 2nd Neutrophils kill pulmonary endothelial cells by a hydrogen-peroxide-dependent pathway. An in vitro model of neutrophil-mediated lung injury. Am Rev Respir Dis. 1984 Aug;130(2):209–213. doi: 10.1164/arrd.1984.130.2.209. [DOI] [PubMed] [Google Scholar]
- Masur H., Jones T. C. The interaction in vitro of Pneumocystis carinii with macrophages and L-cells. J Exp Med. 1978 Jan 1;147(1):157–170. doi: 10.1084/jem.147.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur H. Prevention of Pneumocystis carinii pneumonia. Rev Infect Dis. 1989 Nov-Dec;11 (Suppl 7):S1664–S1668. doi: 10.1093/clinids/11.supplement_7.s1664. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Physiology of fibronectin. Annu Rev Med. 1984;35:561–575. doi: 10.1146/annurev.me.35.020184.003021. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ouaissi M. A., Afchain D., Capron A., Grimaud J. A. Fibronectin receptors on Trypanosoma cruzi trypomastigotes and their biological function. Nature. 1984 Mar 22;308(5957):380–382. doi: 10.1038/308380a0. [DOI] [PubMed] [Google Scholar]
- Pesanti E. L., Shanley J. D. Glycoproteins of Pneumocystis carinii: characterization by electrophoresis and microscopy. J Infect Dis. 1988 Dec;158(6):1353–1359. doi: 10.1093/infdis/158.6.1353. [DOI] [PubMed] [Google Scholar]
- Phair J., Muñoz A., Detels R., Kaslow R., Rinaldo C., Saah A. The risk of Pneumocystis carinii pneumonia among men infected with human immunodeficiency virus type 1. Multicenter AIDS Cohort Study Group. N Engl J Med. 1990 Jan 18;322(3):161–165. doi: 10.1056/NEJM199001183220304. [DOI] [PubMed] [Google Scholar]
- Pottratz S. T., Martin W. J., 2nd Role of fibronectin in Pneumocystis carinii attachment to cultured lung cells. J Clin Invest. 1990 Feb;85(2):351–356. doi: 10.1172/JCI114445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Mosher D. F., Olbrantz P. J. Fibronectin binding to Staphylococcus aureus. J Biol Chem. 1982 Dec 25;257(24):14788–14794. [PubMed] [Google Scholar]
- Radding J. A., Armstrong M. Y., Ullu E., Richards F. F. Identification and isolation of a major cell surface glycoprotein of Pneumocystis carinii. Infect Immun. 1989 Jul;57(7):2149–2157. doi: 10.1128/iai.57.7.2149-2157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin J. I., Guerrant R. L. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J Clin Invest. 1981 Nov;68(5):1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sharon N. Bacterial lectins, cell-cell recognition and infectious disease. FEBS Lett. 1987 Jun 15;217(2):145–157. doi: 10.1016/0014-5793(87)80654-3. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Beachey E. H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983 Jan;39(1):275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueishi K., Hisano S., Sumiyoshi A., Tanaka K. Scanning and transmission electron microscopic study of human pulmonary pneumocystosis. Chest. 1977 Aug;72(2):213–216. doi: 10.1378/chest.72.2.213. [DOI] [PubMed] [Google Scholar]
- Tamkun J. W., DeSimone D. W., Fonda D., Patel R. S., Buck C., Horwitz A. F., Hynes R. O. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986 Jul 18;46(2):271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Tanabe K., Takasaki S., Watanabe J., Kobata A., Egawa K., Nakamura Y. Glycoproteins composed of major surface immunodeterminants of Pneumocystis carinii. Infect Immun. 1989 May;57(5):1363–1368. doi: 10.1128/iai.57.5.1363-1368.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J Exp Med. 1985 Mar 1;161(3):514–525. doi: 10.1084/jem.161.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K., Walzer P. D. Attachment of Pneumocystis carinii to type I alveolar cells studied by freeze-fracture electron microscopy. Infect Immun. 1983 May;40(2):812–815. doi: 10.1128/iai.40.2.812-815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K., Walzer P. D. Mechanism of pulmonary alveolar injury in experimental Pneumocystis carinii pneumonia in the rat. Br J Exp Pathol. 1981 Aug;62(4):339–346. [PMC free article] [PubMed] [Google Scholar]