Abstract
Oxidative damage is a major cause of lung injury during systemic inflammatory response syndrome. In the present study, expression of an anti-oxidant enzyme, extracellular superoxide dismutase (EC-SOD), and its protective role against pulmonary oxidative damage were investigated using mouse models of systemic inflammation. Intraperitoneal injection with bacterial endotoxin lipopolysaccharides (LPS, 20 mg/kg) caused oxidative damage in lungs as assessed by increased tyrosine nitration in proteins. LPS administration also resulted in a rapid and significant loss of pulmonary EC-SOD more than 80% in a time- and dose-dependent manner, but other types of SODs, cytoplasmic CuZn-SOD and mitochondrial Mn-SOD, were not affected. EC-SOD protein is most abundant in lungs but also present at high levels in other tissues such as heart and white fat; however, the LPS-mediated decrease in this enzyme was most apparent in the lungs. Intravenous injection of mice with tumor necrosis factor α (10 μg per mouse) also caused 60% decrease in EC-SOD in lungs, suggesting that the EC-SOD down-regulation is mediated by this LPS-inducible inflammatory cytokine. A protective role of EC-SOD against LPS-mediated systemic inflammation was shown by an increased survival rate (75% vs.29% in 5 days) and decreased pulmonary oxidative damage in EC-SOD transgenic mice that overexpress the human EC-SOD gene. These results demonstrate that the inflammation-mediated EC-SOD down-regulation has a major pathophysiological impact during the systemic inflammatory response syndrome.
Keywords: EC-SOD, oxidative stress, nitro tyrosine, systemic inflammation, superoxide dismutase, endotoxemia
Introduction
Systemic inflammatory response syndrome (SIRS), characterized by hyper- or hypothermia, increased heart and respiratory rate, and abnormally high or low white blood cell number, occurs during sepsis and trauma-mediated injury [1, 2]. SIRS is accompanied with increased plasma levels of such inflammatory cytokines as tumor necrosis factor α (TNFα), interleukin 1β (IL-1β) and interleukin 6 (IL-6). Severe systemic inflammation leads to refractory hypotension and multiple organ failure which is a significant cause for the increased morbidity and mortality [1]. One of the major pathological consequences of SIRS is acute lung injury which is mainly caused by oxidative damage due to the inflammatory response [2]. During the inflammatory response, neutrophils undergo a respiratory burst and produce superoxide (O2• −). The superoxide anion reacts with nitric oxide (NO) and produces peroxynitrite (ONOO−), a toxic oxidant and nitrating agent. Breakdown of peroxynitrite can produce highly toxic hydroxyl radicals (•OH). These reactive oxygen species (ROS) can react with a variety of cellular macromolecules such as lipids, proteins, and DNA. Though these ROS have physiologically essential roles in host defense, overproduction of ROS during SIRS is highly toxic to host tissues resulting in severe pathophysiological consequences [3–5].
Superoxide dismutases (SODs) are the only antioxidant enzymes which can scavenge superoxide (O2• −) by converting this free radical to oxygen and hydrogen peroxide; the hydrogen peroxide can be further scavenged by several antioxidant enzymes including catalase or glutathione peroxidases. By scavenging superoxide (O2• −), SODs prevent production of peroxynitrite (ONOO−) [6, 7]. Three genetically distinct isoforms of SOD have been identified in mammals. CuZn-SOD or SOD1, is a copper- and zinc-containing enzyme which is localized primarily to the cytoplasm [8]. Mn-SOD or SOD2, is a manganese-containing enzyme and found predominantly in mitochondria [9]. The most recently discovered mammalian SOD is extracellular SOD (EC-SOD) or SOD3, which is primarily found in the extracellular matrix of tissues and to a lesser extent in extracellular fluid. EC-SOD, which also contains copper and zinc, is the predominant extracellular antioxidant enzyme [10–14]. EC-SOD is strongly expressed in lungs particularly in blood vessels and airways [15, 16].
Several studies, using either EC-SOD-null mice or transgenic mice overexpressing EC-SOD, have shown that this enzyme plays important roles in protecting lung tissues from certain types of physiological stress such as hyperoxia [17, 18] and hemorrhage [19, 20]. However, a role of EC-SOD in lungs during SIRS has not been clearly defined.
The purpose of our present study was to determine the expression pattern and the role of EC-SOD during endotoxin-mediated acute systemic inflammation as a SIRS model. Here, we show that the EC-SOD levels are significantly decreased in the lungs during the systemic inflammation and that the enzyme plays a protective role against inflammation-mediated oxidative damage and mortality; thus, the EC-SOD down-regulation is causally associated with pathophysiology of SIRS.
Materials and Methods
Animals
Two-month-old C57BL/6 mice and Swiss-Webster mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were kept in a 12:12-h light-dark cycle with free access to water and regular chow diet before and during experiments. EC-SOD transgenic mice with a genetic background of C57BL/6, which overexpress human EC-SOD constitutively under a β-actin promoter [21], were obtained from the National Jewish Medical and Research Center (Dennver, CO). Average body weight of the transgenic mice was equivalent to that of the wild-type control mice. To elicit systemic inflammation, mice were injected intraperitoneally (i.p.) with bacterial endotoxin lipopolysaccharide (LPS, derived from Pseudomonas aeruginosa, L8643, Lot number 045K 4057, Sigma Chemical, St. Louis, MO) dissolved in physiological saline. In some experiments, Swiss Webster mice were injected intravenously with a carrier-free form of mouse recombinant cytokines, TNFα or IL-6 (R&D Systems, Minneapolis, MN). Swiss Webster mice were chosen for the intravenous injection study because their tail veins are more clearly visible and thus easier to inject. Body temperature was assessed by monitoring rectal temperature with a digital thermometer (Precision Thermometer 4600, YS1 Inc. Dayton, OH). For collecting blood and tissues, mice were anesthetized with 2% isoflurane, blood was collected from the inferior vena cava with a heparin-treated syringe needle. The inferior vena cava was cut, and tissues perfused with 30 ml of saline: first through the right ventricle and next through the left ventricle. Lungs, heart, kidneys, liver and abdominal white fat pads were collected, snap-frozen in liquid nitrogen and stored at −80°C until use. All procedures were approved by the University of Texas Medical Branch (UTMB) Institutional Animal Care and Use Committee (IACUC).
Breeding and Genotyping of EC-SOD Transgenic Mice
For breeding the EC-SOD transgenic mice, male transgenic mice were matched with female wild-type C57BL/6 mice, and offspring were genotyped by PCR analysis using tail DNA isolated from a tail-end tissue (0.5 mm length) by digestion with 0.5% SDS (in 10 mM Tris-HCl, pH8.0, 10 mM EDTA, 100μg/ml Proteinase K) at 42°C overnight, followed by phenol extraction and ethanol precipitation. The precipitated DNA was resuspended in 50 μl of TE buffer (10 mM Tris-HCl, pH8.0, 1 mM EDTA), and 10 μl were used for PCR with a pair of human EC-SOD gene-specific primers: 5′-AGACACCTTCCACTCTGAGG -3′ and 5′-GTTTCGGTACAAATGGAGGC -3′. Mice whose tail DNA yielded a 172-bp PCR product were considered to be EC-SOD transgenic.
Preparation of Protein Samples and Western Blot Analysis
Pulverized frozen tissues were homogenized in ice-cold lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA, 1% Triton-X and 0.1% SDS) containing protease inhibitors (4-[2-aminoethyl] benzenesulfonyl fluoride, E-64, bestatin, leupeptin, aprotinin, mixed as Protease Inhibitor Cocktail, P2714, Sigma), 1 mM phenylmethanesulfonyl fluoride (PMSF) and phosphatase inhibitors (1 mM sodium orthovanadate, 20 mM β-glycerophosphate, 50 mM sodium fluoride, and 10 μM sodium molybdate; all purchased from Sigma). The homogenates were centrifuged and the protein concentrations were determined by Bradford’s method [22] using protein assay reagent (Bio-Rad, Hercules, CA). Equal amounts of proteins (40 μg per lane) were resolved on Nu-PAGE Bis-Tris gels (Invitrogen, Carlsbad, CA) and electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad). After being blocked with 5% nonfat milk, each membrane was incubated with individual primary antibody and 1% nonfat milk in TBS/T (20mM Tris-HCl, pH 7.6, 136 mM NaCl, and 0.05% Tween 20) at 4°C for overnight. The primary antibodies used in the present study were antibodies against EC-SOD (1:10,000 dilution [23]), CuZn-SOD (1:10,000 dilution, SOD-101, Stressgen, Canada), and Mn-SOD (1:10,000 dilution, SOD-111, Stressgen). The membranes were then incubated with a secondary antibody conjugated with horseradish peroxidase (1:5000 dilution, anti-rabbit), and the immunoreaction was visualized by the enhanced chemiluminescence (ECL Plus or ECL Western Blotting Detection System, Amersham, Arlington Heights, IL). For detection of tyrosine nitration, nitrocellulose membranes (Bio-Rad) were used instead of PVDF membrane, and the membranes were blocked with 1% bovine serum albumin (A4503 Sigma) in TBS/T and incubated with a horseradish peroxidase-conjugated antibody against nitrotyrosine (1:2,000 dilution, 16-207, Upstate, Lake Placid, NY) in TBS/T with 1% bovine serum albumin, washed with TBS/T, and directly subjected to ECL. All membranes were reprobed with anti-β-actin antibody (1: 5,000 dilution, A1978, Sigma) to confirm equal loading of the protein samples. Intensity of autoradiograph Protein levels were determined by densitometric analysis using Kodak one-dimensional software (version 3.6). Level of each protein was normalized to the control β-actin protein.
Cytokine Analysis
Plasma was separated from each blood sample by brief centrifugation, and the cytokine concentration was determined by enzyme-linked immunosorbent assay (ELISA). For TNFα assay, Endogen Minikit was used (Pierce Endogen, Rockford, IL). For IL-6 assay, anti-mouse IL-6 monoclonal antibody (MP520F3) and biotinylated anti-mouse IL-6 antibody (both from R&D systems) were used.
RNA Isolation and Northern Blot Analysis
Total RNA was isolated from tissues using guanidine/phenol solution [24]. In brief, each frozen lung tissue was placed in 5 ml of TRIzol Reagent (Invitrogen) and immediately processed with a homogenizer. Total RNA was purified from the homogenates by phenol extraction and alcohol precipitation. The RNA samples (20 μg per lane) were electrophoretically fractionated through 1.0% agarose gels containing 3% formaldehyde buffered with 20mM MOPS and 1mM EDTA at pH 7.4. The integrity of the RNA and the equality of loading were verified by the intensities of rRNA in ethidium bromide-stained gels. The RNAs were transferred to Zeta-Probe nylon membranes (Bio-Rad) and fixed by ultraviolet cross-linker Strataliker (Stratagene, La Jolla, CA). To prepare a probe DNA, a 264-bp EC-SOD cDNA fragment was amplified by reverse transcriptase-polymerase chain reaction (RT-PCR) from mouse lung RNA, and purified with QIAquick purification kit (QIAGEN, Valencia, CA). The primer oligo-DNA used for the PCR were 5′-TTGGCCTTCTTGTTCTACGG-3′ (sense) and 5′-AGTGGTCTTGCACTCGCTCT-3′ (anti-sense). Radiolabeled probe was prepared from the EC-SOD cDNA by the random priming method [25] with [α-32P]-dCTP (BLU513A250UC, Perkin Elmer, Boston, MA) using DECAprime II (Ambion, Austin, TX). The methods for hybridization and washing were described by Church and Gilbert [26]. The washed filter was exposed to autoradiography film in the presence of an intensifying screen at −80°C.
Statistical Analyses
Relative EC-SOD level was analyzed using one-way classification analysis of variance (ANOVA) followed by Bonferroni adjustment for multiple comparison. Results from the mortality experiment were analyzed using a log rank test from the StatView software (SAS Institute, Cray, CA). A P value of less than 0.05 was considered significant.
Results
Oxidative Damage Accumulated in Lung Proteins during LPS-mediated Systemic Inflammation
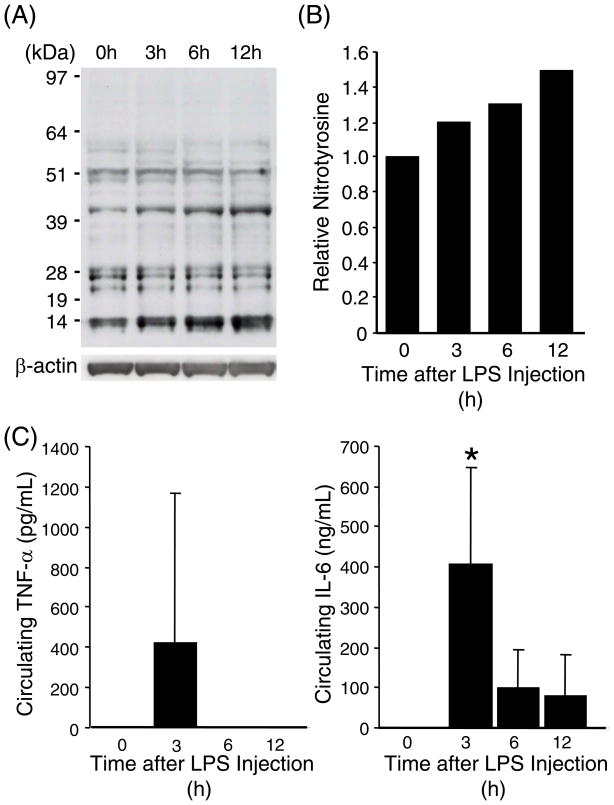
Accumulation of nitrotyrosine has been shown to be a good marker of oxidative damages in animal tissues [27, 28]. In order to assess pulmonary oxidative damage during acute systemic inflammation, mice were injected intraperitoneally with a dose of LPS (20 mg/kg), sacrificed at various time points, and the accumulation of nitrotyrosine in lung proteins was assessed by Western blot analysis. As shown in Fig. 1A, multiple bands with sizes ranging from 14- to 97-kDa, were detected with the anti-nitrotyrosine antibody. Total intensity of these bands was increased approximately 50% by 12 h after LPS injection (Fig. 1B). Presence of acute systemic inflammation was confirmed by induction of two inflammatory cytokines, TNFα and IL-6, in circulating blood (Fig. 1C). These results demonstrate that acute systemic inflammation causes rapid oxidative damage to the lung proteins.
Fig. 1. Increased tyrosine nitration in the lung proteins during systemic inflammation.
(A) Immuno-reactive nitrotyrosine was detected by Western blot analyses on whole lung protein samples from C57BL/6 mice (2-mo-old, male) that were sacrificed at indicated time points after LPS injection (20mg/kg, i.p.). Each lane represents pooled protein samples from 3–4 mice. Immuno-reaction with an anti-β-actin antibody confirms equal loading of the protein samples. (B) Densitometric analysis of (A). Total intensity throughout each lane was normalized to β-actin level. The normalized intensity value of the control lane (0 hour) was set at 1. (C) Plasma concentrations of TNFα and IL-6 were determined in each mouse used for (A). * indicates p<0.05 as compared to 0 hour samples.
Time- and Dose-dependent Decreases in EC-SOD in Lungs after LPS Administration
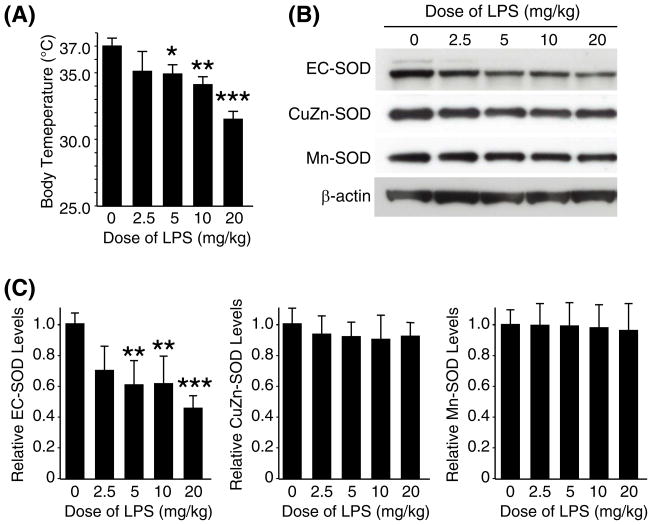
The effects of systemic inflammation on antioxidant enzymes in the lungs have not been clearly defined. Since systemic administration of LPS caused pulmonary oxidative damage, we then analyzed levels of the three SOD enzymes in the lungs after LPS injection. Mice were injected with various doses of LPS, ranging from 2.5 to 20 mg/kg, sacrificed 6 h later, and whole lungs processed for protein analysis. Western blot analysis was performed to examine whether LPS administration altered expression of the three SODs in the lungs. At the time of sacrifice, body temperature of each mouse was measured to assess the severity of systemic inflammation [29].
The mice developed hypothermia in a LPS dose-dependent fashion indicating that the severity of systemic inflammation was dependent on the LPS dose (Fig. 2A). As shown in Fig. 2B and C, lung EC-SOD protein levels clearly decreased by LPS injection in a dose-dependent fashion. In contrast, protein levels of the other two types of SODs, Cu/Zn-SOD and Mn-SOD, were not affected by LPS administration. There was a strong correlation between reduction of EC-SOD and the severity of hypothermia. For example, the smallest dose of LPS (2.5 mg/kg) caused a mild hypothermia (35.1°C) and an approximate 30% reduction of EC-SOD. A higher dose of LPS (20 mg/kg) caused a more severe hypothermia (31.5°C) and an approximate 54% reduction of EC-SOD. While LPS doses of 10 mg/kg or less were non-lethal for these mice, 20mg/kg of LPS resulted in more than 60% mortality within 5 days (data not shown).
Fig. 2. LPS dose-dependent decrease in EC-SOD in the lungs.
C57BL/6 mice (2-mo-old, male) were sacrificed 6 h after intraperitoneal injection with various doses of LPS; lung protein samples were used for Western blot analysis. (A) Body temperature of each mouse at the time of sacrifice. (B) Western blot analyses on pooled protein samples. Each lane represents pooled protein samples from 4 mice. The control mice received no injection (indicated as 0). (C) Western blot analyses were also performed on each protein sample from the individual mice, and results of densitometric analysis is shown. Each protein level in the control group (0 mg/ml) was set at 1. Statistical significance as compared to the control group is shown with *, ** and *** which indicates p < 0.05, 0.01 and 0.001, respectively.
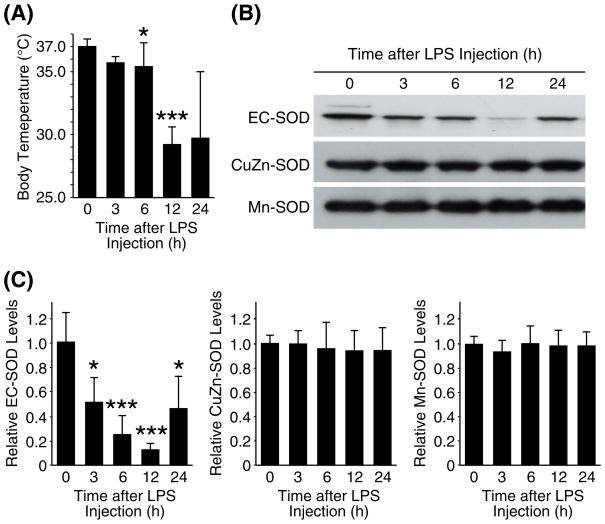
We also examined the kinetics of the LPS effects on the levels of the three SODs in the lungs. For this study, mice were sacrificed at various time points after injection with LPS (20 mg/kg), and whole lung proteins were extracted. At the time of sacrifice, body temperature of each mouse was measured to assess the degree of hypothermia. Average body temperature of the mice dropped from 37.0°C to 29.2°C by 12 h after LPS injection, confirming an acute systemic inflammation in this mouse model (Fig 3A). By 24 h post injection, some mice showed recovery of the body temperature to near normal levels while others showed progressive hypothermia (Fig 3A). Western blot analyses on the lung protein samples showed a time-dependent decrease in EC-SOD as shown in Fig 3B and C. The EC-SOD levels decreased progressively until 12 h post injection. By 24 h, EC-SOD levels increased close to the basal level in some mice while the levels remained low in other mice. In contrast, two other types of SODs did not show noticeable changes in their protein levels during the time course. Those mice that recovered to their normal body temperature by 24 h post injection showed recovery of the EC-SOD levels, and those that remained hypothermic showed sustained low levels of EC-SOD at 24 h post injection (data not shown), thus further suggesting the association between the decrease in the lung EC-SOD and severity of systemic inflammation.
Fig. 3. A time-course of the three SOD levels in the lungs during systemic inflammation.
C57BL/6 mice (2-mo-old, male) were injected with LPS (20mg/kg, i.p.), sacrificed at various time points; lung protein samples were used for Western blot analysis. (A) Body temperature of each mouse at the time of sacrifice. (B) Western blot analyses on pooled protein samples. Each lane represents pooled protein samples from 4 mice. (C) Western blot analyses were also performed on each protein sample from the individual mice, and results of densitometric analysis is shown. Each protein level in the control group (0 h) was set at 1. Statistical significance as compared to the control group is shown with * and *** which indicates p < 0.05 and 0.001, respectively.
Taken together, we conclude that protein levels of EC-SOD, but not other types of SODs, decreased rapidly in the lungs during LPS-mediated systemic inflammation. We also conclude that the decrease in the lung EC-SOD was dependent on the severity of systemic inflammation.
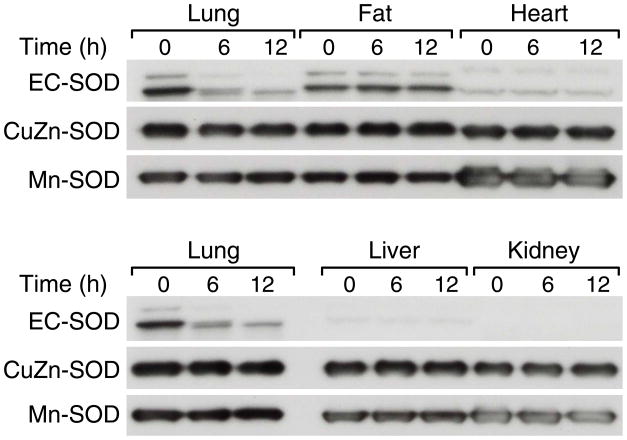
Tissue-specific Decreases in EC-SOD
Systemic inflammation resulting from intraperitoneal injection with LPS involves inflammatory responses in almost all tissues in the body [30, 31]. Although EC-SOD is most abundant in the lungs, several other organs including heart and adipose tissues are known to express EC-SOD at high levels [7, 32]. Thus, we examined whether the decrease in EC-SOD occurs in other tissues as well. Western blot analysis was performed on protein samples from lungs, heart, liver, kidneys and fat from mice that were sacrificed at 6 or 12 h after LPS injection; control mice received no LPS (0 h). Among these five tissues, the lungs showed the highest basal EC-SOD level, the adipose tissue was the second highest, and the heart the third highest in the control mice. Barely detectable levels of basal EC-SOD were present in the liver and the kidneys. In contrast, CuZn-SOD and Mn-SOD proteins did not show such a distinct tissue-specific expression pattern except that Mn-SOD was more abundant in the heart than in other tissues. After LPS injection, the EC-SOD levels decreased in the lungs confirming our initial observation; however, the EC-SOD reduction in other tissues was not as apparent as in the lungs. (Fig. 4). The protein levels of CuZn-SOD and Mn-SOD were not affected by LPS injection in the tissues examined. These results suggest that the LPS-induced decrease in EC-SOD may be an unique phenomenon to the lung which is the major source of this enzyme in the body.
Fig. 4. A lung-specific decrease in EC-SOD during systemic inflammation.
C57BL/6 mice (2-mo-old, male) were sacrificed at the indicated time points after LPS injection (20mg/kg, i.p.), and protein samples from five different tissues were used for Western blot analysis. Each lane represents pooled protein samples from 3 mice. Equal loading of the protein samples were confirmed by the CuZn-SOD levels.
Transcriptional Down-regulation of EC-SOD
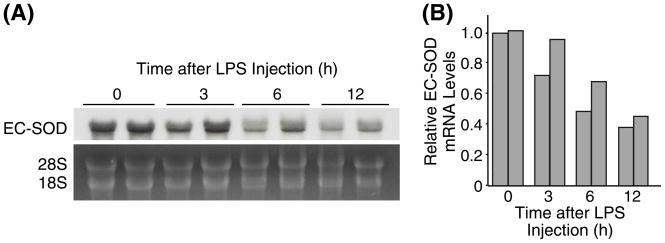
To determine whether the decrease in EC-SOD during systemic inflammation was due to a transcriptional or post-translational regulation, pulmonary EC-SOD mRNA levels were examined after LPS injection. Northern blot analysis showed that the lung EC-SOD mRNA decreased progressively with systemic inflammation (Fig 5); Average EC-SOD mRNA level decreased to 40% of the initial level by 12 h post-LPS injection.. These results suggest that lung EC-SOD levels are down-regulated at least partly by a transcriptional mechanism during systemic inflammation.
Fig. 5. Down-regulation of EC-SOD gene expression in the lungs during systemic inflammtion.
(A) Mice were injected with LPS (20mg/kg i.p.), sacrificed at indicated time points, and their total lung RNA isolated and used for Northern blot hybridization analysis. Each lane represents RNA sample from individual mouse. (B) Densitometric analysis of (A).
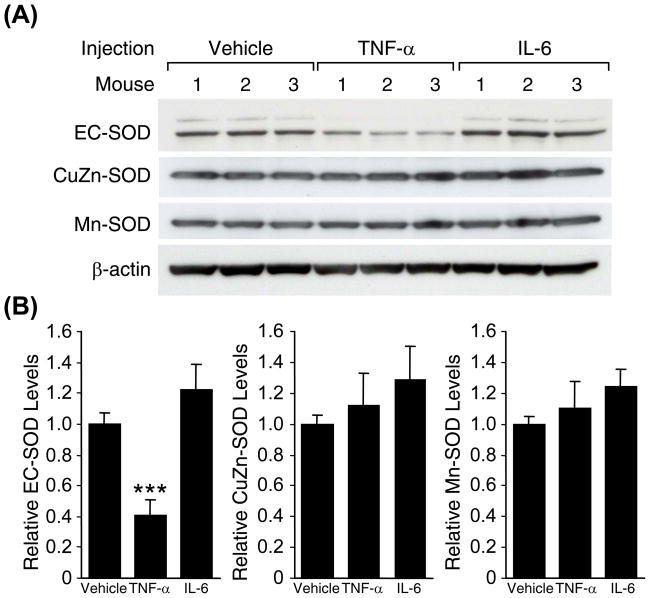
Decrease of Lung EC-SOD by TNFα
TNFα is an important cytokine that mediates the early phase of LPS-induced systemic inflammatory response including induction of IL-6. To assess a role of these two cytokines in the LPS-mediated down-regulation of EC-SOD, mice were injected intravenously with mouse recombinant TNFα (10 μg per mouse) or IL-6 (5 μg per mouse) and lung proteins isolated for Western blot analysis. As shown in Fig 6A, all mice treated with TNFα, but not IL-6, showed decreased levels of EC-SOD in the lungs, demonstrating that TNFα alone can elicit the down-regulation of EC-SOD. The average decrease of EC-SOD levels in the TNFα-injected mice was approximately 60% (Fig. 6B). The experiment was repeated with higher doses of IL-6 (up to 20 μg per mouse) confirming that this cytokine alone does not down-regulate EC-SOD (data not shown).
Fig. 6. Effects of inflammatory cytokines on lung EC-SOD levels during systemic inflammation.
(A) Western blot analysis demonstrating that lung EC-SOD protein levels decrease after injection of recombinant TNFα, but not IL-6. Swiss Webster mice (2-mo-old, male) were intraveneously injected with recombinant mouse TNFα (10 μg) or IL-6 (5 μg), and sacrificed 5 h after injection. (B) Densitometric analysis of (A). Each protein level in the control (vehicle-injected group) was set at 1. Statistical significance as compared to the control group is shown with *** which indicates p < 0.001.
Increased Tolerance to LPS by EC-SOD Overexpression
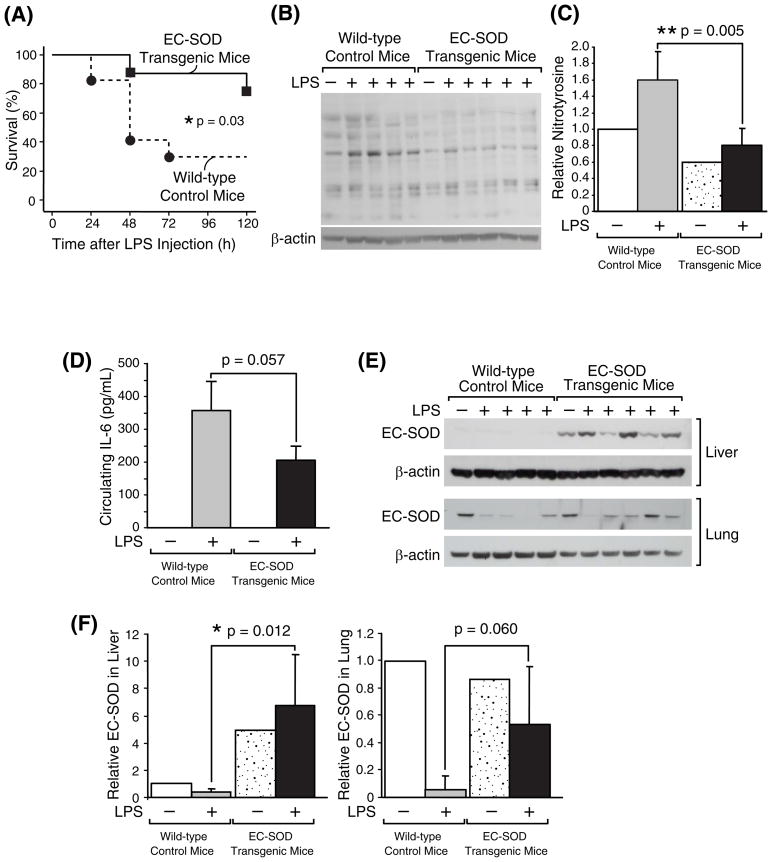
The roles of EC-SOD during systemic inflammation is not known. We hypothesized that EC-SOD may have a protective role against oxidative damage and conversely that down-regulation of pulmonary EC-SOD may have pathological significance in acute lung injury during systemic inflammation. To test these hypotheses, survival rates after LPS injection was compared in wild-type control mice vs. transgenic mutant mice which constitutively express exogenous human EC-SOD [21]. In the five days after injection with a lethal dose of LPS (20 mg/kg), 6 of 8 EC-SOD transgenic mice survived (75%) while only 5 of 17 wild-type mice survived (29%) (Fig. 7A). The increased survival of the EC-SOD transgenic mice was statistically significant (p < 0.05) as analyzed with a log rank test.
Fig. 7. Increased resistance to systemic inflammation in EC-SOD transgenic mice.
(A) Survival of 4-month-old male EC-SOD transgenic mice (n = 8) and wild-type control mice (n = 17) was monitored for 5 days after LPS injection (20 mg/kg, i.p.). (B) Western blot analysis demonstrating that EC-SOD transgenic mice have less nitro-tyrosine in lung proteins after LPS injection. The EC-SOD transgenic mice (n = 5) and wild-type control mice (n = 4) were intraperitoneally injected with LPS (20 mg/kg, indicated as LPS +), sacrificed 12 h later, and their lung proteins were subjected to Western blot analysis. Each lane represents a protein sample from individual mice. (C) Densitometric analysis of (B). Total intensity throughout each lane was normalized to β-actin level. The normalized intensity value of the control lane (Wild-type without LPS) was set at 1. Statistical analysis showed a significant difference with p < 0.01 between LPS-injected EC-SOD transgenic mice and wild-type control mice. (D) Plasma concentration of IL-6 determined in each mouse. (E) Western blot analysis comparing EC-SOD levels in the transgenic mice vs. wild-type mice. (F) Densitometric analyses of (E). The EC-SOD level in the control mouse without LPS was set at 1. Statistical analysis showed a significant difference in hepatic EC-SOD levels between LPS-injected EC-SOD transgenic mice and wild-type control mice.
To compare the oxidative damage in these mice during systemic inflammation, another set of mice were sacrificed 12 h after LPS injection, and the whole lung proteins were processed for Western blot analysis. As shown in Fig. 7B and 7C, LPS-induced accumulation of nitrotyrosine in the whole lung proteins was significantly lower in the EC-SOD transgenic mice compared to wild-type control mice. Plasma cytokine analysis showed that IL-6 levels 12 h after LPS injection appeared to be lower in the transgenic mice as compared to the control mice although statistical significance was not obtained (p=0.057) (Fig. 7D). TNFα levels in the same plasma samples were also analyzed; however, the levels were all below the detection limit (data not shown) confirming that this cytokine rapidly disappears after maximum induction (Fig. 1C).
To confirm the EC-SOD overexpression in the transgenic mice, Western blot analysis was performed and EC-SOD level was assessed in the lungs and liver of these mice. Without LPS injection, the lung EC-SOD levels in the transgenic and control mice were equivalent. After LPS injection, all four control mice showed clear down-regulation of EC-SOD; the EC-SOD transgenic mice showed relatively modest down-regulation as compared to the control mice although the difference was not statistically significant (p=0.060) (Fig. 7E and F). In contrast, the liver tissues of the transgenic mice showed apparent overexpression of EC-SOD. While endogenous hepatic EC-SOD levels in the control mice were almost undetectable, there was a strong hepatic EC-SOD expression in the transgenic mice, and the expression did not appear to be decreased by LPS (Fig. 7E and F). These results demonstrate that EC-SOD transgenic mice have an increased tolerance to LPS-mediated oxidative damage. Thus, we conclude that EC-SOD plays a protective role against acute lung injury during systemic inflammation.
Discussion
In the present study, we demonstrated for the first time that total lung EC-SOD protein levels significantly decreased during systemic inflammation. This decrease was rapid, occurring within 3 h after intraperitoneal administration of endotoxin, and strongly associated with the severity of the endotoxemia. It was previously reported that the EC-SOD mRNA levels decreased in lungs of rats after LPS injection [36]. In our study, we also confirmed that the lung EC-SOD mRNA levels are down-regulated in mice with LPS administration. By comparing the kinetics of the EC-SOD mRNA and protein levels, it is noted that protein levels are decreased faster than the mRNA level (Fig. 3C vs. Fig. 5B), suggesting that the rapid decrease in the protein is not solely due to transcriptional down-regulation.
EC-SOD has a heparin-binding domain which is sensitive to proteolysis [33]. With certain stresses such as exposure to hyperoxia or asbestos, EC-SOD is subjected to a proteolytic regulation and cleaved from the lung cell surface, causing a depletion or redistribution of this enzyme [34, 35]. For example, exposure to 100% oxygen causes a significant decrease in EC-SOD levels in the lung without a change in the mRNA levels [34]. Unlike this hyperoxia-induced EC-SOD down-regulation, inflammation-mediated decrease in EC-SOD is seen at both mRNA and protein levels, suggesting that this decrease involves both transcriptional and proteolytic regulations. Whether the down-regulation of the EC-SOD mRNA is mediated by a reduction of transcriptional activation or solely by a rapid destabilization of the mRNA, has yet to be determined. A previous study has identified myeloid zinc finger 1 and gut-enriched Kruppel-like-factor-like nuclear transcription factors as potential repressors of EC-SOD expression [37], and thus, these factors may play a role in LPS-mediated down-regulation of the gene. One limitation in the current study is that activities of each SOD enzyme were not measured. It remains to be determined whether the enzyme activity parallels with the down-regulation of the EC-SOD mRNA and protein.
It is intriguing that the LPS-mediated down-regulation of EC-SOD in the heart and adipose tissues was not apparent as compared to lungs. The mechanism for this tissue-specific gene regulation is unknown at present. EC-SOD levels in the kidney are also reportedly decreased during LPS-mediated endotoxemia [38]; however, the basal level of EC-SOD in the kidney was very low in our study and we could not confirm the LPS-mediated loss of EC-SOD in this organ (Fig 4). In contrast, the lung is the major source of this enzyme in the body as previously described [39] and also as confirmed in our present study. Since cleaved forms of EC-SOD would circulate systemically throughout the body, reduced production of EC-SOD from the lung may have a significant physiological impact on other organs as well.
TNFα is an early inflammatory cytokine induced rapidly upon inflammatory stimuli. In our mouse endotoxemia model, circulating levels of TNFα, usually undetectable in non-treated young animals, are increased as early as 30 minutes after LPS administration, peaking at 1.5 h and diminish rapidly thereafter [31, 40]. We also show that treatment of mice with TNFα decreases lung EC-SOD. Previous studies have shown that in vitro production of EC-SOD by cultured human skin fibroblasts and vascular smooth muscle cells are reduced with TNFα treatment, increased by interferon γ, and unchanged by several other cytokines including IL-6 [41, 42]. These results agree with our in vivo data that lung EC-SOD levels are decreased by intravenous administration with TNFα but not IL-6. We have repeated these studies with intraperitoneal injection of these cytokines and obtained similar results; TNFα, but not IL-6, down-regulated EC-SOD in the lung (data not shown). Thus, it is reasonable to conclude that the down-regulation of EC-SOD is mediated mainly by TNFα after LPS injection. A previous study showed that EC-SOD inhibits LPS-stimulated release of TNFα in macrophages [43]. Thus, there appears to be a mutual negative-regulating mechanism between pro-inflammatory TNFα and anti-inflammatory EC-SOD in the lung.
Transgenic mice with human EC-SOD regulated by the β-actin promoter overexpress the EC-SOD in various organs including heart, brain, muscle, and lungs [21]. These mutant mice have increased resistance to global cerebral ischemia and aging-induced cognitive impairment [44, 45]. Injection of LPS into EC-SOD null mutant mice increased levels of inflammatory cytokines/chemokines, including TNFα, compared to wild-type control mice [43]. While this study has demonstrated an anti-inflammatory role for EC-SOD, it was not clear whether EC-SOD has a protective effect against LPS-mediated systemic inflammation and acute lung injury. In the present study, we showed that the EC-SOD overexpressing transgenic mice have an increased survival rate as compared to the wild-type mice during endotoxemia. This is in contrast to a previous study showing that transgenic mice overexpressing human CuZn-SOD were not resistant to endotoxemia [46]. Thus, EC-SOD, rather than CuZn-SOD, appears to play a major protective role against systemic inflammation. We also demonstrated that LPS-mediated lung protein oxidation, as assessed by formation of nitrotyrosine, was reduced in the transgenic mice compared to control mice. As noted above, both superoxide anion (O2• −) and nitric oxide (NO) are produced during systemic inflammation, and these two react with each other to form a toxic peroxynitrite (ONOO−) while SODs prevent the peroxynitrite formation by scavenging the superoxide before it reacts with nitric oxide. Thus, overexpression of EC-SOD is likely to prevent peroxynitrite formation and resulting tissue damage during acute lung injury. Our study also found a modest decrease in LPS-mediated IL-6 production in the EC-SOD transgenic mice (Fig. 7D), confirming a possible anti-inflammatory effect of EC-SOD [43]. Thus, an anti-inflammatory effect of EC-SOD may be a possible alternative explanation for the reduced mortality and oxidative damage in the EC-SOD transgenic mice; an overexpression of EC-SOD may have suppressed inflammation which resulted in reduction of oxidative stress and mortality. However, the decrease in IL-6 production was relatively insignificant (Fig. 7D) as compared to the statistically significant decrease in nitro-tyrosine in the transgenic mice (Fig. 7C), raising a possibility that the anti-oxidant effect rather than the anti-inflammatory effect of EC-SOD may be the direct contributing factor for the increased tolerance to LPS in the transgenic mice. The EC-SOD overexpression in the transgenic mice used in the current study was not limited to the lungs. Our analysis indicated that EC-SOD overexpression was more apparent in the liver than in the lungs of the transgenic mice. Thus, it is highly possible that overexpressed EC-SOD from tissues other than lungs may have protected lungs from oxidative damage. To examine such possibility, transgenic mice with lung-specific overexpression of EC-SOD would be useful [20]. Taken together, it would be reasonable to conclude that the inflammation-mediated loss of EC-SOD is causally associated with the pulmonary oxidative damage and mortality during endotoxemia. Our findings support use of anti-oxidant therapies with SOD or SOD-like drugs to reduce lung injury in SIRS.
With the endotoxemia model used in our present study, no mice died until 12 h after LPS injection, and approximately 20% and 70% died by 24 h and 120 h, respectively. Therefore, the kinetics of the EC-SOD levels must be interpreted with some caution as only surviving mice were selectively representing in such late time points. For example, in our time course experiment, the average EC-SOD levels appeared to have recovered to the basal level by 24 h (Fig. 3C). However, one mouse with the most severe hypothermia in the group died before 24 h, and thus was not included in the 24 h time point; the EC-SOD level in this mouse would have been possibly very low if this mouse had survived by 24 h. Such possibility is supported by the strong association between the decrease in the lung EC-SOD levels and severity of endotoxemia as assessed by the degree of hypothermia.
In summary, EC-SOD, the predominant extracellular antioxidant enzyme which is most abundantly expressed in the lungs, has a protective role against acute pulmonary oxidative damage caused by systemic inflammation. The lung EC-SOD level is significantly reduced by TNFα during acute systemic inflammation. The inflammation-mediated reduction of EC-SOD appears to be causally linked to the increased pulmonary oxidative damage and mortality in SIRS.
Acknowledgments
This work was supported by the National Institutes of Health Grant RO1-AG025908.
Authors thank Karen Martin for manuscript preparation and Tatsuo Uchida for statistical analysis. We also thank Dr. Naseem Ansari at University of Texas Medical Branch for her thoughtful advice on analysis of oxidative stress. Dr. James Crapo holds patents on antioxidant mimetic compounds that demonstrate antioxidant activity similar to the EC-SOD protein. This work was supported by the National Institutes of Health Grant RO1-AG025908.
List of Abbreviations
- EC-SOD
extracellular superoxide dismutase
- IL-1β
interleukin 1β IL-6, interleukin 6
- i.p
intraperitoneal injection
- LPS
lipopolysaccharide
- NO
nitric oxide
- ROS
reactive oxygen species
- SIRS
systemic inflammatory response syndrome
- TNFα
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsiotou AG, Sakorafas GH, Anagnostopoulos G, Bramis J. Septic shock; current pathogenetic concepts from a clinical perspective. Med Sci Monit. 2005;11:RA76–85. [PubMed] [Google Scholar]
- 2.Roth E, Manhart N, Wessner B. Assessing the antioxidative status in critically ill patients. Curr Opin Clin Nutr Metab Care. 2004;7:161–168. doi: 10.1097/00075197-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharyya J, Biswas S, Datta AG. Mode of action of endotoxin: role of free radicals and antioxidants. Curr Med Chem. 2004;11:359–368. doi: 10.2174/0929867043456098. [DOI] [PubMed] [Google Scholar]
- 4.Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. 2003;35:236–256. doi: 10.1016/s0891-5849(03)00275-2. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232. doi: 10.1093/bja/aeg034. [DOI] [PubMed] [Google Scholar]
- 6.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 7.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 8.Crapo JD, Oury T, Rabouille C, Slot JW, Chang LY. Copper, zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci U S A. 1992;89:10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisiger RA, Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973;248:4793–4796. [PubMed] [Google Scholar]
- 10.Marklund SL. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982;79:7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marklund SL, Holme E, Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982;126:41–51. doi: 10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- 12.Sandstrom J, Karlsson K, Edlund T, Marklund SL. Heparin-affinity patterns and composition of extracellular superoxide dismutase in human plasma and tissues. Biochem J. 1993;294(Pt 3):853–857. doi: 10.1042/bj2940853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folz RJ, Crapo JD. Extracellular superoxide dismutase (SOD3): tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics. 1994;22:162–171. doi: 10.1006/geno.1994.1357. [DOI] [PubMed] [Google Scholar]
- 14.Folz RJ, Guan J, Seldin MF, Oury TD, Enghild JJ, Crapo JD. Mouse extracellular superoxide dismutase: primary structure, tissue-specific gene expression, chromosomal localization, and lung in situ hybridization. Am J Respir Cell Mol Biol. 1997;17:393–403. doi: 10.1165/ajrcmb.17.4.2826. [DOI] [PubMed] [Google Scholar]
- 15.Oury TD, Chang LY, Marklund SL, Day BJ, Crapo JD. Immunocytochemical localization of extracellular superoxide dismutase in human lung. Lab Invest. 1994;70:889–898. [PubMed] [Google Scholar]
- 16.Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radic Biol Med. 1996;20:957–965. doi: 10.1016/0891-5849(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 17.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci U S A. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest. 1999;103:1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowler RP, Arcaroli J, Abraham E, Patel M, Chang LY, Crapo JD. Evidence for extracellular superoxide dismutase as a mediator of hemorrhage-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;284:L680–687. doi: 10.1152/ajplung.00191.2002. [DOI] [PubMed] [Google Scholar]
- 20.Bowler RP, Arcaroli J, Crapo JD, Ross A, Slot JW, Abraham E. Extracellular superoxide dismutase attenuates lung injury after hemorrhage. Am J Respir Crit Care Med. 2001;164:290–294. doi: 10.1164/ajrccm.164.2.2011054. [DOI] [PubMed] [Google Scholar]
- 21.Oury TD, Ho YS, Piantadosi CA, Crapo JD. Extracellular superoxide dismutase, nitric oxide, and central nervous system O2 toxicity. Proc Natl Acad Sci U S A. 1992;89:9715–9719. doi: 10.1073/pnas.89.20.9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Fattman CL, Enghild JJ, Crapo JD, Schaefer LM, Valnickova Z, Oury TD. Purification and characterization of extracellular superoxide dismutase in mouse lung. Biochem Biophys Res Commun. 2000;275:542–548. doi: 10.1006/bbrc.2000.3327. [DOI] [PubMed] [Google Scholar]
- 24.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 25.Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- 26.Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristof AS, Goldberg P, Laubach V, Hussain SN. Role of inducible nitric oxide synthase in endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1998;158:1883–1889. doi: 10.1164/ajrccm.158.6.9802100. [DOI] [PubMed] [Google Scholar]
- 28.Gorg B, Wettstein M, Metzger S, Schliess F, Haussinger D. Lipopolysaccharide-induced tyrosine nitration and inactivation of hepatic glutamine synthetase in the rat. Hepatology. 2005;41:1065–1073. doi: 10.1002/hep.20662. [DOI] [PubMed] [Google Scholar]
- 29.Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev. 2003;124:1047–1058. doi: 10.1016/j.mad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Alam T, An MR, Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J Biol Chem. 1992;267:5021–5024. [PubMed] [Google Scholar]
- 31.Saito H, Patterson C, Hu Z, Runge MS, Tipnis U, Sinha M, Papaconstantinou J. Expression and self-regulatory function of cardiac interleukin-6 during endotoxemia. Am J Physiol Heart Circ Physiol. 2000;279:H2241–2248. doi: 10.1152/ajpheart.2000.279.5.H2241. [DOI] [PubMed] [Google Scholar]
- 32.Ookawara T, Imazeki N, Matsubara O, Kizaki T, Oh-Ishi S, Nakao C, Sato Y, Ohno H. Tissue distribution of immunoreactive mouse extracellular superoxide dismutase. Am J Physiol. 1998;275:C840–847. doi: 10.1152/ajpcell.1998.275.3.C840. [DOI] [PubMed] [Google Scholar]
- 33.Sandstrom J, Carlsson L, Marklund SL, Edlund T. The heparin-binding domain of extracellular superoxide dismutase C and formation of variants with reduced heparin affinity. J Biol Chem. 1992;267:18205–18209. [PubMed] [Google Scholar]
- 34.Oury TD, Schaefer LM, Fattman CL, Choi A, Weck KE, Watkins SC. Depletion of pulmonary EC-SOD after exposure to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2002;283:L777–784. doi: 10.1152/ajplung.00011.2002. [DOI] [PubMed] [Google Scholar]
- 35.Tan RJ, Fattman CL, Watkins SC, Oury TD. Redistribution of pulmonary EC-SOD after exposure to asbestos. J Appl Physiol. 2004;97:2006–2013. doi: 10.1152/japplphysiol.00480.2004. [DOI] [PubMed] [Google Scholar]
- 36.Loenders B, Van Mechelen E, Nicolai S, Buyssens N, Van Osselaer N, Jorens PG, Willems J, Herman AG, Slegers H. Localization of extracellular superoxide dismutase in rat lung: neutrophils and macrophages as carriers of the enzyme. Free Radic Biol Med. 1998;24:1097–1106. doi: 10.1016/s0891-5849(97)00434-6. [DOI] [PubMed] [Google Scholar]
- 37.Zelko IN, Folz RJ. Myeloid zinc finger (MZF)-like, Kruppel-like and Ets families of transcription factors determine the cell-specific expression of mouse extracellular superoxide dismutase. Biochem J. 2003;369:375–386. doi: 10.1042/BJ20021431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W, Jittikanont S, Falk SA, Li P, Feng L, Gengaro PE, Poole BD, Bowler RP, Day BJ, Crapo JD, Schrier RW. Interaction among nitric oxide, reactive oxygen species, and antioxidants during endotoxemia-related acute renal failure. Am J Physiol Renal Physiol. 2003;284:F532–537. doi: 10.1152/ajprenal.00323.2002. [DOI] [PubMed] [Google Scholar]
- 39.Marklund SL. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito H, Papaconstantinou J. Age-associated differences in cardiovascular inflammatory gene induction during endotoxic stress. J Biol Chem. 2001;276:29307–29312. doi: 10.1074/jbc.M103740200. [DOI] [PubMed] [Google Scholar]
- 41.Marklund SL. Regulation by cytokines of extracellular superoxide dismutase and other superoxide dismutase isoenzymes in fibroblasts. J Biol Chem. 1992;267:6696–6701. [PubMed] [Google Scholar]
- 42.Stralin P, Marklund SL. Multiple cytokines regulate the expression of extracellular superoxide dismutase in human vascular smooth muscle cells. Atherosclerosis. 2000;151:433–441. doi: 10.1016/s0021-9150(99)00427-x. [DOI] [PubMed] [Google Scholar]
- 43.Bowler RP, Nicks M, Tran K, Tanner G, Chang LY, Young SK, Worthen GS. Extracellular superoxide dismutase attenuates lipopolysaccharide-induced neutrophilic inflammation. Am J Respir Cell Mol Biol. 2004;31:432–439. doi: 10.1165/rcmb.2004-0057OC. [DOI] [PubMed] [Google Scholar]
- 44.Levin ED, Christopher NC, Lateef S, Elamir BM, Patel M, Liang LP, Crapo JD. Extracellular superoxide dismutase overexpression protects against aging-induced cognitive impairment in mice. Behav Genet. 2002;32:119–125. doi: 10.1023/a:1015201823417. [DOI] [PubMed] [Google Scholar]
- 45.Sheng H, Kudo M, Mackensen GB, Pearlstein RD, Crapo JD, Warner DS. Mice overexpressing extracellular superoxide dismutase have increased resistance to global cerebral ischemia. Exp Neurol. 2000;163:392–398. doi: 10.1006/exnr.2000.7363. [DOI] [PubMed] [Google Scholar]
- 46.de Vos S, Epstein CJ, Carlson E, Cho SK, Koeffler HP. Transgenic mice overexpressing human copper/zinc-superoxide dismutase (Cu/Zn SOD) are not resistant to endotoxic shock. Biochem Biophys Res Commun. 1995;208:523–531. doi: 10.1006/bbrc.1995.1370. [DOI] [PubMed] [Google Scholar]