Abstract
Research over the last 25 years has demonstrated that animals are able to organize sequences in memory and retrieve ordered sequences without language. Qualitative differences have been found between the serial organization of behavior in pigeons and monkeys. Here the authors test serial ordering abilities in ring-tailed lemurs, a strepsirrhine primate whose ancestral lineage diverged from that of monkeys, apes, and humans approximately 63 million years ago. Lemurs’ accuracy and response times were similar to monkeys, thus suggesting that they may share mechanisms for serial organization that dates to a common primate ancestor.
Keywords: serial learning, ordinal memory, distance effect, lemurs
Much of human behavior requires the serial organization of information in memory. We rely on memorized lists such as the months of the year, the days of the week, the counting sequence, and the alphabet. The order of events is essential when we tell a story, recite the events of the day, or describe how an experiment was conducted. Over the last 25 years, the study of serial order processing in nonverbal organisms has demonstrated that information can be serially organized and retrieved without language (e.g., Sands & Wright, 1980; Straub & Terrace, 1981). For example, when monkeys are shown a series of successively presented photographs, and are then asked to determine whether a probe picture was presented in the original list, monkeys tend to show the same qualitative response patterns commonly observed in serial order processing tasks performed by humans. Specifically, accuracy is higher for items at the ends of the list, and lower for items in the middle of the list (Sands & Wright, 1980). Several studies have also shown that nonverbal organisms can solve transitivity problems by inferring the order of a list based on relationships between individual adjacent-pairs of items in the list. For example, when taught that A<B, B<C, C<D, D<E, and so forth, various non-human species have been able to properly order previously un-trained test pairs such as BD (Bond et al., 2003; D’Amato & Colombo, 1990; Gillan, 1981; Lazareva et al., 2004; McGonigle & Chalmers, 1977; Paz-Y-Mino et al., 2004; Roberts & Phelps, 1994; Treichler & Van Tilburg, 1996; von Fersen, Wynne, Delius, & Staddon, 1991). Finally, in the simultaneous chaining paradigm (SCP) both monkeys and pigeons are able to select a series of arbitrarily ordered photographs according to a consistent arbitrary order (e.g., D’Amato & Colombo, 1988, 1989; Swartz, Chen, & Terrace, 1991; Terrace, 1984, 1991; Terrace & Chen, 1991a, 1991b; Terrace, Son, & Brannon, 2003).
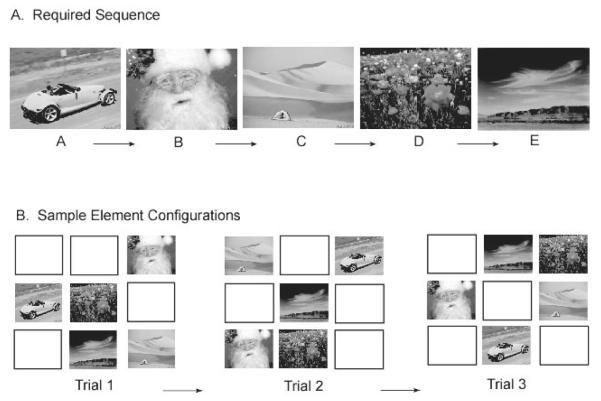
The SCP has proven particularly useful for studying the non-verbal serial organization of memory. In the SCP, a series of arbitrary stimuli (such as color photographs) are simultaneously presented in random spatial positions on a touch-sensitive monitor. To obtain reward the subject is required to respond to each stimulus in a prespecified arbitrary order without making an error. An important component of the paradigm is that no differential cues are provided after each correct response to indicate which item is next in the sequence. Therefore, the subject’s responses must be guided by an internal representation of the sequence (see Figure 1).
Figure 1.
An example trial in a simultaneous chaining paradigm. Items appear on the screen in random spatial positions. A correct response requires that all items are selected in the proper order with no errors. See text for additional details.
An explicit comparison of performance by monkeys and pigeons with the SCP revealed qualitative differences in the two species’ understanding of serial order (Terrace, 1991, 1993; D’Amato & Colombo, 1988). Pigeons and monkeys were trained to a similar level of accuracy on a five-item simultaneous chain. However, when pigeons were subsequently tested with all possible pairs derived from the five-item list (AB, AC, AD, AE, BC, BD, BE, CD, CE, DE) they were only able to accurately order pairs that conformed to a set of simple decision rules: (a) Respond to A first, (b) respond to E last, and (c) respond to any other item by default (Straub & Terrace, 1981; Terrace, 1991; Terrace & Chen, 1991a, 1991b). Thus pigeons were able to order pairs that contained a beginning or end item (or both), but they responded at chance with interior pairs (e.g., BD). One explanation for the discrepancy between the five-item performance and the subset performance in pigeons is that each item is able to cue the next as long as those items are physically present on the screen. However, when items are missing (as in the subset tests), pigeons are unable to internally generate the cue for the next item, and must therefore rely on a set of decision rules.
Unlike pigeons, cebus and rhesus monkeys were able to correctly order all interior (nonend) subset items selected from the list (Chen, Swartz, & Terrace, 1997; D’Amato & Colombo, 1988). Another important difference between pigeons and monkeys was seen in their reaction times. Response times for pigeons were unrelated to the position of the items in the original list (Terrace, 1991). Monkeys on the other hand, showed monotonic increases in reaction time to the first item as its distance increased from the beginning of the list. For example, responses to B in a BC test pair were faster than responses to C in a CD pair. In addition, for monkeys but not pigeons, reaction time increased to the second item as its distance from the first item increased (e.g., responses to C in a BC pair were faster than responses to D in a BD pair). The pattern of reaction times exhibited by monkeys suggests that monkeys may access an internal associative chain whereby reaction times reflect the time needed to sequentially access each item in the memory chain (D’Amato & Colombo, 1988). This idea is functionally similar to Ebbinghaus’ (1885/1964) associative chaining mechanism, except that the “stimuli” are internal representations rather than external cues. The onset of the trial may cue the representation of A, which then cues the representation of B and so forth, until the monkey proceeds through the entire list. A response is made when a match is found between the represented item, and the item on the screen.
If monkeys use an internal associative chain (see D’Amato & Colombo, 1988), associative links between items should strengthen as the monkeys learn the list. The associative strength between items is based on their proximity in the list. Items that are close (e.g., AB) have stronger associative links than items that are farther apart (e.g., AD). Therefore, it would be expected that when errors occur, a larger proportion of them will be single-skip errors (e.g., A → C or A → B → A) as opposed to multiskip errors for example, A → D or A → B → C → A (see Slamecka, 1985). These patterns have been observed in both old world monkeys (rhesus macaques) and new world monkeys (cebus) (D’Amato & Colombo, 1988; Swartz, Chen, & Terrace, 1991).
Another error trend that emerges with increased list experience is a reduction in backward relative to forward errors. Forward errors are those in which the correct item is skipped in favor of an item that occupies a position later in the list (e.g., A → B → D). In contrast, backward errors are those in which the correct item is skipped in favor of a previous item that has already been selected (e.g., A → B → A). D’Amato and Colombo (1988) found that when cebus monkeys learned a five-item simultaneous chain, the vast majority of their errors were forward errors, and of those, 94.3% were of the one-skip variety. Backward errors were much less common, but for those that did occur, 82.1% were of the one-skip variety (D’Amato & Colombo, 1988). Similarly, Swartz, Chen, and Terrace (1991) found that with experience, the proportion of backward errors relative to forward errors decreased, suggesting that monkeys learned to avoid selecting previously selected stimuli. Terrace, Son, and Brannon (2003) have argued that with increasing levels of expertise, monkeys become more efficient and make more “logical errors” in the sense that they learn to avoid making backward errors (e.g., A → B → A), as well as repeating previous mistakes.
Although an internal associative chain can account for much of the monkey accuracy and reaction time data, some evidence suggests that, with experience, monkeys acquire additional representational strategies for ordering pairs. For example, Chen, Swartz, and Terrace (1997) found that rhesus monkeys were able to correctly order subset pairs that contained individual items selected from different four-item lists (e.g., B from List 1 before D from List 2). Similarly, monkeys with extensive list learning expertise (multiple 3, 4, 5, and 7 item lists) exhibit distance effects for both accuracy and reaction time (Terrace, Son, & Brannon, 2003). As distance between the first and second item increases, reaction time to the first item in the pair decreases, and accuracy on that pair increases (e.g., faster and more accurate ordering of BE than BC). An internal associative chain cannot account for accurate between-list ordinal judgments, and neither can it account for the distance effects shown by the expert monkeys. Thus, it may be that monkeys with extensive list-learning experience have multiple representational tools available by which they organize serial events.
The complexity and apparent flexibility by which monkeys are capable of solving sequential order tasks raises the question of whether monkeys share sequential processing mechanisms with humans. Like monkeys, humans tested in the SCP show monotonic increases in reaction time to the first subset item as the distance of that item from the beginning of the list increases (Colombo & Frost, 2001). Humans also show distance effects for both accuracy and reaction time. A minor difference is that unlike monkeys, humans do not show increases in reaction time to the second item in the subset pair as distance between the first and second item increases. It seems likely that humans have simply learned that the second item can be responded to by default without any additional retrieval or decision processes (Colombo & Frost, 2001). Thus, performance in monkeys and humans on SCP is overwhelmingly similar and qualitatively different from that of pigeons.
In summary, it appears that old and new world monkeys have representational structures available to them that differ qualitatively from those of pigeons. Although pigeons rely on a set of simple decision rules to solve novel subset pairs selected from the list, monkeys and humans may access an internal representation of the list to solve novel subset pairs. These fundamental differences between species are useful not only in terms of understanding the means by which various cognitive problems can be solved, but also in shedding light on the phylogenetic pathways by which these processes evolved. Given that, humans and rhesus/cebus monkeys show considerable qualitative similarities in patterns of serial order production, and given that these patterns may be governed by similar underlying mechanisms, one possibility is that serial ordering capacities in monkeys and humans are homologous and therefore trace back to a common ancestor. Thus, an interesting question is when, in evolutionary time, this ability developed.
The primate order is composed of two suborders; Strepsirrhines and Haplorrhines. Strepsirrhine primates (lemurs, lorises, and galagos) diverged from the common ancestor of haplorrhines approximately 63 million years ago and therefore can potentially answer whether the observed behaviors shared by humans and monkeys (both haplorrhines) were likely extant in a primate ancestor before the divergence of strepsirrhines and haplorrhines in primate evolution (Yoder, 2003). Of course, if strepsirrhines do show the monkey and human pattern, additional out-groups would need to be tested to determine whether this pattern is primate unique or dates to an even earlier common ancestor.
Very little research has been conducted on strepsirrhine cognition, and much of what has been done does not lend itself to a comparative analysis of underlying cognitive mechanisms. This is largely because many of the studies involved simple binary problem-solving tasks (e.g., flipping a lid to obtain food, or manipulating a locked box) or the focus of these studies was not on the cognitive mechanisms per se, but rather on other factors such as social relationships that influence the outcome of the task (e.g., Anderson, Fornasieri, Ludes, & Roeder, 1992; Fornasieri, Anderson, & Roeder, 1990; Kappeler, 1987). An exception is a study by Ohta, Ishida, and Matano (1984) in which ring-tailed lemurs exhibited learning sets and first trial learning. In a more recent study, Santos, Mahajan, and Barnes (2005) examined the representation of tool functionality in two species of lemurs and found that lemurs attend to the functional properties of tools (e.g., tool orientation, ease of use) as opposed to the superficial nonfunctional features (e.g., color, texture). In addition, two recent studies (Lewis, Jaffe, & Brannon, 2005; Santos, Barnes, & Mahajan, 2005) found evidence of non-verbal number processing in lemurs. Despite these successes, other data suggest that lemurs are poor relational learners. Rumbaugh and McCormack (1967) found that when lemurs were trained to learn a specific relation (e.g., A + B-), that they performed much more poorly than monkeys and apes on reversal trials in which the reward contingency was reversed (e.g., A-B+).
In the present set of experiments, we trained two ring-tailed lemurs on three-, four-, and five-item simultaneous chains. In the first experiment, the lemurs learned five 3-item lists and five 4-item lists. The goal was to examine whether lemurs, like monkeys, show increased efficiency in learning to order new sets of photographs as they become more experienced with the task (e.g., learning sets, Harlow, 1949), and also to determine whether shifts in error patterns over time mirrored those of rhesus monkeys. In the second experiment, lemurs learned a five-item list and were subsequently tested with all possible subset pairs drawn from the list (AB, AC, AD, AE, BC, BD, BE, CD, CE, DE). The purpose was to determine whether lemurs could properly order test pairs selected from the list, and if so, to determine whether the lemurs showed error and latency patterns that corresponded to the previously observed “monkey pattern,” or whether those patterns were more similar to the previously observed “pigeon pattern.” In other words, do lemurs access an internal representation of a list when ordering pairs derived from a previously learned five-item sequence, or do they use a set of simple decision rules?
Experiment 1
Two ring-tailed lemurs were trained on five 3-item and five 4-item lists to examine whether lemurs show improvements in learning with list-learning experience. Our main question was whether lemurs would show a learning set whereby successive lists require fewer sessions to reach a performance criterion. Another goal was to examine whether the errors lemurs made as they learned lists changed over the course of learning in a way similar to that observed in new and old world monkeys.
Method
Subjects
The subjects were two ring-tailed lemurs (Lemur catta), Teres and Aristides, aged 10 and 12 years. Ring-tailed lemurs belong to the strepsirrhine suborder of primates that diverged from the lineage of monkeys, apes, and humans approximately 63 million years ago (Yoder et al., 2003). Among the strepsirrhines, ring-tailed lemurs are a highly social and terrestrial species. Both lemurs were experimentally naïve and were housed in indoor enclosures at the Duke Lemur Center. Water access was unlimited and fresh fruit and Purina monkey chow was provided daily.
Apparatus
Lemurs were tested in their home enclosures. All equipment for stimulus presentation, data acquisition, and reward delivery was housed in a custom-built, stainless steel, portable testing station (86 cm × 43 cm × 35 cm). The list items consisted of digitized color photographs (5 cm × 6 cm) of natural and fabricated objects (e.g., people, cars, landscapes) and were displayed on a 15-inch touch-sensitive computer monitor. No photograph was used in more than one list for the same animal. The photographs could appear in any of 9 equally spaced positions arranged in a 3 × 3 matrix. Custom-built Java™ and REALbasic® programs presented the stimuli and recorded responses. Correct Responses were rewarded with 190-mg fruit punch-flavored sucrose reward pellets.
Procedure
Subjects began list training immediately after being shaped to press single images on the touchscreen for reward pellets. Lemurs were then trained on five 3-item and five 4-item lists. Lists were trained using the simultaneous chaining paradigm (Terrace, 1984). Although previous training methodologies used incremental training procedures in which training began with one item (A), then two items (A→B), and so forth; we used a method similar to that of Terrace, Son, & Brannon (2003) in which lemurs were presented with all three items from the very beginning of training. A trial began with the simultaneous presentation of all list items. The spatial configuration of the list items varied randomly from trial to trial to prevent the list from being learned as a motor sequence (see Figure 1). During a trial, each correct selection was followed by a .3-second tone and a .5 cm green border around the image that lasted for .7 seconds. If the subject selected all of the items in the correct order, a reward was delivered once the terminal item was selected. All images remained on the screen throughout the trial until the subject either completed the trial correctly, or until the subject made an error. Any error, in either the forward (e.g., A→B→ D) or backward (e.g., A→B→A) direction, immediately terminated the trial and was followed by a 2 second timeout during which the screen was white. Multiple presses to the same item (e.g., A→B→B→C) were ignored. Training sessions were terminated after 1 hour or 60 correct responses, whichever came first.
To maintain motivation in early phases of learning a subset of trials were comprised of only the first two or three items of the complete list. For example, a training session for a three-item list included some trials with only the AB stimuli. Likewise, training for a four-item list included some trials with only the AB or ABC stimuli. These partial list trials constituted approximately 50% of the trials in a session and were gradually eliminated as a subject’s performance on the full target sequence improved. Partial list trials were briefly reintroduced at later stages of training if subjects exhibited a lack of motivation for the task. Partial list trials were excluded from all analyses and did not count toward criterial performance. With the exception of the first three-item list, each list was trained to a 35% accuracy criterion before a subject was advanced to the next list. After criterial performance on each of five 3-item lists, lemurs were trained in an identical manner with five 4-item lists. Chance performance on the three-item list was 16.67% correct and chance performance on the four-item list was 4.17% correct. These values were based on a conservative estimate of chance in which the possibility of backward errors was not included in the calculation. We eliminated backward errors because we expected them to be low in frequency and also because it is possible to learn nothing about the order of the list per se, yet still score above true chance by learning to avoid previously selected responses.
Results and Discussion
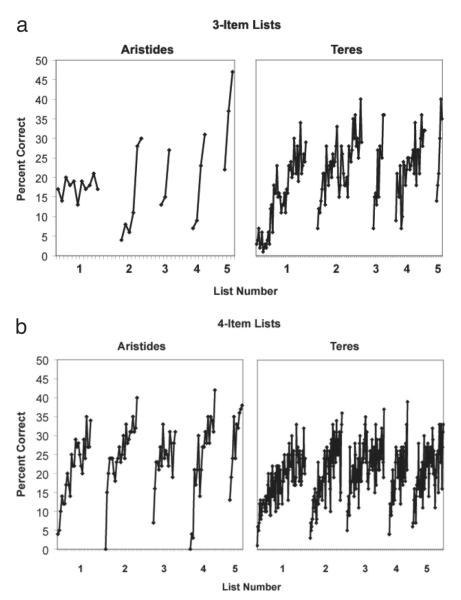
Both subjects showed evidence of “learning to learn” becoming more efficient list learners with each successive list (Harlow, 1949). As shown in Figures 2a and b, there was a decrease in the number of training sessions required to reach criterial performance with each additional list for both three- and four-item lists. Regression analyses were conducted on the three- and four-item lists with subject and list as independent predictors of trials to criterion. The partial slope for list was significantly different from zero in both the three and four-item list conditions (three item, [t(7) = −3.19, p < .05; eta2 = 0.59]; four item, [t(7) = −2.52, p < .05; eta2 = 0.48]).
Figure 2.
(A) Learning curves for the five 3-item lists. Each tick-mark represents accuracy for a 100-trial block. (B) Learning curves for the five 4-item lists. Each tick-mark represents accuracy for a 200-trial block.
The overall number of forward and backward errors averaged across all sessions is shown in Table 1. The negative numbers represent backward skips, and the positive numbers represent forward skips. To assess changes in the relative frequency of forward and backward errors, multiple regressions were performed to predict the proportion of forward/backward errors, and the shift from multiskip to single skip errors (in both forward and backward directions) with trials and list as independent predictors. T tests were conducted on the partial slopes to determine whether they differed significantly from zero. Although the majority of the errors for both lemurs were of the forward variety, neither subject showed any evidence of a proportionate decrease in backward errors relative to forward errors. Specifically, Aristides showed no change [F(2, 103) = .353, p > .05], whereas Teres actually showed the opposite pattern, with the proportion of backward to forward errors increasing across both trials [t(357) = 2.70, p < .05; eta2 = 0.02], and lists [t(357) = 4.90, p < .05; eta2 = 0.06].
Table 1.
Proportion of Forward and Backward Errors During Multiple Four-Item List Learning
| Error type |
−2 | −1 | +1 | +2 | +3 | Total Backward |
Total Forward |
|---|---|---|---|---|---|---|---|
| Aristides | 0.04 | 0.11 | 0.71 | 0.11 | 0.03 | 0.15 | 0.85 |
| Teres | 0.04 | 0.11 | 0.66 | 0.15 | 0.03 | 0.15 | 0.85 |
Teres and Aristides did however, show a tendency to shift from multiskip forward errors to single-skip errors with list experience, and Teres also showed this pattern with backward errors. The proportion of multiskip forward errors decreased for Teres across both lists [t(357) = 5.83, p < .05; eta2 = 0.09] and trials [t(357) = 8.82, p < .05; eta2 = 0.18]. Aristides showed a similar decrease across trials [t(103) = 4.58, p < .05; eta2 = 0.17], but not across lists [t(103) = 0.51, p > .05]. For backward errors, Teres showed a proportional decrease in multiskip backward errors in favor of one-skip backward errors across lists [t(357) = 2.15, p < .05; eta2 = 0.01], but Aristides actually showed a proportional increase in multiskip backward errors relative to one-skip backward errors [t(103) =−2.23, p < .05; eta2 = 0.05]. Neither lemur showed any proportional changes in multiskip relative to single-skip backward errors across trials [Aristides, t(103) = 0.94, p > .05; Teres, t(357) = 1.21, p > .05]. In summary, the reduction in number of sessions required to reach the performance criterion and the reduction in multiskip errors relative to single-skip errors suggests that with list experience there is strengthening in an associative chain. However, unlike the monkey pattern, lemurs did not show a reduction in backward errors.
Experiment 2a
The results of Experiment 1 demonstrate that lemurs become better list-learners over time. Our next goal was to train a longer list that would allow a meaningful subset test with multiple interior pairs to allow a comparison of lemur performance with that of monkeys and pigeons. In Experiment 2a, the same two ring-tailed lemurs were trained to execute a five-item list, and once criterion performance was reached, they were tested with all possible subset pairs derived from the list.
Method
Subjects and apparatus
The subjects and apparatus were the same as in Experiment 1.
Procedure
After each subject completed Experiment 1, a novel photograph was added to the final four-item sequence to form a five-item list. The newly introduced item was assigned to the 5th position in the sequence and subjects were trained to a 50% accuracy criterion. Although this performance criterion was lower than the 70% criterion used with pigeons and monkeys, we stopped at 50% accuracy because of concern that Teres would be unable to reach the 70% accuracy criterion. Knowledge of ordinal position was then assessed by presenting all possible pairs that could be created from the five-item sequence. Each subset pair was tested on a total of 20 trials that were administered across three test sessions. Subjects were rewarded for responding to items in an order consistent with their ordinal positions in the original list (e.g., B→D). Incorrect responses (e.g., selecting D first in a B & D pairing) terminated the trial and were followed by a 2-second timeout during which the screen was white.
Results and Discussion
When the 5th item was added to the final four-item list it took Aristides 1,080 and Teres 29,525 trials to reach a 50% performance criterion. Teres’ slower acquisition is consistent with the acquisition data for the three- and four-item training. Although, Aristides learned lists more quickly than Teres (Figures 2a and b), both animals showed very similar reaction times and accuracies for subset pairs. Therefore, the data were combined for analyses.
Pairwise accuracy
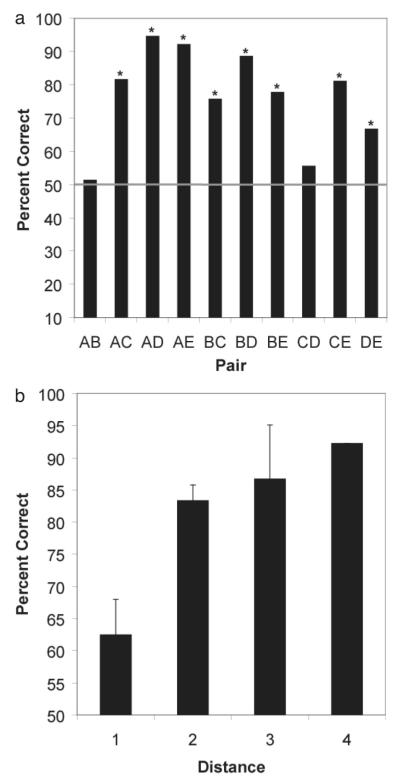
As shown in Figure 3a accuracy on subset pairs was considerably above chance levels (binomial, p < .05) with the exception of pairs AB and CD (binomial, p > .05). It is noteworthy that the lemurs were able to solve internal test pairs that did not contain a beginning- or end-item anchor. This suggests that unlike pigeons, lemurs were not relying on end-item cues to make decisions about how to respond to subset items, but rather, may have relied on an internal representation of the list to solve the task. However, the consistently poor performance on the AB pair was particularly surprising and unlike the performance of pigeons or monkeys.
Figure 3.
(A) Accuracy for subset pairs during testing. Asterisks indicate that the accuracy was significant at the α = .05 level using a binomial test. (B) Accuracy for subset pairs during testing as a function of the distance between the items in a pair (Distance 1, M = 62.5, 95% Confidence Interval [CI] = ± 10.8; Distance 2, M = 83.3, ± 4.7; Distance 3, M = 86.7, ± 16.5; Distance 4, M = 92.2, ± 0.
As shown in Figures 3a and b, lemurs showed a trend toward a distance effect, with accuracy increasing as distance between the items increased [one-tailed t test for slope, t(3) = 3.19, p = .08; eta2 = 0.77]. Most of this effect seems to be the result of lower performance on adjacent pairs relative to the nonadjacent pairs. This is not surprising given that one-skip forward errors account for the majority of all errors.
Given that the test pairs were reinforced, one possible alternative explanation is that the lemurs were able to quickly learn the individual test pairs. To test for this possibility, accuracy was assessed on the first trial for all test-pairs. On the first trial, Aristides was correct on 7 of the 10 test pairs, and Teres was correct on 8 of the 10 test pairs. The combined data show that the lemurs were significantly above chance on the very first trial (15/20, binomial, p < .05).
Pairwise latency
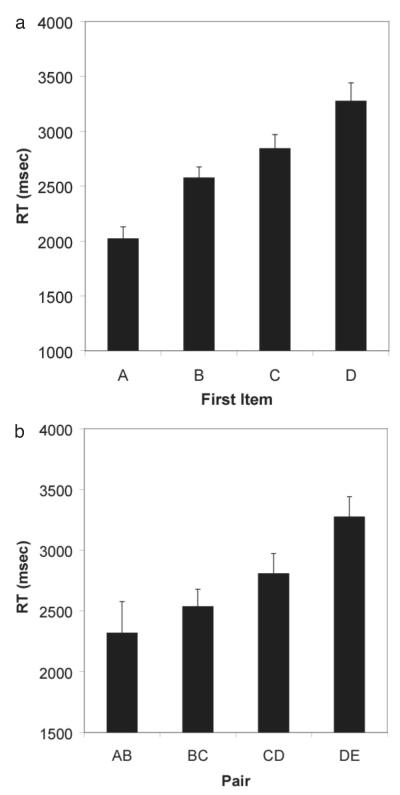
Response latencies to all subset pairs are shown in Figure 4. Only response times from correct trials were included for analysis. In general, lemurs showed the monkey pattern in response latencies. Figure 5a shows the response latencies to the first item as a function of its distance from the beginning of the list. There is a significant monotonic increase in reaction time to the first subset item as that item’s distance increases from the beginning of the list. Given that first-item position is confounded with the distance between the first and second subset items, it is possible that average increases in reaction time to the first item were due because of decreases in average distance between the first and second items (e.g., pairs that start with C have a smaller average distance than pairs that start with A). However, Figure 5b shows that for pairs with a fixed distance of 1, reaction time to the first item still increases with its position in the list. In addition, a partial correlation shows that even when distance between items is controlled, there is a statistically significant relationship between reaction time and first item position [r(336) = 0.25, p < .05]. Distance between the first and second item had very little bearing on response latency to the first item [r(336) = −0.06, p > .05]. However, as shown in Figure 6, there was a monotonic increase in reaction time to the second item as its distance from the first item increased [r(390) = 0.42, p < .05].
Figure 4.
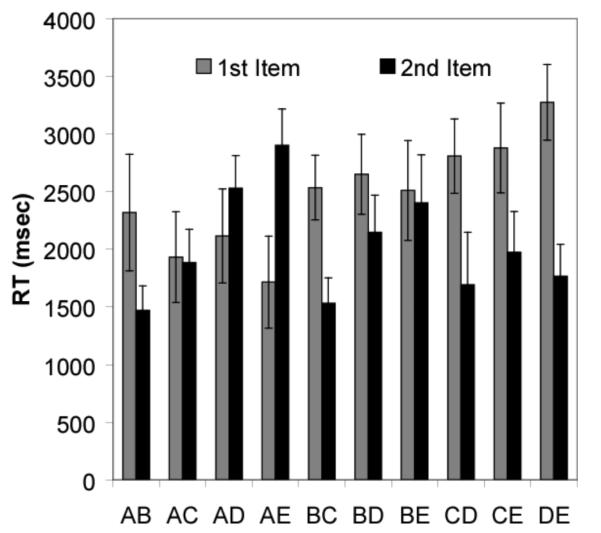
Reaction time to the individual subset pairs. The error bars in the figure represent 95% CI.
Figure 5.
(A) Reaction time to the first item in a subset pair as a function of its distance from the beginning of the list (Item A, M = 2020.9, 95% [CI] = ± 213.6; Item B, M = 2575.7, ± 197.4; Item C, M = 2843.1, ± 250.11; Item D, M = 3274.3, ± 327.9). (B) Reaction time to the first item in a subset pair as a function of its distance from the beginning of the list when distance between the first and second item is held constant at 1 (Pair AB, M = 2318.5, 95% [CI] = ± 505.4; Pair BC, M = 2535.1, ± 279.7; Pair CD, M = 2808, ± 323.5; Pair DE, M = 3274.3, ± 327.9).
Figure 6.
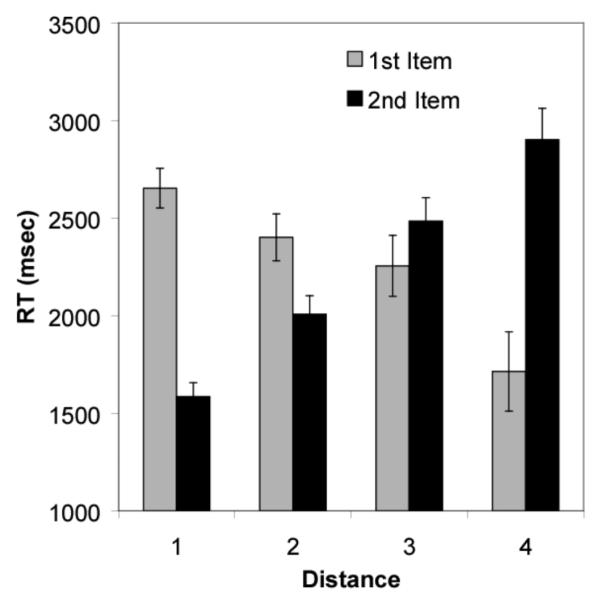
Reaction time to the first and second items in the subset test pair as a function of their distance from one another. It should be noted that the apparent decrease in reaction time to the first item as distance increases is because of the first items being, on average, closer to the beginning of the list (First Item Distance [FID] 1, M = 2652.9, 95% [CI] = ± 198.6; FID 2, M = 2401.7, ± 235.7; FID 3, M = 2255.5, ± 306.6; FID 4, M = 1715.0, ± 399.3; Second Item Distance [SID] 1, M = 1585.7, ± 138.5; SID 2, M = 2007.4.1, ± 186.0; SID 3, M = 2484.6, ± 234.1; SID 4, M = 2901.4, ± 316.2).
In summary, the accuracy and reaction time patterns of lemurs closely resemble those of monkeys and demonstrate that lemurs do not use a set of simple decision rules to solve this task. The data are consistent with the use of an internal associative chain where items are accessed one by one, starting at the beginning of the list, and responses are made once the item on the screen matches the current item in memory. The reaction times to individual items are a direct result of the time it takes to travel through the list and find a match. The fact that reaction time to the first item was relatively independent of its distance from the second item supports this argument.
Experiment 2b
As we saw in Experiment 2a, accuracy and reaction time on subset pairs generally followed the patterns observed previously for monkeys as opposed to those shown by pigeons. Lemurs successfully ordered many subset pairs that did not contain an end item. However, a surprising finding was that both lemurs performed poorly on the AB pair. This finding is at odds with previous data obtained for both monkeys and pigeons in which performance on the AB pair was uniformly high. To examine whether this surprisingly poor performance on the AB pair was idiosyncratic and specific to the stimuli and methods used in Experiment 2a, we replicated Experiment 2a with a new set of pictures with Aristides. An important change in the design of Experiment 2b was that all five-items were presented from the beginning of training instead of adding a 5th item to a four-item sequence. Another important difference was that Aristides was trained to a criterion of 70% (rather than the 50% criterion used in Experiment 2a) to closely match the criteria used in previous experiments with monkeys and pigeons.
Method
Subjects and apparatus
Only Aristides participated in Experiment 2b. The apparatus was the same as in Experiment 1.
Procedure
A novel set of five digital photographs was used. All five items were presented from the beginning of training until a 70% accuracy criterion was reached. Aristides was then tested on all 10 subsets derived from the five-item sequence. Each pair was tested a total of 30 times across five sessions.
Results and Discussion
Aristides required 12,284 trials to reach the 70% performance criterion. The accuracies across the different test pairs were remarkably similar to those shown by both Aristides and Teres in Experiment 2a. Binomial tests revealed that all test pair accuracies were above chance with the exception of AB and DE. Aristides successfully ordered interior pairs, which is consistent with Experiment 2a performance, and consistent with the overall monkey pattern observed for interior subset pairs. However, as in Experiment 2a, Aristides did not perform above chance expectation on the AB pair (18/30; binomial, p > .05). Thus, the poor performance on the AB pair in Experiment 2a was not the result of the particular pictures or methods used. Poor performance on AB is difficult to account for given that it is at odds with all current models of pigeon and monkey performance.
General Discussion
The lemurs in our study demonstrated that they were capable of learning three-, four-, and five-item lists. As their experience in the list-learning paradigm increased, they also showed an increase in the efficiency with which they acquired novel lists in that it took fewer trials to reach criterion with each new list. Although their overall errors decreased over time, unlike monkeys, lemurs did not show any changes in the ratio of forward to backward errors over the course of learning a single list or multiple lists. However, like monkeys, they did show proportional decreases in multiskip forward errors with increased list experience.
A central finding was that lemurs showed remarkable similarity to monkeys in reaction time and accuracy patterns in subset tests. Consistently, lemurs were above chance at ordering 8 of the 10 test pairs, which included two of the three internal pairs. Reaction time patterns also closely matched those of monkeys. Reaction time to the first subset item increased monotonically with its distance from the beginning of the list. Likewise, reaction time to the second subset item increased as its distance from the first item increased. These results are highly consistent with the use of an internal associative chain (D’Amato & Colombo, 1988), and inconsistent with the use of a set of simple decision rules similar to those used by pigeons. Therefore, it appears that although there are some subtle differences between performance by lemurs and monkeys, lemurs may have similar fundamental mechanisms for representing ordinal information.
It is important to note that monkeys’ reaction time and accuracy patterns change when they have extended list experience. Terrace, Son, and Brannon (2003) demonstrated that when rhesus monkeys are trained to learn many three-, four-, five-, and seven-item lists, that they show magnitude-like effects in reaction time and distance effects in reaction time and accuracy. These effects are also found when humans and nonhuman animals make numerical comparisons. For example, when monkeys are required to choose the larger of two numerosities, they show magnitude and distance effects for both accuracy and reaction time (e.g., Brannon & Terrace, 2000; Cantlon & Brannon, 2006). Accuracy is higher and reaction time is lower as the numerical disparity between values in a pair increases (e.g., 1 vs. 9 compared to 1 vs. 2). When holding distance constant, accuracy is lower and reaction time is higher as overall magnitude increases (e.g., 8 vs. 9 compared to 1 vs. 2).
In contrast, our lemurs showed a moderate effect of distance on accuracy and no effect of distance on response latency. This finding is consistent with the use of an internal associative chain, but is inconsistent with the notion that analog magnitudes underlie list positions. In addition, although the lemurs showed magnitude-like effects in terms of reaction time; those effects were wholly dependent on the position of the first item in the test pair, and completely independent of the second item (see Figure 5b). This differs from number discrimination tasks where reaction time patterns vary as a function of the ratio between the two numerical values. Thus, although the distance and apparent “magnitude” reaction time functions shown by our lemurs resemble those observed in numerical discrimination tasks, the underlying mechanisms governing those processes are likely to be very different.
As reported earlier, one puzzling aspect of the data is the consistently poor performance on pair AB. The difficulty with this particular subset is hard to explain with models used to account for performance in pigeons and monkeys. A decision rule such as that used by pigeons, should allow high accuracy to the pair AB because it includes an end item. Likewise, AB accuracy should be high if an associative chain underlies performance because the onset of the trial is thought to cue a response to item A, which then cues a response to item B. Whereas we do not fully understand why lemurs perform poorly on the AB pair, we would like to offer some speculation. One possibility is that the AB pair may be particularly sensitive to the degraded contextual cue of the onset of the trial. On a five-item trial, the subject presses a “start” stimulus to begin the trial. Next, five pictures appear on the screen simultaneously. Those pictures, combined with the trial onset, may serve as a contextual cue to choose A. Given that forward errors are the most common type of errors, it may be that the degraded contextual cue (having only two items present on the screen instead of five) may increase the likelihood of one-skip forward errors at the beginning of the list. Multiskip forward errors are much less common, so the other pairs beginning with A would be less affected (e.g., AC, AD, AE). Pairs later in the list would be less susceptible to forward skip errors simply because they are less dependent on the trial onset cue (e.g., BC, BE, CD, CE, DE). It is unclear why the degraded contextual cue of providing only two elements as opposed to five negatively influences lemurs and not monkeys on the AB pair.
There are several ways to test whether the contextual degradation of providing only two stimuli as opposed to five was the critical factor in poor AB performance for lemurs. One possibility is to manipulate context information at the beginning of the trial. Additional items from the list could be presented (e.g., having a total of three or four items) to determine whether accuracy on the AB pair varies continously with the number of items present. Alternatively, it would be interesting to determine the effect on AB accuracy if all five pictures were briefly presented before each subset trial, or if the AB pair was presented with three additional nonpicture stimuli. Future research should address these possibilities.
Overall, the bulk of the reaction time and accuracy patterns observed for lemurs match those demonstrated by monkeys in previous studies. Both species are able to organize arbitrary stimuli in an ordinal sequence in memory and execute a chain of responses in which only internal cues can guide responses. Although the lemurs in our experiments did not show a reduction in the proportion of back-ward errors over training and exhibited surprisingly poor performance on the AB pair during Experiment 2, it must be noted that for Experiment 2a, Teres and Aristides were trained to a lower performance criterion than monkeys in similar studies. However, this is unlikely to explain the differences because Aristides was trained to a 70% accuracy criterion before subset tests in Experiment 2b, yet again was at chance on the AB pair. Thus, although the lemurs show patterns that are very similar to those of monkeys, it is important to keep in mind that there are some notable differences as well. Further testing may reveal important differences in the way lemurs and monkeys process ordinal information.
As noted earlier, monkeys with extensive list learning experience have shown magnitude and distance effects in pairwise tests. They have also shown knowledge of the ordinal positions of items within a list such that they can correctly order list items drawn from two separate lists (e.g., “B” from List 1 and “C” from List 2; Chen, Swartz, & Terrace, 1997; Terrace, Son, & Brannon, 2003). Neither of these findings can be explained by an associative chain, which suggests that list-experienced monkeys have multiple ways of accessing and responding to list items. Although lemurs’ performance in the present experiments fits well with an associative chaining model, it may be that with sufficient experience or more sensitive testing paradigms, that they too will demonstrate other ways of solving serial order problems. Additional research to probe differences between strepsirrhine and haplorrhine cognition will shed light on the evolutionary history of humans’ cognitive abilities. Investigating these processes in nonprimate outgroups will be essential to determining which cognitive adaptations are unique to our primate lineage.
Acknowledgments
We thank the Duke Lemur Center and the many Duke University undergraduates who assisted with this research. We also thank all members of the Brannon lab for their helpful discussion of these data. This material is based upon work supported by an NSF CAREER award (#0448250), National Institute of Child Health and Human Development Grant R01 (HD49912), and a John Merck Fund fellowship to EMB. Duke Lemur Center (publication #1116).
References
- Anderson JR, Fornasieri I, Ludes E, Roeder JJ. Social processes and innovative behavior in changing groups of lemur-fulvus. Behavioural Processes. 1992;27:101–112. doi: 10.1016/0376-6357(92)90020-E. [DOI] [PubMed] [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Social complexity and transitive inference in corvids. Animal Behaviour. 2003;65:479–487. [Google Scholar]
- Brannon EM, Terrace HS. Representation of the numerosities 1–9 by rhesus macaques (Macaca mulatta) Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:31–49. doi: 10.1037//0097-7403.26.1.31. [DOI] [PubMed] [Google Scholar]
- Cantlon JF, Brannon EM. Shared system for ordering small and large numbers in monkeys and humans. Psychological Science. 2006;17:402–407. doi: 10.1111/j.1467-9280.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- Chen SF, Swartz KB, Terrace HS. Knowledge of the ordinal position of list items in rhesus monkeys. Psychological Science. 1997;8:80–86. [Google Scholar]
- Colombo M, Frost N. Representation of serial order in humans: A comparison to the findings with monkeys (Cebus apella) Psychonomic Bulletin & Review. 2001;8:262–269. doi: 10.3758/bf03196160. [DOI] [PubMed] [Google Scholar]
- D’Amato MR, Colombo M. Representation of serial order in monkeys (Cebus apella) Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:131–139. [PubMed] [Google Scholar]
- D’Amato MR, Colombo M. Serial learning with wild card items by monkeys (Cebus apella): Implications for knowledge of ordinal position. Journal of Comparative Psychology. 1989;103:252–261. doi: 10.1037/0735-7036.103.3.252. [DOI] [PubMed] [Google Scholar]
- D’Amato MR, Colombo M. The symbolic distance effect in monkeys (Cebus-apella) Animal Learning & Behavior. 1990;18:133–140. [Google Scholar]
- Ebbinghaus H. Memory: A contribution to experimental psychology. Dover; New York: 1885/1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornasieri I, Anderson J, Roeder J. Responses to a novel food acquisition task in three species of lemurs. Behavioral Processes. 1990;21:143–156. doi: 10.1016/0376-6357(90)90021-7. [DOI] [PubMed] [Google Scholar]
- Gillan D. Reasoning in chimpanzees II. Transitive inference. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:150–164. [Google Scholar]
- Harlow H. The formation of learning sets. Psychological Review. 1949;56:51–65. doi: 10.1037/h0062474. [DOI] [PubMed] [Google Scholar]
- Kappeler PM. The acquisition process of a novel behaviour pattern in a group of ring-tailed lemurs (Lemur catta) Primates. 1987;28:225–228. [Google Scholar]
- Lazareva OF, Smirnova AA, Bagozkaja MS, Zorina ZA, Rayevsky VV, Wasserman EA. Transitive responding in hooded crows requires linearly ordered stimuli. Journal of the Experimental Analysis of Behavior. 2004;82:1–19. doi: 10.1901/jeab.2004.82-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KP, Jaffe S, Brannon EM. Analog number representations in mongoose lemurs (Eulemur mongoz): Evidence from a search task. Animal Cognition. 2005;8:247–252. doi: 10.1007/s10071-004-0251-x. [DOI] [PubMed] [Google Scholar]
- Mcgonigle BO, Chalmers M. Are monkeys logical? Nature. 1977;267:694–696. doi: 10.1038/267694a0. [DOI] [PubMed] [Google Scholar]
- Ohta H, Ishida H, Matano S. Learning set formation in ring-tailed lemurs (Lemur catta) Folia Primatologica. 1984;43:53–58. [Google Scholar]
- Paz-Y-Mino G, Bond AB, Kamil AC, Balda RP. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430:778–781. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- Roberts WA, Phelps MT. Transitive inference in rats: A test of the spatial coding hypothesis. Psychological Science. 1994;5:368–374. [Google Scholar]
- Rumbaugh D, Mccormack D. The learning skills of primates: A comparative study of apes and monkeys. In: Stark D, Schneider R, Kuhn HJ, editors. Progress in primatology. Gustav Fischer; Stuttgart: 1967. pp. 289–306. [Google Scholar]
- Sands SF, Wright AA. Primate memory: Retention of serial list items by a rhesus monkey. Science. 1980;209:938–940. doi: 10.1126/science.6773143. [DOI] [PubMed] [Google Scholar]
- Santos LR, Barnes JL, Mahajan N. Expectations about numerical events in four lemur species (Eulemur fulvus, Eulemur mongoz, Lemur catta and Varecia rubra) Animal Cognition. 2005;8:253–262. doi: 10.1007/s10071-005-0252-4. [DOI] [PubMed] [Google Scholar]
- Santos LR, Mahajan N, Barnes JL. How prosimian primates represent tools: Experiments with two lemur species (Eulemur fulvus and Lemur catta) Journal of Comparative Psychology. 2005;119:394–403. doi: 10.1037/0735-7036.119.4.394. [DOI] [PubMed] [Google Scholar]
- Slamecka NJ. Ebbinghaus - some associations. Journal of Experimental Psychology-Learning Memory & Cognition. 1985;11:414–435. [Google Scholar]
- Straub RO, Terrace HS. Generalization of serial-learning in the pigeon. Animal Learning & Behavior. 1981;9:454–468. [Google Scholar]
- Swartz KB, Chen SF, Terrace HS. Serial learning by rhesus monkeys: I. Acquisition and retention of multiple four-item lists. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:396–410. doi: 10.1037//0097-7403.17.4.396. [DOI] [PubMed] [Google Scholar]
- Terrace HS. Simultaneous chaining: The problem it poses for traditional chaining theory. In: Commons ML, Herrnstein RJ, Wagner AR, editors. Quantitative analysis of behavior: Discrimination processes. Ballinger; Cambridge, MA: 1984. pp. 155–138. [Google Scholar]
- Terrace HS. Chunking during serial learning by a pigeon: I. Basic evidence. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:81–93. doi: 10.1037//0097-7403.17.1.81. [DOI] [PubMed] [Google Scholar]
- Terrace HS. The phylogeny and ontogeny of serial memory: List learning by pigeons and monkeys. Psychological Science. 1993;4:162–169. [Google Scholar]
- Terrace HS, Chen SF. Chunking during serial learning by a pigeon: II. Integrity of a chunk on a new list. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:94–106. doi: 10.1037//0097-7403.17.1.94. [DOI] [PubMed] [Google Scholar]
- Terrace HS, Chen SF. Chunking during serial learning by a pigeon: III. What are the necessary conditions for establishing a chunk? Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:107–118. doi: 10.1037//0097-7403.17.1.107. [DOI] [PubMed] [Google Scholar]
- Terrace HS, Son LK, Brannon EM. Serial expertise of rhesus macaques. Psychological Science. 2003;14:66–73. doi: 10.1111/1467-9280.01420. [DOI] [PubMed] [Google Scholar]
- Treichler FR, Van Tilburg D. Concurrent conditional discrimination tests of transitive inference by macaque monkeys: List linking. Journal of Experimental Psychology: Animal Behavior Processes. 1996;22:105–117. [PubMed] [Google Scholar]
- Von Fersen L, Wynne CD, Delius JD, Staddon JE. Transitive inference in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:334–341. doi: 10.1037//0097-7403.17.3.281. [DOI] [PubMed] [Google Scholar]
- Yoder AD, Burns MM, Zehr S, Delefosse T, Veron G, Goodman SM, et al. Single origin of malagasy carnivora from an African ancestor. Nature. 2003;421:734–737. doi: 10.1038/nature01303. [DOI] [PubMed] [Google Scholar]