Abstract
Background
Based on animal experiments and limited data from few human trials, alternate day fasting (ADF) resulted in weight loss; prolonged life; reduced metabolic risk factors for diabetes and cardiovascular diseases; and reduced prevalence of age-related diseases. The present study is the first comprehensive examination of the long-term effects of ADF on general cardiovascular fitness in rats.
Methods and Results
Four months old male Sprague-Dawley rats were started on ADF or continued on ad libitum diets and followed for 6 months with serial echocardiography. A comprehensive hemodynamic evaluation including a combined dobutamine - volume stress test was performed at the end of the study, and hearts were harvested for histological assessment. The six-month long ADF diet resulted in a 9% reduction (p<0.01) of cardiomyocyte diameter and 3 fold increase in interstitial myocardial fibrosis. Left ventricular chamber size was not affected by ADF and ejection fraction was not reduced, but left atrial diameter was increased 16%, and the E/A in Doppler-measured mitral flow was reduced (p<0.01). Pressure-volume loop analyses revealed a “stiff” heart during diastole in ADF rats, while combined dobutamine and volume loading showed a significant reduction in LV diastolic compliance and a lack of increase in systolic pump function, indicating a diminished cardiac reserve.
Conclusion
Chronic ADF in rats results in development of diastolic dysfunction with diminished cardiac reserve. ADF is a novel and unique experimental model of diet-induced diastolic dysfunction. The deleterious effect of ADF in rats suggests that additional studies of ADF effects on cardiovascular functions in humans are warranted.
Keywords: diastolic dysfunction, heart failure, animal model, nutrition
Introduction
Compared to ad libitum (AL) food consumption, caloric restriction, i.e., a reduction of calorie intake by 20–40%, or intermittent fasting, also known as alternate day fasting (ADF), have been reported to prolong life and to result in multiple health benefits, at least in experimental animals (For review see 1–3). Briefly, in addition to extending the life span, these dietary alterations have resulted in reduced risks for diabetes (4) and hypertension (5), and to an increased resistance to experimentally induced degenerative brain diseases (6).
We had previously reported that ADF increased myocardial tolerance to ischemic damage (7). Compared to rats maintained on a usual AL diet, those maintained on an ADF regimen for 6 months sustained a smaller myocardial infarction (MI) induced by a permanent coronary ligation, had a reduced level of apoptosis in the peri-infarct area, and attenuated post-MI left ventricular remodeling. Recently, a similar beneficial effect of ADF was shown in rats: ADF started 2 weeks after the induction of an MI reduced mortality and attenuated the development of chronic heart failure (8).
The long-term effects of chronic ADF on cardiac structure and function in the absence of an experimental MI, however, have not been investigated. In the present study we performed detailed evaluation of the effects of prolonged ADF on the cardiac structure and function in rats.
Methods
All animal procedures were approved by the National Institute on Aging Animal Care and Use Committee. Four month old male Sprague-Dawley rats (n = 40) were randomly assigned to be fed either every day (ad libitum, AL group, n = 20) or to alternate day fasting (ADF group, n=20) with a standard rat diet (NIH-07, Harlan Teklad, Indianapolis, IN). These feeding regimens lasted 6 months. Echocardiographic (Echo) assessment of cardiac morphometry and function was conducted prior to assigning rats to a specific diet, and was repeated at 3 and 6 months following initiation of the diets. Following 6 months on the diets, a subset of animals was subjected to comprehensive hemodynamic studies involving pressure-volume loop analyses that included a hemodynamic stress test (infusion of progressively increased volume of physiological saline containing a constant concentration of Dobutamine). Rats were then euthanized, and their hearts and lungs were harvested and weighed. Hearts were further subjected to a histological evaluation. As an AL weight matched control (ALWM) for 10-mo old ADF rats, five additional, 4-mo old ad libitum fed rats were also euthanized for heart histological evaluation.
Echocardiography
Echocardiography (Sonos 5500, a 12 MHz transducer) was conducted under light sodium pentobarbital anesthesia (30 mg/kg, i.p.) as described previously (7, 9, 10, see also suppl. material). All reported indices of echocardiography were normalized for body weight (BW) either ratiometrically or allometrically (11, 12).
Hemodynamic Measurements
Procedure was similar to previously described (13) and outlined in suppl. material. As with echo parameters all reported indices of hemodynamic measurements were normalized for BW either ratiometrically or allometrically (11, 12)
Dobutamine Stress Test
After completion of hemodynamic evaluation the left femoral vein was cannulated and infusion of Dobutamine, a predominantly β1 AR agonist, in concentration of 50 µg/ml of normal saline was started. The infusion rate was regulated by an infusion pump, which was set at consecutive regimens of 100, 200, 300, and 400 µL/kg/min. Each infusion regimen was continued for 5 minutes. During each consecutive infusion regimen each animal had received 0.5, 1.0, 1.5, and 2.0 ml/kg of saline containing 25, 50, 75, and 100 µg/kg of dobutamine respectively. Therefore, the test actually consisted of two combined stresses: a volume load combined with inotropic stimulation, i.e., a stress similar to that induced by a dynamic exercise. Hemodynamic indices at steady state were collected prior to drug infusion and at the end of each infusion regimen (a total of five measurements).
Gross Pathology and Histological Assessment
Histological staining and analyses were performed as described previously (7, see also suppl. material).
Statistical Analysis
All data are expressed as the mean ± SEM. Echo-derived and hemodynamic indices during the stress test were compared via a two-way ANOVA for repeated measurements. Group differences at specific time points were tested by Bonferroni’s post-hoc test (Prism v3.0, GraphPad Software Inc., San Diego, CA). Histological data were assessed by one-way ANOVA with Bonferroni correction for multiple comparisons. Hemodynamic indices prior to the stress test were compared between the two diet groups using Student’s t-test. Hemodynamic and echocardiographic indices were examined to determine whether they are influenced by a body weight. Parameters that significantly varied with body weight were scaled appropriately to body weight either ratiometrically, or allometrically (SAS, v9, Cary, NC) as described previously (11, 12, see also suppl. material). P<0.05 was considered statistically significant.
Results
Body weight
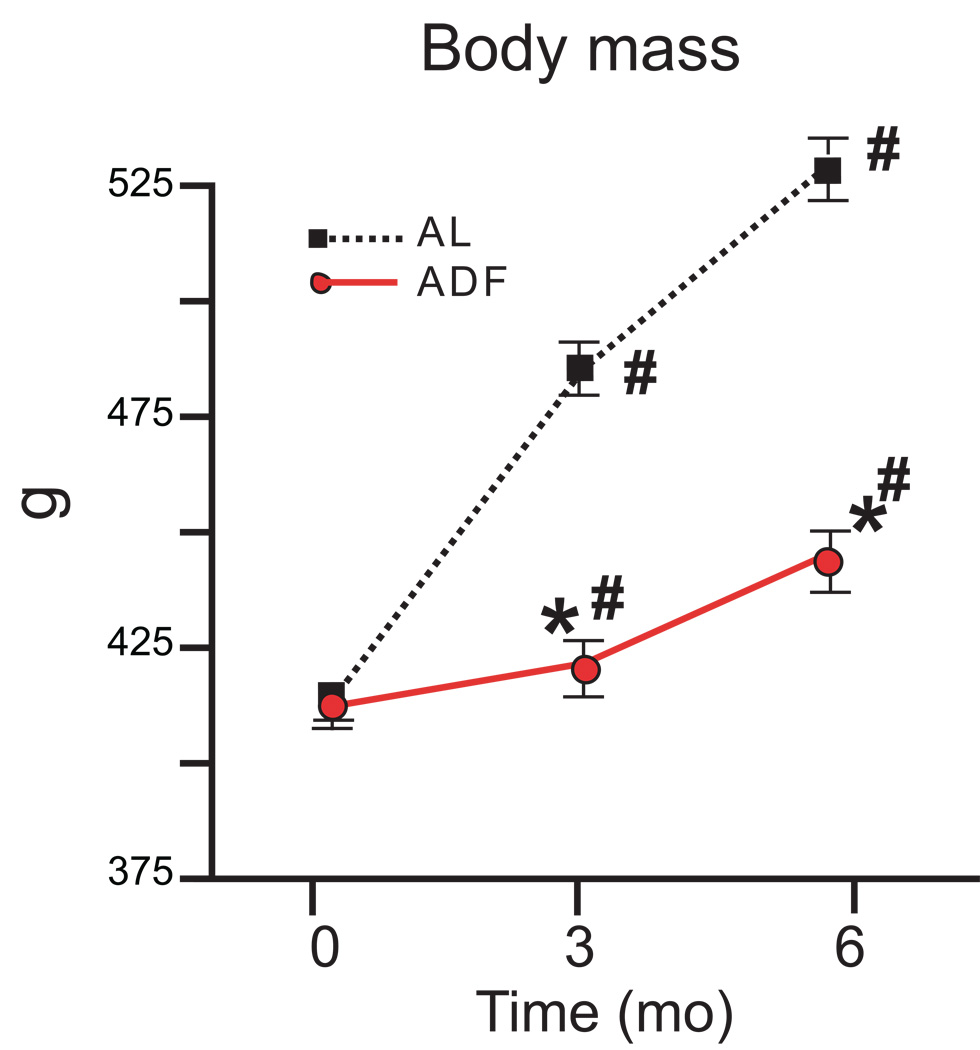
During the 6 mo of observation the body mass in AL increased by 28% (p<0.001). Consistent with the reduced average weekly food consumption, the rate of the weight gain in ADF rats was significantly attenuated, and at the end of the 6 month diet period (when rats were 10 months old), weight of rats in the ADF group increased by only 6% (Fig. 1).
Figure 1.
Body weights of rats during 6 months on AL and ADF dietary regimens. *p<0.05 compared to the AL value at the same time point; #p<0.05 compared to the baseline value for the same group (ANOVA with Bonferroni post hoc comparison).
Echocardiography
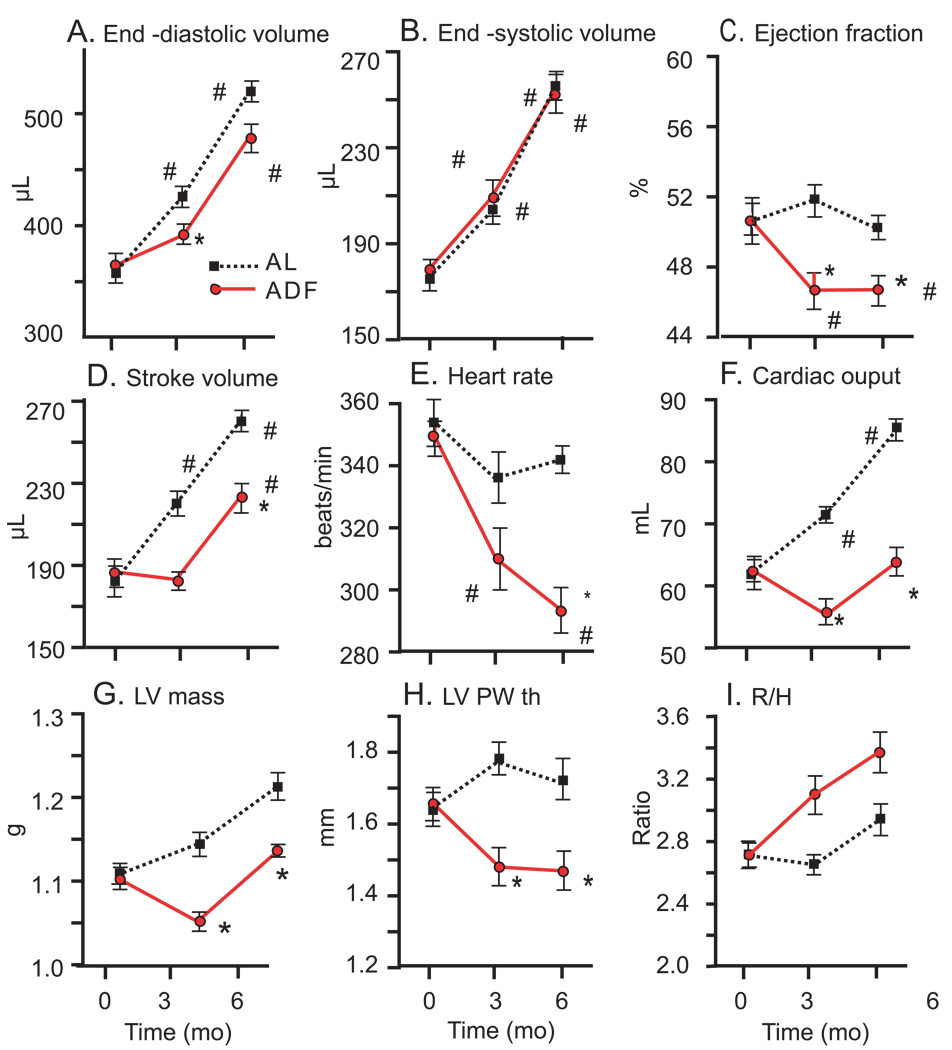
During the 6 month experimental period ESV increased by 49% (p<0.001) similarly in both the AL and ADF diet groups (Fig. 2 B). EDV in AL (Fig. 2A) increased proportionally to ESV (by 52%, p<0.001). The rate and extent of EDV expansion in ADF rats was lower than in AL rats; at 6 months the EDV increased in ADF rats by 33% (p<0.05). However, the differences in EDV between ADF and AL groups at 3 and 6 months did not achieve statistical significance (p<0.07). The lower EDV in rats on the ADF diet was associated with a small (4%) but statistically significant reduction of EF in ADF rats compared to AL rats at both the 3 and 6 month time points (Fig. 2C). In fact, while EF was maintained during the 6 month observation period in AL rats, it significantly dropped after the first 3 months on the ADF diet and remained lower at 6 months (p<0.01). The SV (Fig. 2D) increased in both groups during the 6 month study period (p<0.01), but to a lesser amount in rats on the ADF diet compared to those on the AL diet. The reduction in stroke volume and in HR (Fig. 2E) contributed to a lower cardiac output in rats on the ADF diet compared to those on the AL diet (Fig. 2F). Calculated LV mass (Fig. 2G) and PWth (Fig. 2H) were lower in ADF rats compared to AL rats at both the 3 and 6 month time points. Figure 2I illustrates the ratio of the short radius of LV to posterior wall thickness (r/h). The monotonic increase of the ratio in the ADF group during 6-mo of diet is statistically significant and at 3-mo different from AL rats.
Figure 2.
Echocardiographic Left Ventricular indices (unadjusted for body weight differences) in rats on AL and ADF diets during a 6 month period. LV end-diastolic volume (A), end-systolic volume (B), ejection fraction (C), stroke volume (D), heart rate (E), cardiac index (F), calculated LV mass (G), posterior wall thickness (H), and ratio of short axis LV radius to posterior wall thickness r/h ratio (I). Statistical symbols are same as in Figure 1.
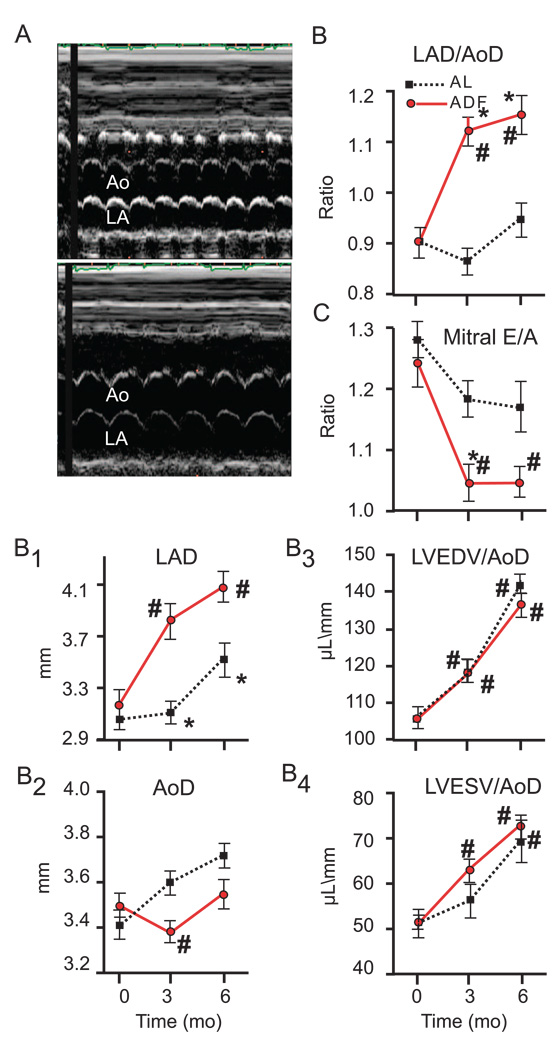
Additional echocardiographic evaluation revealed a substantial increase in left atrial size in rats on the ADF diet compared to the AL diet (Fig. 3 A). In ADF rats the LAD normalized for aortic diameter increased significantly (p<0.001), while it did not change in rats on the AL diet, resulting in a significantly larger LAD in ADF rats compared to AL rats at 3 and 6 months (Fig. 3 B). Fig. 3 B1 and B2 showed that the rate of increase of LAD over time (40%) exceeded the rate of growth of aortic diameter (18%). Fig. 3 B3 and B4 present the LV EDV and ESV, respectively, also normalized for aortic diameter for comparison with LAD. LV volumes normalized for aortic diameter were not different between groups, i.e., the increase of atrial size in ADF rats exceeded that in LV size.
Figure 3.
Echocardiographic indices of Left Atrial dimensions in rats on AL and ADF diets during a 6 month period. Representative M-mode Echos from rats on either the ADF or and AL diets (A), Ao – aorta, LA – left atrium; Ratio of left atral diameter (LAD) to aortic diameter (AoD) (B); LAD (B1); AoD (B2); Ratio of EDV to AoD (B3) and ESV to AoD (B4). Doppler-measured mitral flow velocity (C). Statistical symbols are same as in Figure 1.
While the mitral valve did not exhibit any pathology, the Doppler measured E/A waves (Fig. 3 C) in ADF rats became reduced at 3 months compared to AL rats (p<0.05). The lower E/A in the ADF rats persisted at 6 months. The changes in E and A waves are illustrated in supplemental figure (Fig. 1S). The mitral flow velocity during atrial systole (A wave) did not differ between groups. The early mitral flow (E wave), however, became monotonically reduced during 6 mo of diet in the ADF group and was significantly lower than in AL rats at 6 mos.
In order to separate the effect of ADF per se on cardiovascular function from the effects of reduced body weight, all echocardiographic indices in which a significant correlation with body weight was detected were subjected to a subsequent ratiometric or allometric adjustments (Table 1). After normalization for body weight, at six months all morphometric indices of the LV were similar in both diet groups, HR and CO remained significantly reduced in rats on the ADF diet, while EF became slightly, but statistically significantly elevated in ADF rats compared to AL rats. In contrast to LV dimensions, the left atrial dimensions remained significantly increased in rats in the ADF group compared to those in the AL group even after adjustment for body weight.
Table 1.
Echocardiographic indices at baseline and after 3 and 6 months of respective diets adjusted for body weight, if necessary$.
| Baseline | 3 months | 6 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BWAd | AL | ADF | BWad | AL | ADF | BWad | AL | ADF | |
| HR | U | 350±7 | 346±6 | U | 333±8 | 308±10 | ^0.04 | 263±3.6 | 229±6.2* |
| EDV | U | 357±8 | 365±10 | ^0.51 | 18.1±0.4 | 18±0.5 | ^0.533 | 18.4±0.4 | 18.6±0.5 |
| ESV | U | 175±5 | 179±5 | U | 205±6 | 210±8 | U | 258±7 | 255±9 |
| EF | U | 50.7±1.3 | 50.8±0.9 | U | 51.9±0.9 | 46.7±1.1* | ^0.04 | 39.2±0.6 | 46.7±1* |
| SV | U | 182±5 | 186±7 | ^1 | 0.45±0.01 | 0.43±0.008 | ^1 | 0.49±0.01 | 0.5±0.01 |
| CO | U | 63.2±1.7 | 64±2.2 | ^1 | 149±3.7 | 132±5* | ^1 | 167±3.8 | 147.5±5.1* |
| LVM | U | 1.1±0.01 | 1.1±0.01 | ^0.075 | 0.73±0.009 | 0.68±0.007* | ^0.209 | 0.33±0.005 | 0.32±0.002 |
| PWth | U | 1.64±0.05 | 1.66±0.05 | ^1 | 3.67±0.11 | 3.55±0.13 | U | 2.27±0.1 | 3.34±0.1 |
| LAD | U | 3.04±0.08 | 3.15±0.12 | ^−0.271 | 16.5±0.5 | 19.5±0.7* | ^0.036 | 2.8±0.1 | 3.2±0.1* |
| AoD | U | 3.4±0.06 | 3.49±0.05 | ^−0.105 | 6.9±0.1 | 6.3±0.09* | ^0.52 | 0.14±0.002 | 0.15±0.003 |
Parameters that did not correlate significantly with body weight were not subjected to body weight normalization. Data are mean ± SEM. BWAd (body weight adjustment): U – unadjusted;
1 – adjusted to a body weight at power 1 (ratiometric);
x – adjusted to a body weight at calculated power (allometric).
- p<0.05 vs AL.
HR - heart rate; EDV – end-diastolic volume; ESV – end-systolic volume; EF – ejection fraction; SV – stroke volume; CO – cardiac output; LVM – left ventricular mass; PWth – posterior wall thickness; LAD – left atrial diameter; AoD – aortic diameter.
Gross pathology and histology
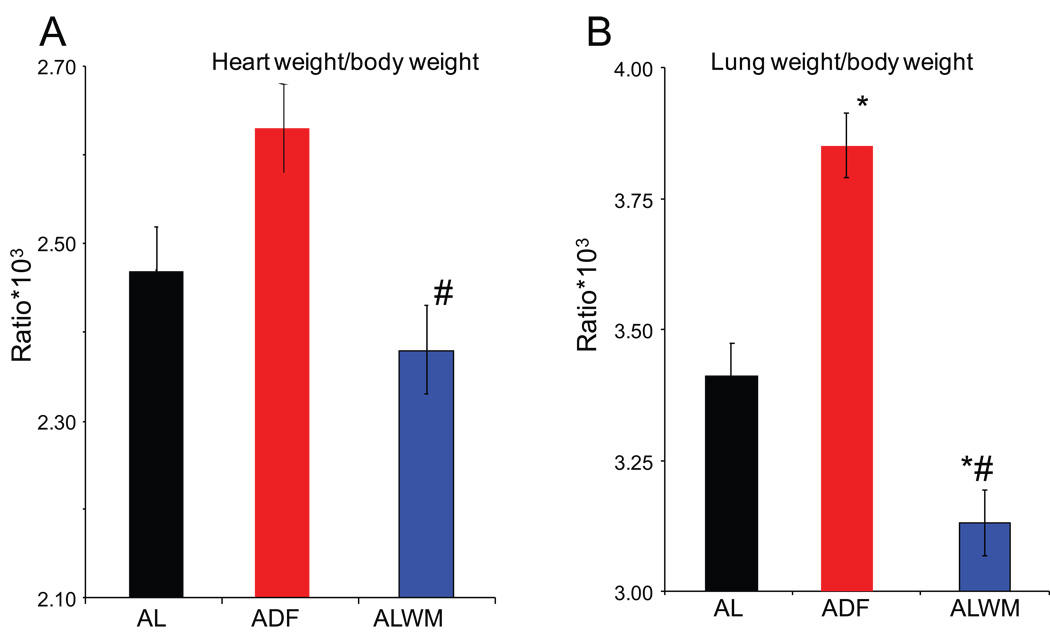
Postmortem heart and lung weights (wet weight) adjusted for body weight (Fig. 4) support echocardiographic findings. In rats on the ADF diet the HW/BW ratio significantly exceeded that in body-weight-matched AL (ALWM) 4 month old rats (Fig. 4 A), but was not significantly higher than that in AL age-matched rats, despite the marked differences in body weight gain. Lung weight/BW ratio in ADF rats (Fig. 4 B) greatly exceeded that of both age-matched and weight-matched AL rats.
Figure 4.
Gross pathology postmortem comparisons of rats on the AL and ADF diets. (A) Heart weight/body weight ratio of AL and ADF groups after 6 months on the respective diets, and of 4 month-old AL rats (AL weight matched control for ADF, ALWM). (B) Lung weight/body weight of the same groups. *p<0.05 vs AL; #p<0.05 compared to the ADF values (ANOVA with Bonferroni post hoc comparison).
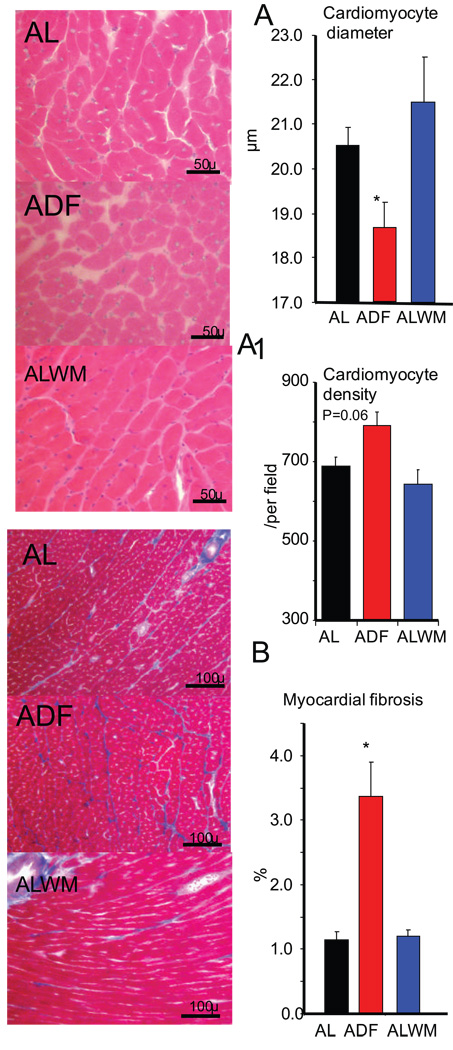
Histological assessment (Fig. 5) showed that cardiomyocyte diameter was significantly smaller in ADF rats compared to age-matched or weight-matched AL rats (Fig. 5 A). Interestingly, the cardiomyocyte density was only marginally increased in ADF rats (Fig. 5A1, p<0.06). ADF rats had 3 times more myocardial fibrosis compared to either age- or weight-matched AL rats (Fig. 5 B).
Figure 5.
Representative histological samples and results of measurements of cardiomyocyte size and density (A, A1, H&E staining) and of myocardial fibrosis (B, Masson staining) for AL, ADF, and ALWM groups. *p<0.05 compared to the ADF and ALWM values (ANOVA with Bonferroni post hoc comparison).
Hemodynamics
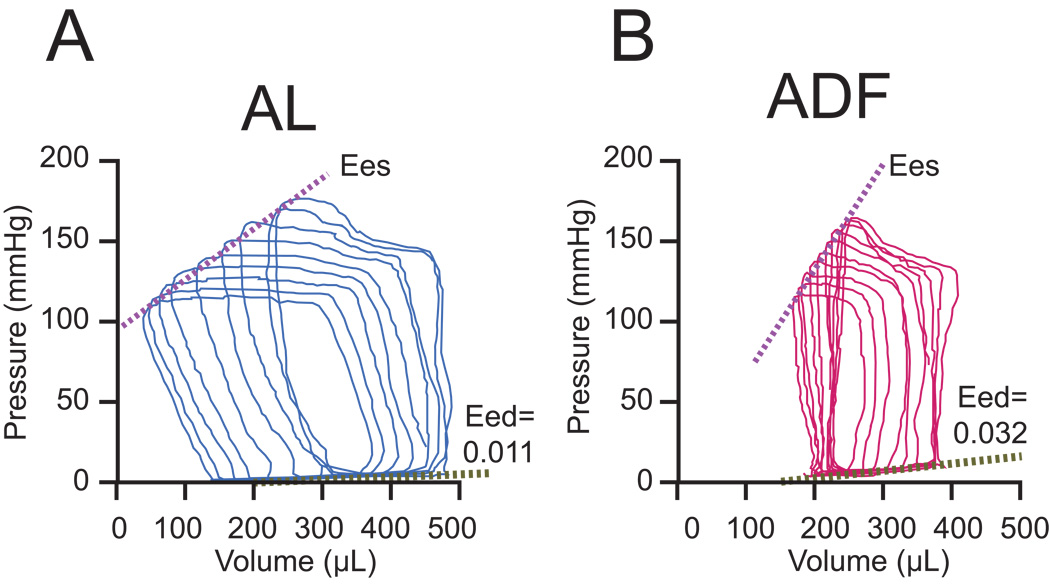
Hemodynamic measurements performed at the end of 6 months of diets are listed in Table 2. In ADF rats there was a statistically significant reduction of HR and aortic systolic pressure resulting in a reduction in pulse pressure compared to AL rats. However, differences between the groups became non-significant after adjustment for body weight (Table 2). Prior to body weight correction SV and CO were reduced in ADF rats, but these differences were not present after adjustment for body weight. Steady state cardiac systolic function in the ADF rats was characterized by a lower ESP and +dp/dt; however a load independent measurement of systolic function, PRSW, and the indices of arterio-ventricular coupling did not differ between the diet groups. ESP in ADF rats remained reduced after adjustment for body weight. All indices of diastolic function, steady state (−dP/dt, Tau, and EDP) and load independent (Eed), were statistically different in ADF rats compared to AL rats, and indicated a reduction of diastolic function and myocardial diastolic stiffness caused by the ADF diet. However, after adjustment for body weight −dP/dt did not differ between groups. Figure 6 illustrates the shifts of PV loops during temporary occlusion of vena cava in representative rats from ADF and AL groups. Note that the slope of the diastolic PV relation is markedly steeper in ADF rats than in AL (see Table 2).
Table 2.
Hemodynamic indices after 6 months of ADF and AL diets adjusted for the effect of body weight if necessary.$
| AL (n=14) | ADF (n=14) | BWAd | AL | ADF | |
|---|---|---|---|---|---|
| BW (kg) | 530±8 | 442±8* | U | N/A | N/A |
| HR (beats/min) | 310±8 | 286±8* | ^0.65 | 5.2±0.14 | 5.4±0.13 |
| BPs (mmHg) | 148±3 | 132±5* | ^0.24 | 32.8±0.9 | 30.6±1.3 |
| BPd (mmHg) | 102±2 | 94±3 | U | N/A | N/A |
| BPm (mmHg) | 116±3 | 107±3 | U | N/A | N/A |
| PP (mmHg) | 46±2 | 38±2* | ^1 | 86±3 | 87±4.4 |
| PVR (mmHg/ml/min) | 1.46±0.05 | 1.74±0.10* | ^−0.85 | 307±12 | 308±17 |
| EDV (µl) | 554±11 | 487±16* | ^0.46 | 1.03±0.02 | 1.1±0.03 |
| ESV (µl) | 295±11 | 264±11(p<0.08) | U | N/A | N/A |
| SV (µl) | 258±6 | 223±8* | ^1 | 0.48±0.01 | 0.5±0.01 |
| EF (%) | 46.8±1.2 | 45.6±1.1 | U | N/A | N/A |
| CO (ml/min) | 80±2 | 63±2* | ^1 | 148±4 | 143±5 |
| Systolic function | |||||
| ESP (mmHg) | 153±3 | 133±5* | ^0.23 | 36±0.6 | 32.8±1.2* |
| (+)dP/dt (mmHg/sec) | 7462±311 | 6524±217* | ^1 | 13.8±0.6 | 14.8±0.6 |
| PRSW (mW/µl) | 73±5 | 59±6 | U | N/A | N/A |
| dP/dt-EDV (mmHg/sec/µL) | 16.8±1.8 | 13.8±1.9 | U | N/A | N/A |
| Diastolic function | |||||
| EDP (mmHg) | 5.5±0.5 | 7.4±0.9(p<0.09) | U | N/A | N/A |
| (−)dP/dt (mmHg/sec) | 8157±253 | 6850±219* | ^1 | 15.2±0.5 | 15.5±0.6 |
| τ (msec) | 12.0±0.2 | 13.2±0.3* | U | N/A | N/A |
| Eed (10−3 mmHg/µl) | 16.4±1.0 | 23.3±2.1* | U | N/A | N/A |
| Arterio-ventricular coupling | |||||
| Ees (mmHg/µl) | 0.39±0.04 | 0.51±0.06 | U | N/A | N/A |
| Ea (mmHg/µl) | 0.60±0.02 | 0.61±0.04 | U | N/A | N/A |
| Ea/Ees | 1.69±0.18 | 1.40±0.16 | U | N/A | N/A |
The same at in Table 1.
BW – body weight; BP – arterial blood pressure; PP – pulse pressure; PVR – peripheral vascular resistance; ESP – end-systolic pressure; PRSW – preload recruitable stroke work; EDP – enddiastolic pressure; Eed – end-diastolic stiffness; Ees – end-systolic elastance; Ea – arterial elastance.
Figure 6.
Pressure-volume loops shift during preload reduction in representative animals from AL (A) and ADF (B) groups. Eed values belong to presented animals.
Dobutamine stress test
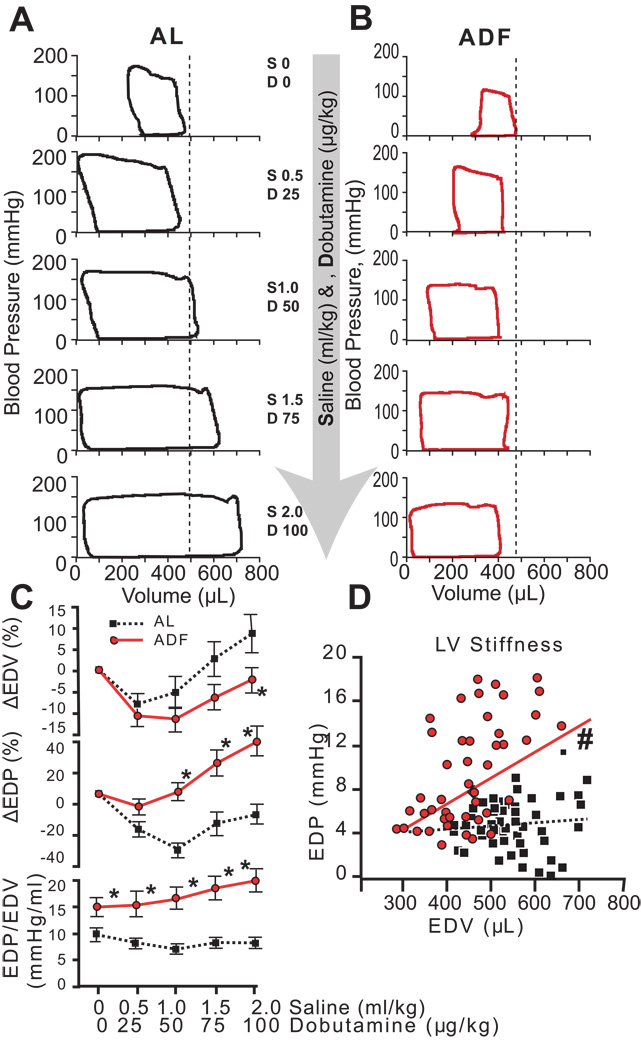
As the rate of infusion increased, the dose of dobutamine increased, so that each sequential measurement reflected a higher volume and higher dobutamine dose. Thus the cardiovascular system was double-stressed, i.e., stressed by both an inotropic stimulation and a volume load. Both groups responded to stress with a similar increase in HR: 20% in AL rats and 27% in ADF rats. Figure 7 illustrates typical shifts of pressure-volume loops in rats in the AL and ADF groups (Figures 7 A and B respectively) in response to increasing inotrope-volume loads. In AL rats (Fig. 7A) the increases in dobutamine/volume stress progressively expanded the end-diastolic volume to accommodate the increased return (right shift), and reduced the end-systolic volume (left shift, i.e., increased systolic performance), also confirmed by increase of stroke volume and stroke work (Fig. 8 C, E). ADF rats (Fig.7 B) also responded to inotropic-volume stress by reducing the end-systolic volume (left shift); however the end-diastolic volume in ADF rats failed to increase. End-diastolic pressure-volume relationships are illustrated in Fig. 7 C and D. While AL rats responded to inotropic-volume stress with greater LV EDV expansion and less of EDP increase compared to ADF rats, ADF rats responded by increasing the end-diastolic pressure (Fig. 7 C, upper and middle panels). Differential responses of AL and ADF rats to inotropic-volume stress are further emphasized in Fig. 7 C, lower panel and 7 D. The dynamics of EDP/EDV ratio in response to stress consistently revealed progressively higher LV stiffness in ADF rats compared to AL rats (Fig. 7 C, lower panel). Additionally, the slope of EDP vs EDV during the stress test (Fig. 7 D), reflecting the LV stiffness, was also markedly different between groups: the slope of regression line for ADF rats was significantly different from zero (p<0.01), whereas the slope of regression line for AL rats was much less steep compared to the ADF rats and statistically not different from zero.
Figure 7.
Hemodynamic measurements during dobutamine-volume stress test. Representative series of pressure-volume loops for AL (A) and ADF (B) groups. Consecutive PV loops from top to bottom represent recordings at baseline (top row) and during cumulative stages of increased stimulation from 0.5 mL/kg of saline (S) and 25 µg/kg of Dobutamine (D, second row) to 2.0 mL/kg of saline and 100 µg/kg of Dobutamine (last row). Vertical dotted lines delineate the end-diastolic volumes at baselines; C (upper and middle panels) - average enddiastolic volume and end-diastolic pressure changes relative to a baseline respectively as a function of dobutamine-volume load intensity in AL and ADF groups; C (lower panel) - average end-diastolic pressure/end-diastolic volume ratio (LV stiffness) during increasing stimulation in AL and ADF groups; D - end-diastolic pressure as a function of end-diastolic volume (LV stiffness) during stress test. Statistical symbols are same as in Figure 1.
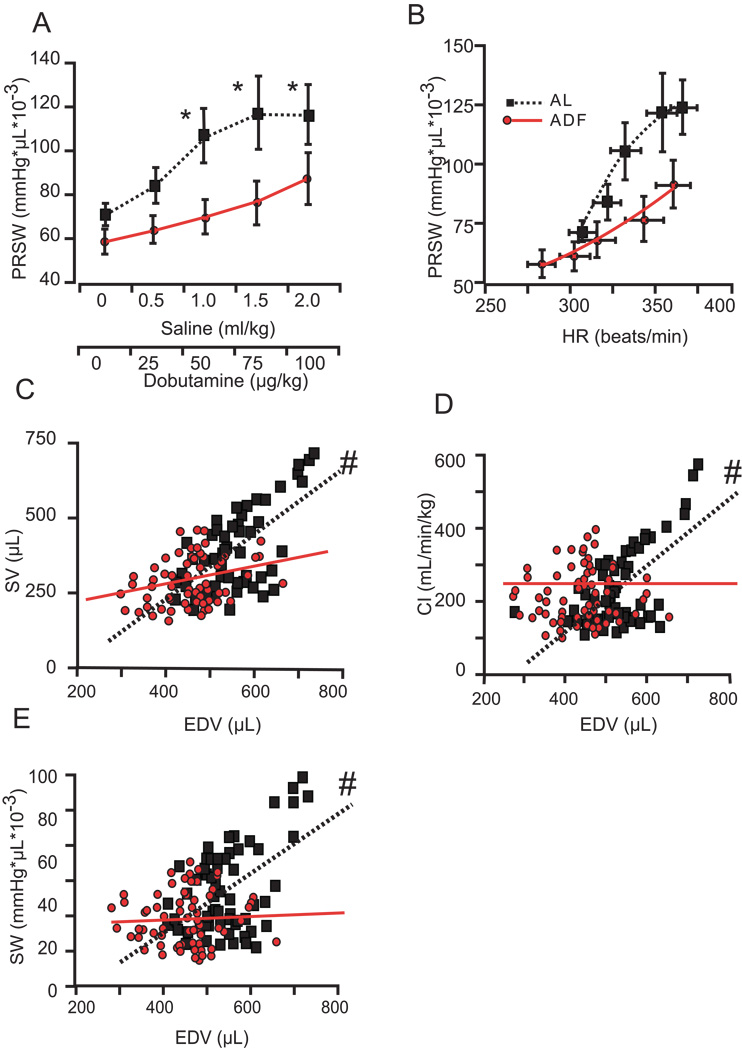
Figure 8.
Additional hemodynamic measurements during a stress test. PRSW (load independent LV work) as a function of dobutamine-volume load intensity (A) or increased HR (B) in AL and ADF groups; C–F - CO, CI, SV, and SW respectively as a function of increased EDV in AL and ADF during the stress test. Statistical symbols are same as in Figure 1.
The indices of cardiac systolic reserve in ADF rats were diminished in comparison to AL rats (Fig. 8). The PRSW, the most integrative index of systolic function, responded to inotropic-volume challenge with significantly less of an increase in ADF compared to AL rats (Fig. 8A). The increase of HR in response to double stress did not differ between groups, and response of PRSW normalized for HR is shown in the Fig. 8 B. In AL rats an increase in HR associated with dobutamine-volume stress corresponded to a steep elevation of PRSW; the response of PRSW to a similar stress-associated increase of HR in ADF rats was substantially blunted compared to AL rats. The diminished cardiac reserve in ADF rats is further illustrated in Figs. 8 C–E, which present the SV, CI, and Stroke Work as a function of the EDV throughout the dobutamine/volume stress. In AL rats an increase of EDV is translated into increases of the SV, CI, and SW: the slopes of regression lines for AL rats are significantly different from zero. On the other hand, in ADF rats the slopes of regressions for these indices remained flat despite a significant increase in EDV.
Discussion
The present results have uncovered a striking deleterious effect of cardiac structure and function in rats maintained on an ADF diet compared to rats on the usual AL diet. Chronic ADF resulted in reduction of cardiomyocyte size and increased myocardial fibrosis. The fibrotic ADF hearts manifested diastolic dysfunction at rest and a diminished diastolic and systolic reserve capacity: During a volume/dobutamine stress the ADF heart was unable to increase its filling volume, despite a greater increase in filling pressure, and this translated into the pump failure.
Considerable previous evidence supports an overall beneficial effect on health of caloric restriction and ADF in rats and mice. Animals maintained on ADF or reduced calorie diets live longer than their AL fed counterparts, and exhibit improved glucose metabolism, decreased tumor incidence and preserved cognitive function and locomotor abilities during aging (see 14 for review). Resting HR was reduced, heart rate variability was increased, and cardiovascular adaptation to stress was improved in animals maintained on an ADF diet for 3–9 months (5, 15). Therefore, it remains to be determined how the alterations in cardiac cytoarchitecture and function caused by long-term ADF in the rats in the present study are related to improved autonomic regulation and increased resistance to ischemic damage reported in previous studies (7, 16).
Echocardiographic measurements indicated that during 6 months of ADF the LV chamber increased less than in control animals, consistent with previous studies which showed a reduction in cardiac weight in rats on a long-term caloric restriction diet (17, 18). The smaller LV chamber in ADF rats was a factor in the reduced LV mass and, additionally, the posterior wall in ADF rats was thinner than in AL rats. These findings were accompanied with reduced SV, EF, and CO. However, adjustment of these parameters for body weight, either ratiometric or allometric, suggested that substantially lower body weight in ADF rats was responsible for most of the differences between them and rats in the AL group: only CO remained lower in ADF rats after BW correction, due to a lower HR. This differs from results of hemodynamic measurements via pressure-volume loop analyses, where BW adjustment eliminated the differences in HR between the diet groups and consequently in CO (Table 2). The reasons might be in different conditions of measurements, namely, open chest (hemodynamics) versus closed chest (echo). Thus, LV echocardiographic findings were mostly unremarkable and attributable to a lower body weight in ADF rats. However, histological assessment showed that cardiac myocytes in ADF rats were smaller than in either age- or weight-matched AL rats, and these findings indicate a real myocardial hypotrophy.
An important Echo finding was a marked increase in left atrial size in ADF rats, which persisted after adjustment for aortic diameter. The increase of atrial size in ADF rats did not correspond to an increase of the LV chamber, which, after adjustment for aortic diameter, was similar in both groups. In the absence of valve disease or specific atrial pathology, left atrial enlargement stems from increased LV filling pressure, indicative of diastolic dysfunction (19–21). It has been suggested (22, 23) that E/A, Doppler index of diastolic dysfunction, reflects elevated filling resistance at the time of measurement, while left atrial enlargement reflects the cumulative effect of habitually increased filling resistance. Since the left atrial contribution to a total LV stroke volume can be as much as 30% (24), atrial enlargement obviously, is a major adaptive mechanism in the setting of diastolic dysfunction. In ADF the E/A was reduced in the absence of evidence of mitral valve disease. Therefore, even in the context of reduced HR in ADF, reduction of E/A in combination with increased left atrial volume and without concomitant increase in LV SV strongly indicates a reduced LV filling and suggests established diastolic dysfunction in rats after 6 months of ADF.
The presence of diastolic dysfunction suggested by Echo findings in ADF rats was confirmed by results of hemodynamic measurements with pressure-volume loop analyses: baseline (prior to volume-dobutamine stress) indices of diastolic performance, EDP, dP/dt min, and Tau were abnormal in ADF rats. Most importantly Eed, the load independent index of myocardial stiffness was elevated. This may be attributable, in part at least, to increased myocardial fibrosis observed in ADF rats. Note, that baseline load independent indices of systolic performance obtained through pressure-volume loop analyses were not reduced in ADF rats, suggesting that intrinsic myocardial contractility was not affected by ADF.
The markedly diminished diastolic reserve in the “stiff” hearts in ADF rats became manifest during the volume/dobutamibe stress test. While AL rats responded to this volume-inotrope load by expanding LV at end-diastole with little increase in EDP, EDV of ADF rats failed to increase, but LV diastolic pressure was elevated in the ADF rats (Fig. 7 C, D). Further analyses of the results of this double stress illustrate the concomitant reduction of systolic reserve in ADF rats (Fig. 8): the increase of preload recruitable stroke work in response to the incremental volume-dobutamine stress (Fig. 8 A, B) that occurred in AL rats was substantially reduced in ADF rats. Moreover, usual increases in LV SV, stroke work, and CO as a function of EDV increase were observed in AL rats (Fig. 8 C–E), but were virtually absent in ADF rats. The findings of gross pathological postmortem examination were consistent with functional changes in that the lung weight indexed for a body weight was increased in ADF compared to AL rats; infused fluid was trapped in the lungs due to lack of systolic cardiac reserve in ADF rats.
Therefore, echocardiographic, hemodynamic, and histological evaluation of ADF rats showed that they developed cardiomyocyte hypotrophy and a “stiff” fibrotic heart manifesting diastolic dysfunction with preserved ejection fraction. Impaired diastolic performance in ADF rats during the volume-dobutamine stress led to the signs of systolic failure. In other words, chronic maintenance of the rats on ADF dietary regimen results in diastolic dysfunction with a diminished cardiac reserve, both during diastole and systole. Although these changes may not adversely affect longevity of the rats under usual laboratory housing conditions, it will be of considerable interest to determine the impact of these changes on the ability of the rats to tolerate vigorous exercise (25).
Although the specific molecular and cellular mechanisms underlying the development of diastolic dysfunction during chronic ADF remain to be determined, our results do lead to mechanistic hypotheses for future studies. A remarkable finding of reduced cardiomyocyte diameter in ADF rats reflects a true hypotrophy of cardiomyocytes, because cardiomyocytes in ADF rats were smaller than in either age- or body weight-matched AL rats. A small drop in ESP in ADF animals cannot be interpreted as leading to a reduction of the LV wall stress, because it was counterbalanced by an increase of r/h ratio. In other words, the mechanism of reduction of cardiomyocyte size in ADF is not similar to the mechanism of cardiomyocyte atrophy associated with mechanical unloading (26). To the best of our knowledge this phenomenon of reduced cardiomyocyte size is not associated with any other experimental model, with the possible exception of a recently described thiamine-deficiency model in rats (27). In that model, rats maintained for one month on a thiamine-depleted, but otherwise standard, diet had smaller cardiomyocytes and reduced thickness of the LV wall, while electrical characteristics of their hearts remained largely intact.
The development of myocardial fibrosis in ADF rats represents another counterintuitive finding of our study, because existing data (28) describe a reduction of myocardial fibrosis in this model, at least in aged rats subjected to life-long ADF. Since preponderance of data does not support the idea of selective mortality in “fibrotic” ADF rats, this finding suggests another direction for mechanistic inquiries. It is widely accepted, although never actually proven, that tissue-protective effects of ADF are related to adaptive stress responses elicited by this dietary regimen. Levels of several stress proteins, such as heat-shock and glucose regulated proteins, were elevated in brain tissues of rats and mice on an ADF diet (1). ADF also results in an elevation of levels of circulating corticosterone (5). Interestingly, activation of mineralocorticoid (MC) receptors, via exogenous administration of MC or via endogenous glucocorticoid stimulation is known to produce cardiac fibrosis (29). Moreover, it has been proposed that elevation of tissue aldosterone level and activation of MC receptors plays a central role in the pathogenesis of diastolic heart failure (30).
Only 4 human studies have been published to date on the effects of ADF (31–34). A decrease in body weight and fat mass, an increase in insulin sensitivity, an increase in plasma HDL cholesterol concentration (women), a decrease in plasma triglyceride concentration (men), and decreases in markers of oxidative stress and inflammation have been observed. While these results are promising, the dietary intervention lasted for a relatively short time periods (1–3 months), involved small sample sizes, and baseline (pre-ADF) measurements were used as control values. The present findings of deleterious effects of ADF on cardiac function in experiment on rodents provide a cautionary note to the adoption of long-term ADF regimens in humans.
In summary, experimental ADF, a dietary regimen, which prolongs life span and promotes numerous health benefits in rodents, including tissue protection from ischemic damage, causes a myocardial hypotrophy, cardiac fibrosis, diastolic dysfunction, and a reduction of cardiac reserve. Therefore, the present findings offer a novel and unique experimental model of dietary induced diastolic dysfunction.
Supplementary Material
Acknowledgments
Authors are grateful to Dr. Paul Chantler for assisting with allometric scaling for body weight, to Ms. Veena Shetty for statistical help, and to Ms. Tina Turner and Mrs. Shannon Marshall for technical assistance.
This work was fully funded by Intramural Research Program, National Institute on Aging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Conflict of Interest to disclosures: none.
References
- 1.Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13. doi: 10.1093/ajcn/86.1.7. Review. [DOI] [PubMed] [Google Scholar]
- 3.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan R, Camandola S, Mattson MP. Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J. 2003;17:1133–1134. doi: 10.1096/fj.02-0996fje. [DOI] [PubMed] [Google Scholar]
- 6.Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- 7.Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- 8.Katare RG, Kakinuma Y, Arikawa M, Yamasaki F, Sato T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3K signaling pathway. J Mol Cell Cardiol. 2009;46:405–412. doi: 10.1016/j.yjmcc.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Moon C, Krawczyk M, Ahn D, Ahmet I, Paik D, Lakatta EG, et al. Erythropoietin reduces myocardial infarction and left ventricular functional decline following coronary artery ligation in rats. Proc Natl Acad Sci USA. 2003;100:11612–11617. doi: 10.1073/pnas.1930406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacologic manipulation of β-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004;110:1083–1090. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- 11.Batterham AM, George KP, Whyte G, Sharma S, McKenna W. Scaling cardiac structural data by body dimensions: a review of theory, practice, and problems. Int J Sports Med. 1999;20:495–502. doi: 10.1055/s-1999-8844. [DOI] [PubMed] [Google Scholar]
- 12.Chantler PD, Clements RE, Sharp L, George KP, Tan LB, Goldspink DF. The influence of body size on measurements of overall cardiac function. Am J Physiol. 2005;289:H2059–H2065. doi: 10.1152/ajpheart.00022.2005. [DOI] [PubMed] [Google Scholar]
- 13.Georgakopoulos D, Mitzner WA, Chen CH, Byrne BJ, Millar HD, Hare JM, et al. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol. 1998;274(4 Pt 2):H1416–H1422. doi: 10.1152/ajpheart.1998.274.4.H1416. [DOI] [PubMed] [Google Scholar]
- 14.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. Review. [DOI] [PubMed] [Google Scholar]
- 15.Mager DE, Wan R, Brown M, Cheng A, Wareski P, Abernethy DR, et al. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- 16.Shinmura K, Tamaki K, Bolli R. Impact of 6-mo caloric restriction on myocardial ischemic tolerance: possible involvement of nitric oxide-dependent increase in nuclear Sirt1. Am J Physiol. 2008;295:H2348–H2355. doi: 10.1152/ajpheart.00602.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walford RL, Harris SB, Weindruch R. Dietary restriction and aging: historical phases, mechanisms and current directions. J Nutr. 1987;117:1650–1654. doi: 10.1093/jn/117.10.1650. Review. [DOI] [PubMed] [Google Scholar]
- 18.Barger JL, Walford RL, Weindruch R. The retardation of aging by caloric restriction: its significance in the transgenic era. Exp Gerontol. 2003;38:1343–1351. doi: 10.1016/j.exger.2003.10.017. Review. [DOI] [PubMed] [Google Scholar]
- 19.Appleton CP, Galloway JM, Gonzalez MS, Gaballa M, Basnight MA. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol. 1993;22:1972–1982. doi: 10.1016/0735-1097(93)90787-2. [DOI] [PubMed] [Google Scholar]
- 20.Douglas PS. The left atrium: a biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J Am Coll Cardiol. 2003;42:1206–1207. doi: 10.1016/s0735-1097(03)00956-2. [DOI] [PubMed] [Google Scholar]
- 21.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 22.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–1289. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 23.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda Y, Toma Y, Ogawa H, Matsuzaki M, Katayama K, Fujii T, et al. Importance of left atrial function in patients with myocardial infarction. Circulation. 1983;67:566–571. doi: 10.1161/01.cir.67.3.566. [DOI] [PubMed] [Google Scholar]
- 25.Kitzman DW, Groban L. Exercise intolerance. Heart Fail Clin. 2008;4:99–115. doi: 10.1016/j.hfc.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razeghi P, Volpini KC, Wang M-E, Youker KA, Stepkowski S, Taegtmeyer H. Mechanical unloading of the heart activates the calpain system. J Mol Cell Cardiol. 2007;42:449–452. doi: 10.1016/j.yjmcc.2006.08.114. [DOI] [PubMed] [Google Scholar]
- 27.Roman-Campos D, Campos AC, Gioda CR, Campos PP, Medeiros MA, Cruz JS. Cardiac structural changes and electrical remodeling in a thiamine-deficiency model in rats. Life Sci. 2009;84:817–824. doi: 10.1016/j.lfs.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Castello L, Froio T, Maina M, Cavallini G, Biasi F, Leonarduzzi G, et al. Alternate-day fasting protects the rat heart against age-induced inflammation and fibrosis by inhibiting oxidative damage and NF-kB activation. Free Radic Biol Med. 2009 Oct 8; doi: 10.1016/j.freeradbiomed.2009.10.003. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Young MJ, Lam EY, Rickard AJ. Mineralocorticoid receptor activation and cardiac fibrosis. Clin Sci (Lond) 2007;112:467–475. doi: 10.1042/CS20060275. Review. [DOI] [PubMed] [Google Scholar]
- 30.Ohtani T, Ohta M, Yamamoto K, Mano T, Sakata Y, Nishio M, et al. Elevated cardiac tissue level of aldosterone and mineralocorticoid receptor in diastolic heart failure: Beneficial effects of mineralocorticoid receptor blocker. Am J Physiol. 2007;292:R946–R954. doi: 10.1152/ajpregu.00402.2006. [DOI] [PubMed] [Google Scholar]
- 31.Heilbronn LK, Civitarese AE, Bogacka I, Smith SR, Hulver M, Ravussin E. Glucose tolerance and skeletal muscle gene expression in response to alternate day fasting. Obes Res. 2005;13:574–581. doi: 10.1038/oby.2005.61. [DOI] [PubMed] [Google Scholar]
- 32.Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81:69–73. doi: 10.1093/ajcn/81.1.69. [DOI] [PubMed] [Google Scholar]
- 33.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allard JS, Heilbronn LK, Smith C, Hunt ND, Ingram DK, Ravussin E, et al. In vitro cellular adaptations of indicators of longevity in response to treatment with serum collected from humans on calorie restricted diets. PLoS One. 2008;3:e3211. doi: 10.1371/journal.pone.0003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.