Abstract
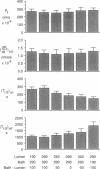
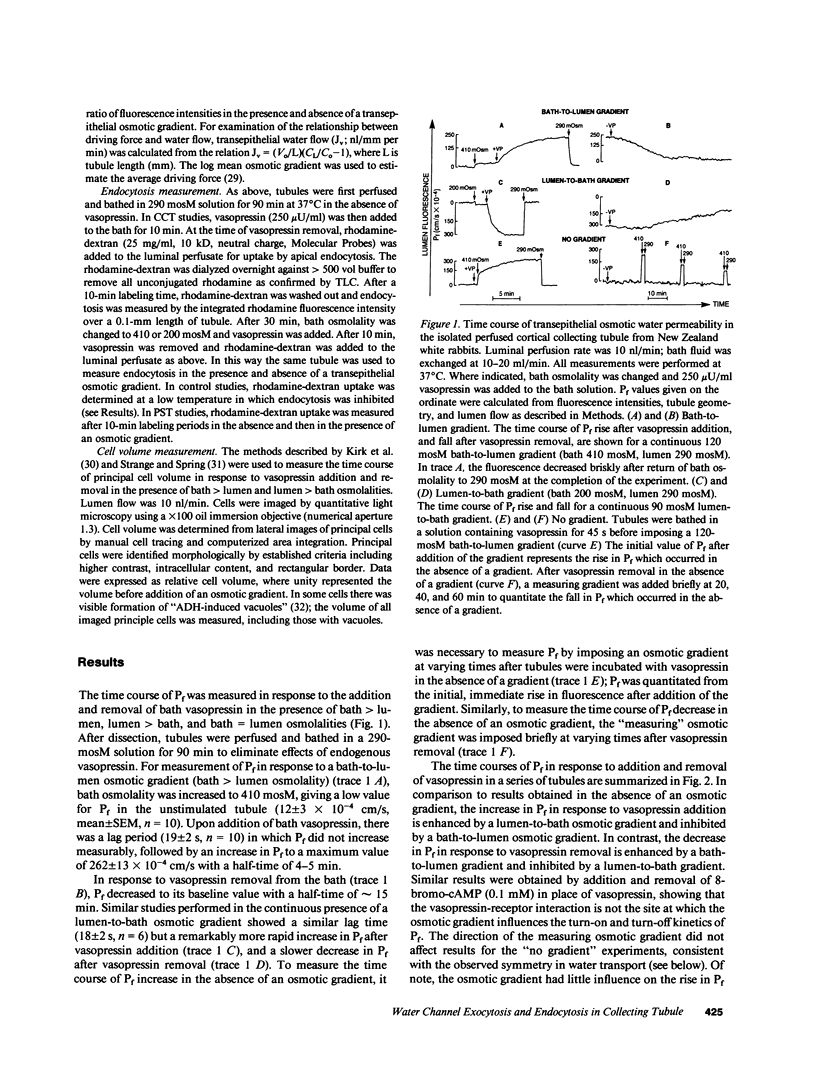
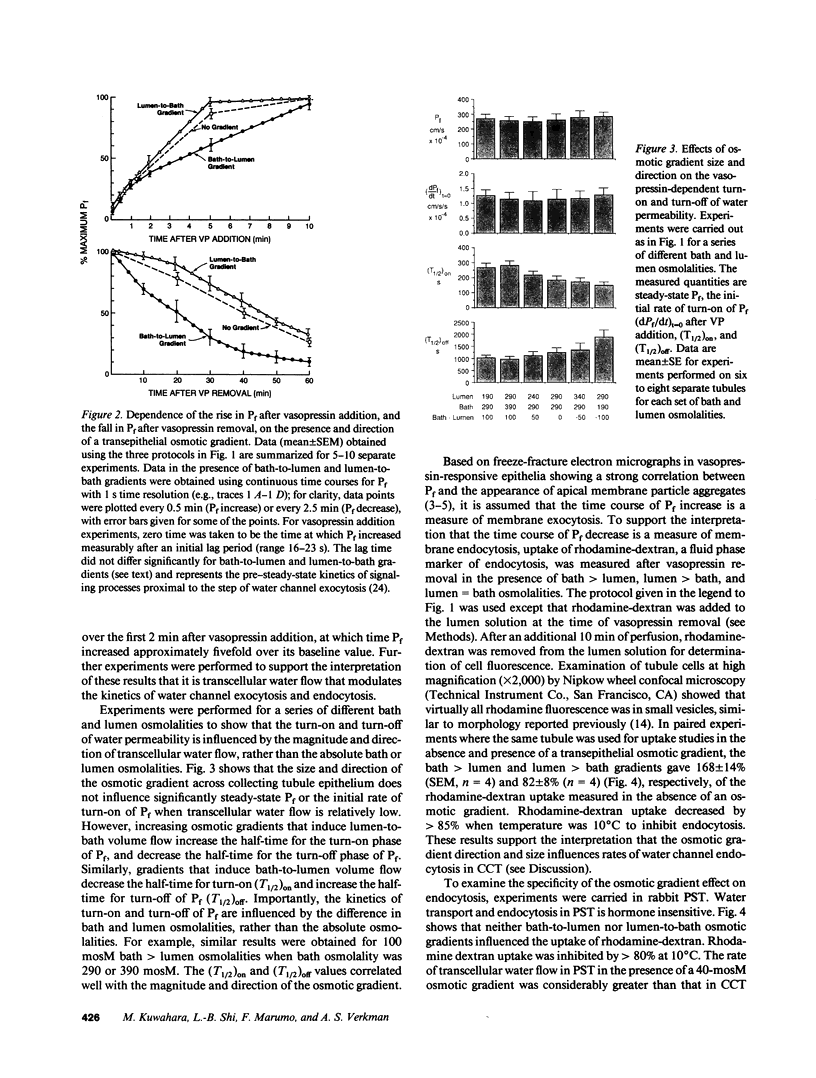
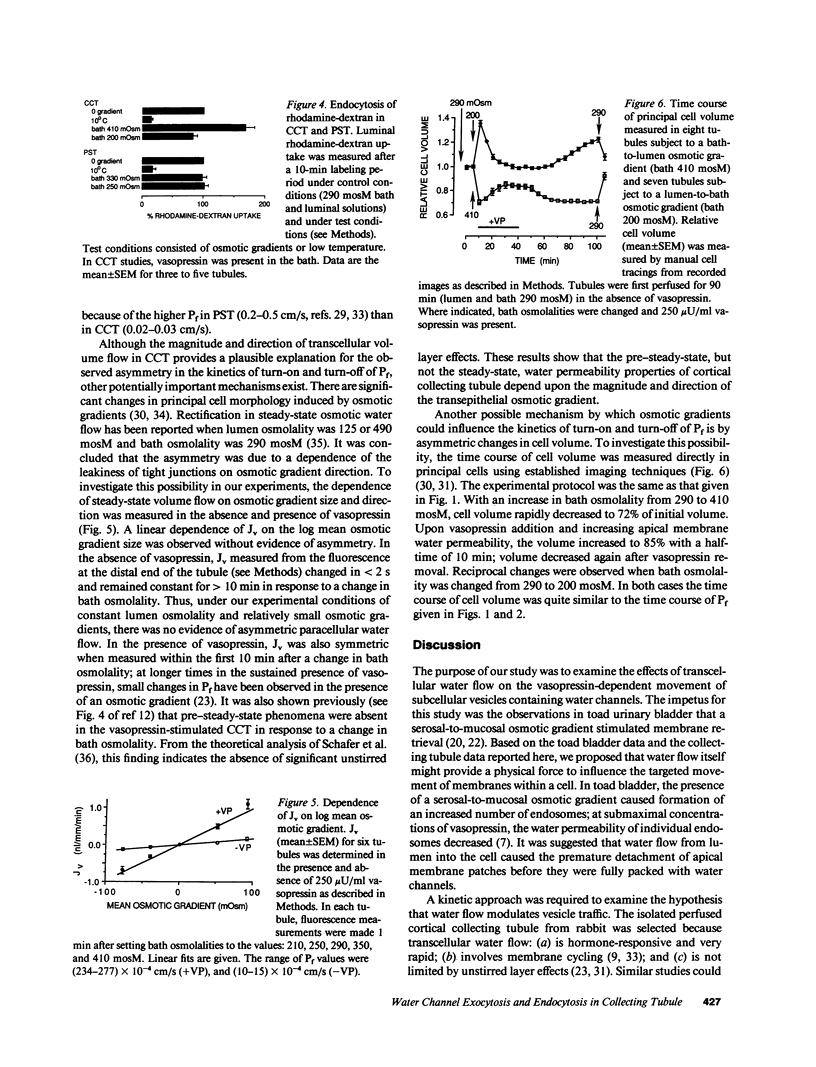
The regulation of osmotic water permeability (Pf) by vasopressin (VP) in kidney collecting tubule involves the exocytic-endocytic trafficking of vesicles containing water channels between an intracellular compartment and apical plasma membrane. To examine effects of transcellular water flow on vesicle movement, Pf was measured with 1-s time resolution in the isolated perfused rabbit cortical collecting tubule in response to addition and removal of VP (250 microU/ml) in the presence of bath greater than lumen (B greater than L), lumen greater than bath (L greater than B), and lumen = bath (L = B) osmolalities. With VP addition, Pf increased from 12 to 240-270 x 10(-4) cm/s (37 degrees C) in 10 min. At 1 min, Pf was approximately 70 x 10(-4) cm/s for B greater than L, L greater than B, and L = B conditions. At later times, Pf increased fastest for L greater than B and slowest for B greater than L osmolalities; at 5 min, Pf was 250 x 10(-4) cm/s (L greater than B) and 158 x 10(-4) cm/s (B greater than L). With VP removal, Pf returned to pre-VP levels at the fastest rate for B greater than L and the slowest rate for L greater than B osmolalities; at 30 min, Pf was 65 x 10(-4) cm/s (B greater than L) and 183 x 10(-4) cm/s (L greater than B). For a series of osmotic gradients of different magnitudes and directions, the rates of Pf increase and decrease were dependent upon the magnitude of transcellular volume flow; control studies showed that paracellular water flux, asymmetric transcellular water pathways, or changes in cell volume could not account for the data. VP-dependent endocytosis was measured by apical uptake of rhodamine-dextran; in paired studies where the same tubule was used for + and - gradients, B greater than L and L greater than B osmolalities gave 168% and 82% of uptake measured with no gradient. In contrast, endocytosis in proximal tubule was not dependent on gradient direction. These data provide evidence that transcellular volume flow modulates the vasopressin-dependent cycling of vesicles containing water channels, suggesting a novel driving mechanism to aid or oppose the targeted, hormonally directed movement of subcellular membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Zahid G., Schafer J. A., Troutman S. L., Andreoli T. E. Effect of antidiuretic hormone on water and solute permeation, and the activation energies for these processes, in mammalian cortical collecting tubules: evidence for parallel ADH-sensitive pathways for water and solute diffusion in luminal plasma membranes. J Membr Biol. 1977 Feb 24;31(1-2):103–129. doi: 10.1007/BF01869401. [DOI] [PubMed] [Google Scholar]
- Bae H. R., Verkman A. S. Protein kinase A regulates chloride conductance in endocytic vesicles from proximal tubule. Nature. 1990 Dec 13;348(6302):637–639. doi: 10.1038/348637a0. [DOI] [PubMed] [Google Scholar]
- Berry C. A., Verkman A. S. Osmotic gradient dependence of osmotic water permeability in rabbit proximal convoluted tubule. J Membr Biol. 1988 Oct;105(1):33–43. doi: 10.1007/BF01871104. [DOI] [PubMed] [Google Scholar]
- Bourguet J., Chevalier J., Hugon J. S. Alterations in membrane-associated particle distribution during antidiuretic challenge in frog urinary bladder epithelium. Biophys J. 1976 Jun;16(6):627–639. doi: 10.1016/S0006-3495(76)85717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Grosso A., DeSousa R. C. Correlation between water flow and intramembrane particle aggregates in toad epidermis. Am J Physiol. 1983 Nov;245(5 Pt 1):C334–C342. doi: 10.1152/ajpcell.1983.245.5.C334. [DOI] [PubMed] [Google Scholar]
- Brown D., Orci L. Vasopressin stimulates formation of coated pits in rat kidney collecting ducts. Nature. 1983 Mar 17;302(5905):253–255. doi: 10.1038/302253a0. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- DiBona D. R. Cytoplasmic involvement in ADH-mediated osmosis across toad urinary bladder. Am J Physiol. 1983 Nov;245(5 Pt 1):C297–C307. doi: 10.1152/ajpcell.1983.245.5.C297. [DOI] [PubMed] [Google Scholar]
- Gronowicz G., Masur S. K., Holtzman E. Quantitative analysis of exocytosis and endocytosis in the hydroosmotic response of toad bladder. J Membr Biol. 1980;52(3):221–235. doi: 10.1007/BF01869191. [DOI] [PubMed] [Google Scholar]
- Handler J. S. Antidiuretic hormone moves membranes. Am J Physiol. 1988 Sep;255(3 Pt 2):F375–F382. doi: 10.1152/ajprenal.1988.255.3.F375. [DOI] [PubMed] [Google Scholar]
- Harmanci M. C., Stern P., Kachadorian W. A., Valtin H., DiScala V. A. Vasopressin and collecting duct intramembranous particle clusters: a dose-response relationship. Am J Physiol. 1980 Dec;239(6):F560–F564. doi: 10.1152/ajprenal.1980.239.6.F560. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Wade J. B., Handler J. S. Transepithelial water flow regulates apical membrane retrieval in antidiuretic hormone-stimulated toad urinary bladder. J Clin Invest. 1986 Sep;78(3):703–712. doi: 10.1172/JCI112630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk K. L. Origin of ADH-induced vacuoles in rabbit cortical collecting tubule. Am J Physiol. 1988 May;254(5 Pt 2):F719–F733. doi: 10.1152/ajprenal.1988.254.5.F719. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., Schafer J. A., DiBona D. R. Quantitative analysis of the structural events associated with antidiuretic hormone-induced volume reabsorption in the rabbit cortical collecting tubule. J Membr Biol. 1984;79(1):65–74. doi: 10.1007/BF01868527. [DOI] [PubMed] [Google Scholar]
- Kuwahara M., Berry C. A., Verkman A. S. Rapid development of vasopressin-induced hydroosmosis in kidney collecting tubules measured by a new fluorescence technique. Biophys J. 1988 Oct;54(4):595–602. doi: 10.1016/S0006-3495(88)82994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara M., Verkman A. S. Direct fluorescence measurement of diffusional water permeability in the vasopressin-sensitive kidney collecting tubule. Biophys J. 1988 Oct;54(4):587–593. doi: 10.1016/S0006-3495(88)82993-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara M., Verkman A. S. Pre-steady-state analysis of the turn-on and turn-off of water permeability in the kidney collecting tubule. J Membr Biol. 1989 Aug;110(1):57–65. doi: 10.1007/BF01870993. [DOI] [PubMed] [Google Scholar]
- Lencer W. I., Verkman A. S., Arnaout M. A., Ausiello D. A., Brown D. Endocytic vesicles from renal papilla which retrieve the vasopressin-sensitive water channel do not contain a functional H+ ATPase. J Cell Biol. 1990 Aug;111(2):379–389. doi: 10.1083/jcb.111.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer W. I., Weyer P., Verkman A. S., Ausiello D. A., Brown D. FITC-dextran as a probe for endosome function and localization in kidney. Am J Physiol. 1990 Feb;258(2 Pt 1):C309–C317. doi: 10.1152/ajpcell.1990.258.2.C309. [DOI] [PubMed] [Google Scholar]
- Levine S. D., Kachadorian W. A. Barriers to water flow in vasopressin-treated toad urinary bladder. J Membr Biol. 1981;61(2):135–139. doi: 10.1007/BF02007640. [DOI] [PubMed] [Google Scholar]
- Masur S. K., Cooper S., Rubin M. S. Effect of an osmotic gradient on antidiuretic hormone-induced endocytosis and hydroosmosis in the toad urinary bladder. Am J Physiol. 1984 Aug;247(2 Pt 2):F370–F379. doi: 10.1152/ajprenal.1984.247.2.F370. [DOI] [PubMed] [Google Scholar]
- Muller J., Kachadorian W. A., DiScala V. A. Evidence that ADH-stimulated intramembrane particle aggregates are transferred from cytoplasmic to luminal membranes in toad bladder epithelial cells. J Cell Biol. 1980 Apr;85(1):83–95. doi: 10.1083/jcb.85.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman J. S., Seidman L., Farquhar M. G. The membrane composition of coated pits, microvilli, endosomes, and lysosomes is distinctive in the rat kidney proximal tubule cell. J Cell Biol. 1986 Jan;102(1):77–87. doi: 10.1083/jcb.102.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J. A., Patlak C. S., Andreoli T. E. Osmosis in cortical collecting tubules. A theoretical and experimental analysis of the osmotic transient phenomenon. J Gen Physiol. 1974 Aug;64(2):201–227. [PMC free article] [PubMed] [Google Scholar]
- Schafer J. A., Troutman S. L., Andreoli T. E. Osmosis in cortical collecting tubules. ADH-independent osmotic flow rectification. J Gen Physiol. 1974 Aug;64(2):228–240. [PMC free article] [PubMed] [Google Scholar]
- Shi L. B., Brown D., Verkman A. S. Water, proton, and urea transport in toad bladder endosomes that contain the vasopressin-sensitive water channel. J Gen Physiol. 1990 May;95(5):941–960. doi: 10.1085/jgp.95.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L. B., Verkman A. S. Very high water permeability in vasopressin-induced endocytic vesicles from toad urinary bladder. J Gen Physiol. 1989 Dec;94(6):1101–1115. doi: 10.1085/jgp.94.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L. B., Wang Y. X., Verkman A. S. Regulation of the formation and water permeability of endosomes from toad bladder granular cells. J Gen Physiol. 1990 Oct;96(4):789–808. doi: 10.1085/jgp.96.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K., Spring K. R. Cell membrane water permeability of rabbit cortical collecting duct. J Membr Biol. 1987;96(1):27–43. doi: 10.1007/BF01869332. [DOI] [PubMed] [Google Scholar]
- Strange K., Willingham M. C., Handler J. S., Harris H. W., Jr Apical membrane endocytosis via coated pits is stimulated by removal of antidiuretic hormone from isolated, perfused rabbit cortical collecting tubule. J Membr Biol. 1988 Jul;103(1):17–28. doi: 10.1007/BF01871929. [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Lencer W. I., Brown D., Ausiello D. A. Endosomes from kidney collecting tubule cells contain the vasopressin-sensitive water channel. Nature. 1988 May 19;333(6170):268–269. doi: 10.1038/333268a0. [DOI] [PubMed] [Google Scholar]
- Verkman A. S. Mechanisms and regulation of water permeability in renal epithelia. Am J Physiol. 1989 Nov;257(5 Pt 1):C837–C850. doi: 10.1152/ajpcell.1989.257.5.C837. [DOI] [PubMed] [Google Scholar]
- Verkman A. S., Weyer P., Brown D., Ausiello D. A. Functional water channels are present in clathrin-coated vesicles from bovine kidney but not from brain. J Biol Chem. 1989 Dec 5;264(34):20608–20613. [PubMed] [Google Scholar]
- Wade J. B., Stetson D. L., Lewis S. A. ADH action: evidence for a membrane shuttle mechanism. Ann N Y Acad Sci. 1981;372:106–117. doi: 10.1111/j.1749-6632.1981.tb15464.x. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. Endocytosis and exocytosis: current concepts of vesicle traffic in animal cells. Int Rev Cytol. 1984;92:51–92. doi: 10.1016/s0074-7696(08)61324-8. [DOI] [PubMed] [Google Scholar]
- Ye R. G., Shi L. B., Lencer W. I., Verkman A. S. Functional colocalization of water channels and proton pumps in endosomes from kidney proximal tubule. J Gen Physiol. 1989 May;93(5):885–902. doi: 10.1085/jgp.93.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]