Abstract
New preparations, fluorescent probes and imaging techniques are providing the means to observe the behavior of cells in the tissue environment of lymphoid organs. In particular, when combined with two-photon laser microscopy, intravital imaging of surgically exposed lymph nodes provides a unique view of lymphocyte migration and antigen presentation as it occurs within the living animal. The view is emerging that lymphocytes migrate randomly within lymphoid organs, and that lymphocyte contact with antigen-presenting cells may be a stochastic process rather than one guided by chemokine gradients.
Introduction
The immune system consists of a distributed network of trillions of cells that must operate independently to provide antigen specificity and yet function in a coordinated manner to defend us from a wide variety of pathogens. Communication between cells can be initiated by direct cell contact, or can take place at some distance within the tissue environment via chemokines.
Over the past 20 years, remarkable progress in molecular immunology has defined the mechanism of antigen recognition and identified a growing cast of molecules and signaling pathways that link the T-cell receptor to the nucleus. However, we still understand very little about the basics of motility, compartmentalization and antigen recognition in vivo, because these events occur within densely packed lymphoid organs [1–3]. How do T cells, B cells and dendritic cells (DCs) move within the native tissue environment? How are T and B cell compartmental boundaries established and maintained? How does a T cell locate antigen within the lymph node — by following chemokine gradients or by random collisions with antigen-presenting cells (APCs)?
There is increasing recognition that events defined in vitro may not correspond to the physiological situation in vivo [4]. For example, contact between a T cell and an APC leads to a redistribution of surface molecules and formation of the ‘immunological synapse’ [5–7]. This type of molecular redistribution has also been studied in vitro in planar lipid bilayers with defined molecular constituents. Yet it is still unclear whether stable synapses occur in the environment of the lymph node, or whether antigen recognition naturally involves short-lived serial encounters.
Lymphoid organs have remained a black box into which defined cell populations can be induced to home, but from which we have been able to obtain only ‘snapshot’ views, by extracting cells or analyzing fixed tissue. Clearly, there exists a strong need for imaging approaches to visualize living cells within intact lymphoid tissue.
Seeing T cells in their native environment: new preparations for imaging
To illuminate the black box of the tissue environment, new in vitro preparations have been developed that more faithfully represent the native tissue environment of intact lymphoid organs. These include 3D collagen gel matrices [8], monolayers of endothelial cells (ECs) bathed with flowing solution to mimic the forces experienced by cells [9], cultures of clusters or reaggregated tissue fragments [10•,11], engineered tissue surrogates [12], and whole lymph node explants (Figure 1; [13,14••,15••]). All of these preparations lack intact vascular and lymphatic vessels, and therefore cannot be applied to investigate processes such as lymphocyte trafficking. Furthermore, the lack of blood and lymphatic vessels may disrupt the distribution of important soluble factors or alter the physiological levels of tissue oxygenation.
Figure 1.
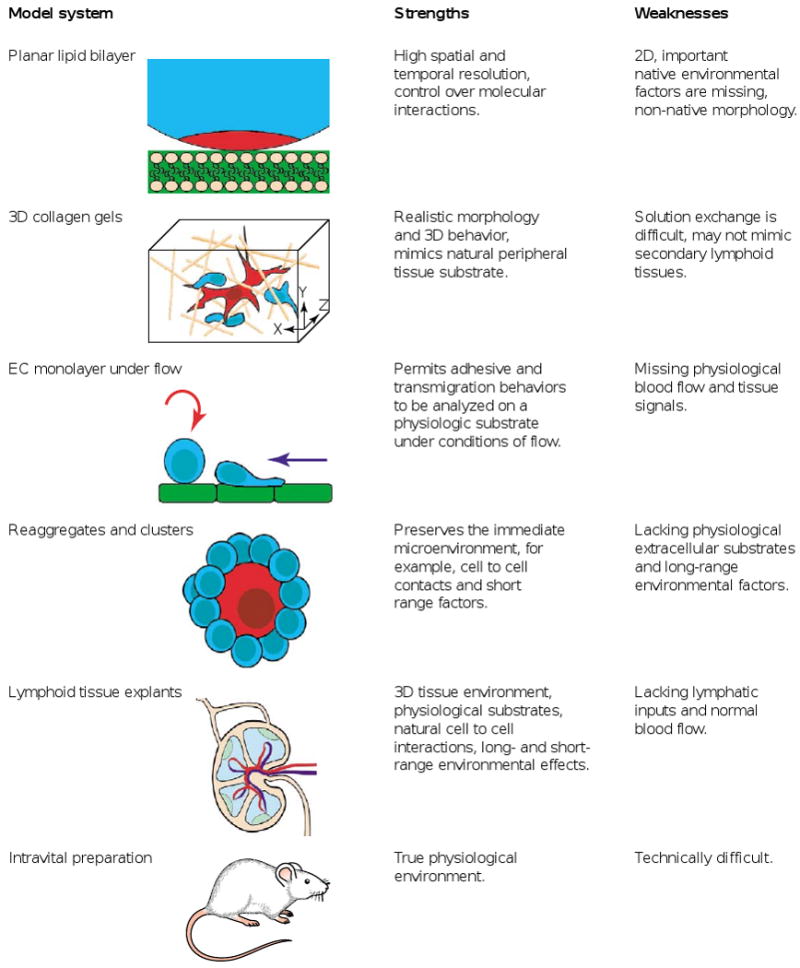
The strengths and weaknesses of model systems in current use for the real-time imaging of lymphocytes. EC, endothelial cell.
To overcome this limitation, several promising methodologies have been developed to visualize cells within the in vivo tissue environment. Non-invasive methods include bioluminescence imaging of cells engineered to express luciferase [16], magnetic resonance imaging microscopy to track cells labeled with superparamagnetic particles [17], and positron-emission tomography (PET; [18]). Although these methods can be applied to intact animals, all three require cell engineering to derive populations that can be detected and presently lack single-cell resolution. Instead, optical techniques offer cellular, and even sub-cellular, levels of resolution. Intravital preparations of exposed lymphoid organs permit light microscopic imaging in the native tissue environment with intact circulatory elements, but require anesthesia and surgery to bring objective lenses close enough to the tissue.
To image cells at depths of more than about 50 μm, two-photon microscopy is the technique of choice. When combined with fluorescent probes, confocal microscopy or two-photon microscopy can reveal single cells at the plane of focus, either by imaging through a pinhole in the case of confocal microscopy, or by selectively exciting the fluorophore only at the plane of focus in the case of two-photon microscopy. Recently, we provided a detailed comparison of confocal and two-photon microscopy as applied to the imaging of T cells in situ [19•]. Advantages of two-photon microscopy include less photodamage, greater sensitivity and deeper imaging within tissue, which is possible because the near-infrared illumination penetrates tissue more effectively.
Imaging T cells in explanted lymph nodes
Advances in imaging techniques and fluorescent markers to label or genetically tag cells or specific proteins are now making it possible to ‘see’ events in real time that could previously only be inferred. Confocal and two-photon imaging approaches have recently provided the first glimpse of lymphocyte dynamics within the tissue environment. We used two-photon microscopy to visualize the behavior of individual T and B lymphocytes in explanted lymph node [14••] and spleen [20] maintained in culture. Using an in vivo adoptive transfer approach (Figure 2), T and B cells labeled with green or red CellTracker™ dyes (Molecular Probes Inc., Eugene, OR, USA) homed to appropriate locations and exhibited vigorous motility within the intact lymph node, with velocities that averaged 12 μm/minute and 6 μm/minute, respectively. T cells were observed to migrate in a ‘stop-and-go’ fashion, similar to the behavior of T cells in a collagen gel matrix culture system, with alternating episodes of rapid motion, when cells were elongated, followed by momentary pauses when cells rounded up. The period of these cycles averaged 1–2 minutes. In the absence of antigen, very few T cells were truly stationary, although pressure on the lymph node or accumulated photodamage caused the cells to stop moving. In healthy preparations, a 3D random walk in all three axes emerges over time because of T-cell turning. In the absence of antigen, naïve T cells from ovalbumin-specific DO11.10 transgenic TCR mice moved randomly without evidence of chemokine gradients.
Figure 2.
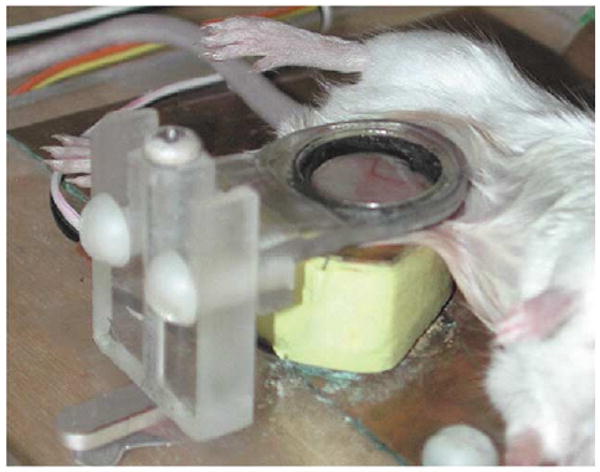
Intravital two-photon microscopy: an anesthetized mouse with surgically exposed lymph node on the microscope stage. Details of the preparation have been described [23].
Very different results were obtained in a parallel study that used a similar adoptive transfer approach, but different imaging and tissue culture conditions. Naïve T cells were immobile when lymph nodes were maintained in culture with atmospheric oxygen tension and imaged using confocal microscopy. Possible reasons for the differences observed have been discussed previously [19•,21,22], and include differences in imaging depth, tissue handling, photodamage and oxygen tension in the tissue. Our study [14••] used lymph nodes immersed in 95% O2, 5% CO2, as previous work on brain slice preparations clearly demonstrated enhanced survival and function when tissue preparations are maintained in vitro under these conditions. Stoll et al. [15••], however, chose to use 20% O2, reasoning that lymph nodes may possess low oxygen tension under physiological conditions [23].
Intravital two-photon imaging
In an effort to resolve the discrepancy regarding the motility of naïve T cells in vivo, we adopted two-photon microscopy to perform intravital imaging of the inguinal lymph node in an anesthetized mouse [24••]. In this preparation, a simple surgical procedure exposed the inguinal node and allowed lymphocytes to be tracked in vivo as they move within the microcirculation, home into the lymph node and migrate within the T-cell zone of the lymph node. Care was taken to maintain intact circulation of blood and lymph, and to avoid microdissection by imaging through naturally occurring windows in the fat pads that lie on top of the node. Under these conditions, T cells exhibited vigorous motility and migrated randomly without evidence for collective drift or motion along putative chemokine gradients. As observed in explanted lymph nodes, naïve T cells moved in a stop-and-go manner, elongating while moving rapidly ahead and then pausing every 1–2 min on average. Overall, the average velocity of movement was 11 μm/min, very similar to that of T cells in explanted nodes at the same temperature. Figure 3 illustrates a field of T cells with four cell tracks highlighted in a depth-encoded representation of T-cell positions. In this instance, the mouse was breathing room air during the entire experiment; in other experiments we used a mask to deliver a stream of 95% O2, 5% CO2 to maintain respiratory drive during long-term measurements. The key point is that vigorous motility in a random walk characterizes the behavior of naïve T cells in vivo. We postulate that T cells distribute autonomously through the T-cell zone, and that the search for antigen is a stochastic process.
Figure 3.
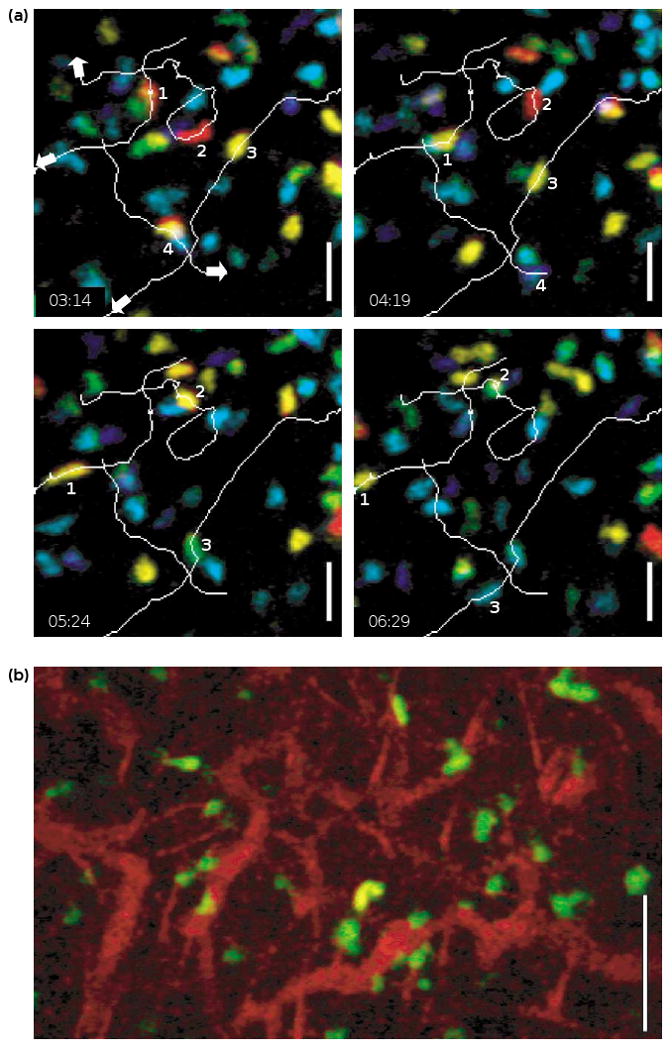
Intravital two-photon images of T cells in the inguinal lymph node of an anesthetized mouse. (a) Trajectories of four separate cells at varying times. The colors represent cells at different depths, ranging from ∼100 to 150 μm below the surface of the lymph node, with blue representing the bottom and red the top of the imaging volume. Scale bar: 25 μm. (b) T cells (green), vessels and fibers (both red) labeled via tail vein injection with tetramethylrhodamine dextran. Scale bar: 50 μm.
Visualizing the interaction between T cells and antigen-presenting cells
Antigen challenge dramatically alters the behavior of T cells, leading to cell enlargement, expression of new genes, secretion of cytokines and cell proliferation. Two different approaches have been used to visualize changes in T-cell dynamics evoked by antigen. When antigen-specific T cells were transferred into animals that had been injected subcutaneously with specific antigen, clusters and swarms of enlarged T cells were observed one day following adoptive transfer [14••]. At later times, cells divided and resumed a vigorous pattern of motility. Using an alternative method of antigen priming, in which APCs were differentiated in vitro from bone marrow cells, pulsed with antigen and then injected subcutaneously, Stoll et al. [15••] observed contact between T cells and APCs that lasted >15 hours in a one-to-one pattern of association.
Recently, we have pursued an in vivo labeling method to visualize antigen-primed DCs interacting with CD4+ T cells (MJ Miller, SH Wei, I Parker, MD Cahalan, unpublished data). If the T cells can be likened to swimming fish, DCs behave effectively as nets; they make contact with T cells by throwing out long membrane tethers and rapidly reeling them back in, constantly changing their shape and greatly expanding their capture radius. It appears from these early studies that the initial encounter between a T cell and a DC relies upon dynamic cell behaviors that are finely tuned to optimize the chance of random collisions.
Conclusions
Two-photon microscopy represents an optimal technique for tracking the behavior of living cells deep within the tissue environment. It is already feasible to image T cells and other cells of the immune system within the circulation, or in the tissue environment of lymph node, spleen, Peyer's patch, thymus and peripheral tissues. Video presentations of the data demonstrate the dynamic behavior of T cells and B cells as they migrate within the lymph node, and of DCs as they interact with T cells during antigen presentation. Two-photon imaging will be adaptable to a wide variety of new probes for second messengers and gene expression, and to a broad range of processes both physiological and pathological. Combined with intravital imaging of surgically exposed lymphoid organs, two-photon imaging is providing a unique view of lymphocyte dynamics in vivo.
Update
A recent study used two-photon microscopy to examine the interaction of dendritic cells labeled in vitro with motile CD8+ T cells in an explanted lymph node preparation [25••]. T cells made stable, long-lasting contacts with antigen-pulsed DCs, rather than a series of short contacts.
Abbreviations
- APC
antigen-presenting cell
- DC
dendritic cell
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Gretz JE, Anderson AO, Shaw S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev. 1997;156:11–24. doi: 10.1111/j.1600-065x.1997.tb00955.x. [DOI] [PubMed] [Google Scholar]
- 2.Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Paper KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 5.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 6.Dustin ML, de Fougerolles AR. Reprogramming T cells: the role of extracellular matrix in coordination of T cell activation and migration. Curr Opin Immunol. 2001;13:286–290. doi: 10.1016/s0952-7915(00)00217-x. [DOI] [PubMed] [Google Scholar]
- 7.Dustin ML, Allen PM, Shaw AS. Environmental control of immunological synapse formation and duration. Trends Immunol. 2001;22:192–194. doi: 10.1016/s1471-4906(01)01872-5. [DOI] [PubMed] [Google Scholar]
- 8.Gunzer M, Schafer A, Borgmann S, Grabbe S, Zanker KS, Brocker EB, Kampgen E, Friedl P. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–332. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 9.Kantele JM, Kurk S, Juntila MA. Effects of continuous exposure to stromal cell-derived factor-1 alpha on T cell rolling and tight adhesion to monolayers of activated endothelial cells. J Immunol. 2000;164:5035–5040. doi: 10.4049/jimmunol.164.10.5035. [DOI] [PubMed] [Google Scholar]
- 10•.Bousso P, Bhakta NR, Lewis RS, Robey E. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science. 2002;296:1876–1880. doi: 10.1126/science.1070945. [DOI] [PubMed] [Google Scholar]; Two-photon microscopy was employed to image motility of and interactions between thymocytes and stromal cells in a reaggregated thymic organ culture system during positive selection.
- 11.Hommel M, Kyewski B. Dynamic changes during the immune response in T cell-antigen-presenting cell clusters isolated from lymph nodes. J Exp Med. 2003;197:269–280. doi: 10.1084/jem.20021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poznansky MC, Evans RH, Foxall RB, Olszak IT, Piascik AH, Hartman KE, Brander C, Meyer TH, Pykett MJ, Chabner KT, et al. Efficient generation of human T cells from a tissue-engineered thymic organoid. Nat Biotechnol. 2000;18:729–734. doi: 10.1038/77288. [DOI] [PubMed] [Google Scholar]
- 13.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and dynamic antigen responses in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]; This paper introduces two-photon microscopy to examine lymphocyte motility and antigen responses in an explanted lymph node preparation. Highly motile T and B cells were observed and shown to have differing velocities in their respective compartments. Changes in response to antigen included T-cell enlargement, formation of stable clusters and swarms, and a resumption of vigorous motility following cell division.
- 15••.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]; Confocal imaging was used to image T cells interacting with DCs in explanted lymph node cultures. Naïve T cells were reportedly immotile, and stable conjugates between T cells and APCs were observed.
- 16.Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 17.Dodd SJ, Williams M, Suhan JP, Williams DS, Koretsky AP, Ho C. Detection of single mammalian cells by high-resolution magnetic resonance imaging. Biophys J. 1999;76:103–109. doi: 10.1016/S0006-3495(99)77182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubey P, Su H, Adonai N, Du S, Rosato A, Braun J, Gambhir SS, Witte ON. Quantitative imaging of the T cell antitumor response by positron-emission tomography. Proc Natl Acad Sci USA. 2003;100:1232–1237. doi: 10.1073/pnas.0337418100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Cahalan MD, Parker I, Wei SH, Miller MJ. Two photon tissue imaging: seeing the immune response in a fresh light. Nature Reviews Immunology. 2002;2:872–880. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review provides a technical description and a comparison of two-photon and confocal imaging methods applied to the immune system.
- 20.Wei SH, Miller MJ, Cahalan MD, Parker I. Two-photon imaging in intact lymphoid tissue. Adv Exp Med Biol. 2002;512:203–208. doi: 10.1007/978-1-4615-0757-4_26. [DOI] [PubMed] [Google Scholar]
- 21.von Andrian UH. Immunology. T cell activation in six dimensions. Science. 2002;296:1815–1817. doi: 10.1126/science.296.5574.1815. [DOI] [PubMed] [Google Scholar]
- 22.Delon J, Stoll S, Germain RN. Imaging of T-cell interactions with antigen presenting cells in culture and in intact lymphoid tissue. Immunol Rev. 2002;189:51–63. doi: 10.1034/j.1600-065x.2002.18906.x. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- 24••.Miller MM, Wei SH, Parker I, Cahalan MD. Autonomous T cell trafficking examined in vivo using intravital two-photon microscopy. Proc Natl Acad Sci USA. 2003;100:2604–2609. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]; The combination of two-photon microscopy with an intravital preparation allowed naïve T cells to be tracked inside the inguinal lymph node of an anesthetized mouse. T-cell migration was randomly distributed in three dimensions, leading to the suggestion that the default antigen recognition algorithm consists of an autonomous random walk through the T-cell zone.
- 25••.Bousso P, Robey E. Dynamics of CD8(+) T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]; The authors of this study used two-photon microscopy to examine the interaction of dendritic cells, labeled in vitro with motile CD8+ T cells, in an explanted lymph node preparation. T cells made stable, long-lasting contacts with antigen-pulsed DCs, rather than a series of short contacts.


