Summary
Objective
GH is usually the first pituitary hormone to be affected following a pathological insult to the pituitary; however, data on the prevalence of GH deficiency in patients with nonsecreting pituitary microadenomas and normal serum IGF-1 levels are scarce. This study aims to evaluate the prevalence of GH and other anterior pituitary hormone deficiencies, and to determine whether microadenomas per se could be associated with reduced GH response rates to GHRH–arginine stimulation.
Design
Analytical, retrospective, two-site case-control study.
Patients and methods
Thirty-eight patients with nonsecreting pituitary microadenomas (mean size 4·2 mm) and normal serum IGF-1 levels were studied. Anterior pituitary function testing, including the GHRH–arginine test to examine GH reserve, was performed in all patients. Serum IGF-1 levels and peak GH levels in the patients that passed the GHRH–arginine test were compared with 22 age- and BMI-matched healthy controls.
Results
Nineteen patients (50%) failed the GHRH–arginine test and had higher body mass index (BMI) than those that passed the GHRH–arginine test and healthy controls. Peak GH levels in patients that passed the GHRH–arginine test were lower compared to healthy controls and 19 patients (50%) had at least one other pituitary hormone deficit. A negative correlation (r = −0·42, P < 0·01) between peak GH levels and BMI was identified, but no correlations were found between peak GH and serum IGF-1 levels.
Conclusions
Our data demonstrated that a substantial number of patients with nonsecreting pituitary microadenomas failed the GHRH–arginine test despite normal serum IGF-1 levels, and had at least one other pituitary hormone deficiency, suggesting that nonsecreting microadenomas may not be clinically harmless. We therefore recommend long-term follow-up with periodic basal pituitary function testing, and to consider dynamic pituitary testing should clinical symptoms arise in these patients.
Introduction
Previous autopsy studies have demonstrated that the prevalence of clinically nonfunctioning pituitary lesions range from 1·5% to 27%,1–4 but with the widespread use of imaging modalities like computed tomography (CT) and magnetic resonance imaging (MRI) scanning, the incidental finding of pituitary masses is increasing in frequency. 5,6 Additionally, some centres are now advocating formal pituitary imaging as part of the full evaluation for pituitary function, including central hypothyroidism 7 and central hypogonadism. 8 The most frequent abnormalities found on imaging are adenomas of the pituitary gland,9,10 and in most cases, the tumour size is < 10 mm (microadenomas). As these lesions tend to be asymptomatic and nonsecreting, they usually escape early detection and previously were only recognized when they become large enough to exert local pressure symptoms to surrounding neurological tissues.
If these pituitary microadenomas are hormonally active, then diagnosis and treatment usually becomes straightforward. ACTH-secreting tumours are usually treated surgically, whereas GH-secreting tumours are treated surgically and/or medically with or without radiation therapy. In contrast, PRL-secreting tumours are almost exclusively treated medically. However, clear guidelines on the diagnosis and management of hormonal deficiencies associated with pituitary microadenomas are not fully established. Following a pathological insult to the pituitary gland such as tumour growth or cranial irradiation, GH is usually the first anterior pituitary hormone to become deficient.11 This could therefore explain the high prevalence of GH deficiency observed in patients with pituitary macroadenomas.12
Adult GH deficiency is now a well-recognized clinical entity13 associated with symptoms of increased central obesity, psychological dysfunction, muscle weakness, increased fatigue, difficulty concentrating and impaired general well-being.14,15 Adult-onset GH deficiency usually results from damage to the pituitary gland that is most commonly caused by surgery and/or radiotherapy for benign pituitary adenomas.16 However, current guidelines proposed by the recently published GH Research Society Consensus Guidelines,17 and the Endocrine Society Clinical Practice Guidelines18 recommend that provocative GH testing within an appropriate clinical context is mandatory for confirmation of the diagnosis and that normal serum IGF-1 levels alone do not necessarily exclude the possibility of adult GH deficiency. For patients with nonsecreting pituitary microadenomas presenting with normal serum IGF-1 levels within the age- and sex-appropriate reference range, no detailed data are available for the prevalence of GH and other pituitary hormone deficiencies in these patients. This study aimed to obtain information on the prevalence of GH and other pituitary hormone deficiencies in patients with nonsecreting pituitary microadenomas presenting with normal serum IGF-1 levels, and to simultaneously determine whether microadenomas per se could be associated with reduced GH response rates to GHRH–arginine stimulation.
Subjects and methods
Subjects
The Endocrinology Department at Oregon Health and Science University (OHSU), Portland, OR and T.C.F’s Endocrinology clinic affiliated with the Charles Drew University (CDU), Los Angeles, CA are two tertiary academic endocrine referral centres. We receive patient referrals from general endocrinologists, primary care physicians, neurologists, rheumatologists and internists primarily for further endocrinological work-up. In certain cases, some of the basal pituitary function testing such as measurement of TSH, free T4, FSH, LH, testosterone, IGF-1, cortisol and MRI of the brain have been performed by the referring physicians. Thus, the referred patients can be divided into (i) patients with biochemical evidence of basal pituitary dysfunction and MRI of the brain that revealed a microadenoma; (ii) patients with MRI of the brain that revealed a microadenoma but their basal pituitary function has not been fully assessed by their referring physician; and (iii) patients with biochemical evidence of basal pituitary dysfunction but not had an MRI of the brain. At presentation to OHSU and the CDU, all patients underwent thorough re-evaluation that included the following: clinical assessment that included visual field acuity and visual field testing by Goldman perimetry, complete basal pituitary function testing that included serum levels of IGF-1, 09·00 h cortisol, PRL, TSH, free T4, FSH, LH, E2 in women and testosterone in men, and MRI of the brain in cases where it has not been previously performed by their referring physicians. All patients with nonsecreting pituitary microadenomas underwent the GHRH–arginine test regardless of their serum IGF-1 levels, whereas in those that had 09·00 h serum cortisol levels < 140 nmol/l in the absence of glucocorticoid therapy, a low-dose 1–24 ACTH stimulation test with 1 µg Cortrosyn® (Amphastar Pharmaceuticals, CA) was subsequently performed.
LH/FSH deficiency was diagnosed on the basis of low or ‘inappropriately normal’ LH and FSH levels combined with serum testosterone below the reference values in adult men and with low serum E2 and oligo-/amenorrhoea in adult women; ACTH deficiency was defined as peak cortisol levels < 500 nmol/l following low dose 1–24 ACTH stimulation testing; and TSH deficiency was defined by low or ‘inappropriately normal’ TSH with free T4 levels below the normal reference values. Patients with microadenomas that had serum IGF-1 levels less than IGF-1 standard deviation score (SDS) −2 or more than +2 were excluded from the study.
Although the insulin tolerance test (ITT) is generally considered the ‘gold standard’ test of choice for evaluating GH reserve, its use has been challenged because of its contraindications particularly in patients with ischaemic heart disease and seizure disorders. With that in mind, we elected to use the GHRH–arginine test to evaluate GH reserve, as this test is considered by many to be the best diagnostic alternative to the ITT.19,20 With evidence accumulating that the diagnostic cut-off levels of the GHRH–arginine test is influenced by body mass index (BMI),21–23 we chose stringent GH cut-off levels based on the most current guidelines proposed by the GH Research Society.17 Thus, for our study, GH deficiency was defined by GH peak ≤ 11·0 µg/l (1 µg/l = 3 mU/l) in lean subjects (BMI < 25 kg/m2), ≤ 8·0 µg/l in overweight subjects (BMI ≥ 25 and < 30 kg/m2) and ≤ 4·0 µg/l in obese subjects (BMI ≥ 30 kg/m2). On the other hand, we did not adjust for gender as its effects on GH response rates to GH stimulation testing has been reported to be inconclusive.24 In addition, other investigators have also proposed that for lean patients with peak GH responses between 9 and 16·5 µg/l, there is a ‘grey area’ that they classify as partial GH deficiency;25,26 however, this classification of partial GH deficiency has not been defined for nonlean individuals.
We also compared the peak GH responses of those that passed the GHRH–arginine test (GH-sufficient patients) with 22 healthy controls, matched for age (± 5 years) and BMI (± 2 kg/m2) status. The healthy control subjects comprised of subjects recruited from advertisements placed in bulletin boards at OHSU, and subjects recruited from a previous study.21 All of the healthy control subjects were thoroughly evaluated to avoid including any subject with other underlying disorders and were not on any other investigational drugs within 6 months of the study. All control subjects had undergone normal growth and development and were not taking any medications.
The Institutional Review Board at OHSU and the CDU deemed that this study was exempt from formal Institutional Review Board review because the study was conducted in accordance with federal regulations [45CFR 46·101(b) (4)]; however, all patients and healthy control subjects whose data were analysed provided written informed consent to the study.
GHRH–arginine protocol
Following a 10-h overnight fast, blood samples were collected for the measurement of serum IGF-1 levels and the subjects then underwent intravenous GHRH–arginine testing between 08·30 and 09·00 h. An indwelling catheter was inserted into a cubital vein and blood was sampled at the time of catheter insertion (0 min). GHRH [1–29 (Geref, Serono, Inc., Norwell, MA); 1 µg/kg] was then administered as an i.v. bolus, and i.v. arginine hydrochloride (30 g) was simultaneously infused from 0 to 30 min. Further blood samples were drawn at 30, 60, 90 and 120 min. All of the blood samples were centrifuged on-site, and the aliquoted serum samples were then sent to Esoterix Laboratory Services, Inc. (Calabasas Hills, CA) in dry ice and stored at −20 °C until assayed for IGF-1 and GH.
Assays
Serum IGF-1 levels were measured by a double antibody radioimmunoassay (RIA) after ethanol extraction with the addition of IGF-2 as a blocking agent. The assay sensitivity was 10 ng/ml, and the coefficient of variation (CV) was 5·4%. The normal age-related range (years) of IGF-1 levels in males from 18 to 20, 21 to 30, 31 to 40, 41 to 50, 51 to 60, 61 to 70, and 71 to 80 was 36·7 to 66·7, 20·3 to 56·5, 17·3 to 43·5, 15·8 to 31·0, 8·9 to 32·0, 7·8 to 28·8 and 4·7 to 28·1 nmol/l, respectively, whereas in women it was 28·4 to 62·1, 11·4 to 48·1, 13·9 to 48·1, 15·4 to 39, 6·9 to 375, 9·8 to 37, 7·1 to 26·8 nmol/l, respectively. For the 17 patients and 22 controls recruited from OHSU, their serum GH levels were measured using an ICMA specific for 22-kDa human GH with a sensitivity of 0·05 µg/l. The intra- and interassay CV ranges were 3·8%–9·1% and 8%–10%, respectively. This assay is calibrated against the World Health Organization 80/505 international GH standard (human pituitary-derived GH) but uses native-sequence recombinant human GH as standard (Eli Lilly and Co., Indianapolis, IN). For the other 21 patients recruited from the CDU, their serum GH levels were measured by a standard double antibody RIA. The sensitivity was 0·3 µg/l, and the intra-assay CV was 3·4% at 8·1 µg/l and 10% at 0·46 µg/l, whereas the interassay CV was 7·2% at 5·6 µg/l and 13% at 0·92 µg/l.
Calculations
The IGF-1 SDS was calculated in relation to age-specific values according to the formula: (patient IGF-1 value – mean of control group)/standard deviation of control group.27 The IGF-1 age-specific reference range comprised of IGF-1 SDS between −2 and +2 (i.e. 2·5–97·5%ile). BMI was calculated by dividing a subject’s weight (in kilograms) by the square of their height (in meters). For the assays used to measure serum GH levels, regression analysis revealed the following relationship between the two assays: GH (ICMA) = 0·52 × GH (RIA) + 0·56 (r2 = 0·94; P < 0·001; n = 116). Therefore, serum GH levels that were measured using an ICMA yields results that are approximately half of those obtained with a standard double antibody RIA (Chandler D.W., Esoterix Laboratory Services, Inc., personal communication).21,28 Hence, the GH levels obtained from patients recruited from the CDU that were analysed using the standard double antibody RIA method were halved to standardize the data with those obtained from OHSU, where all of the GH levels were analysed using ICMA methodology.
Statistical analyses
All statistical analyses were performed using spss for Windows (v10·0, SPSS, Chicago, IL). Distributions of residuals were examined for normality by graphical methods. For peak GH levels, tumour size and IGF-1 SDS data where the residuals were not normally distributed, the Mann–Whitney U-test was employed. For normally distributed residuals, the Student’s unpaired t-test was used. Correlations were sought using Pearson’s test and P-values < 0·05 were considered statistically significant.
Results
Fifty-four patients (45 females, age range 30–68 years) with pituitary microadenomas and no clinical/biochemical evidence of hormonal hypersecretion were identified from the two academic centres (OHSU and the CDU). Of the 54 patients, 38 patients fulfilled the criteria of having pituitary microadenomas and serum IGF-1 levels that were within the age-appropriate normal reference range (IGF-1 SDS between −2 and +2). In 21 out of the 38 patients (55%), pituitary microadenomas were discovered on brain imaging carried out by referring physicians for investigation of symptoms/signs not relevant to a pituitary tumour (indications for imaging included visual symptoms, localized headaches, dizziness, vertigo, migraine, investigation for infertility, investigation for lethargy, weight gain and episodes of loss of consciousness). The remaining 17 patients underwent pituitary imaging based on abnormal laboratory values suggesting underlying pituitary dysfunction. We found that although serum IGF-1 levels between all the patients with pituitary microadenomas and the healthy controls were comparable (14·3 ± 3·5 vs. 15·7 ± 4·6 µg/l), peak GH levels following the GHRH–arginine test (10·2 ± 11·6 vs. 30·9 ± 26·6 µg/l, P < 0·001) and IGF-1 SDS (−1·34 ± 0·51 vs. −0·93 ± 0·53, P < 0·005) were significantly lower in patients with microadenomas.
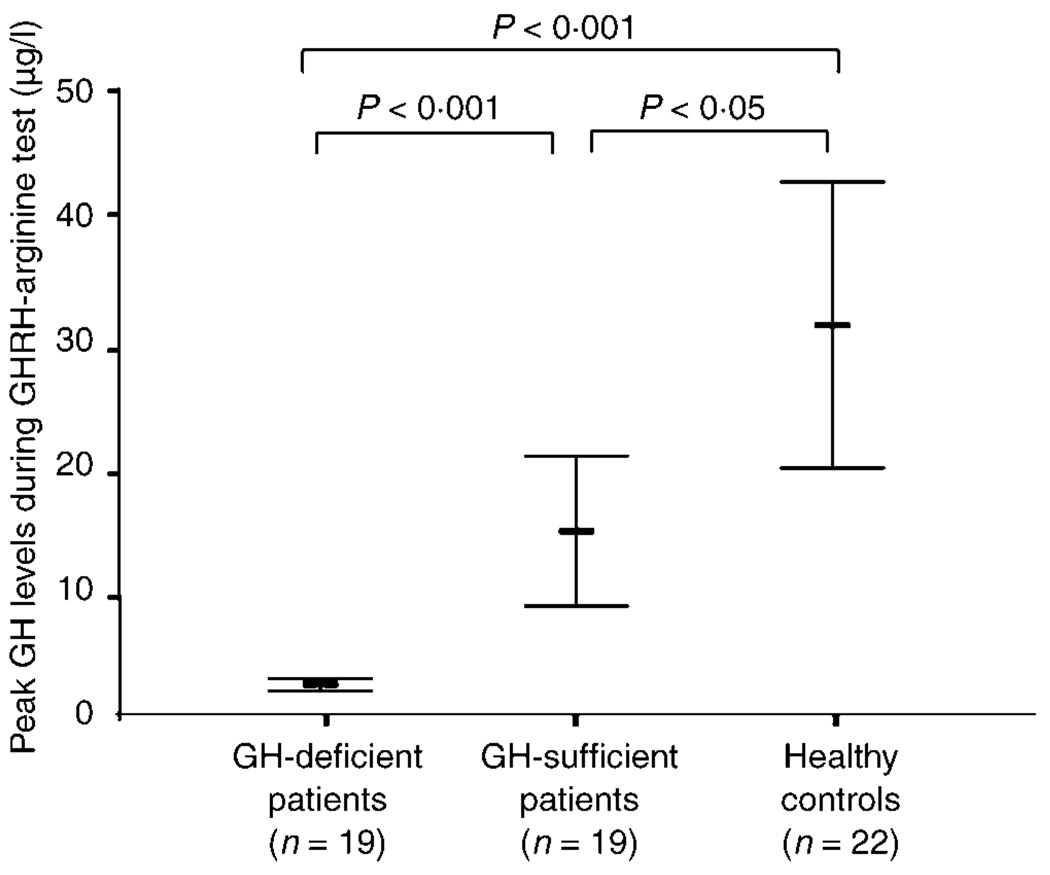
Out of the 38 patients who underwent the GHRH–arginine test, 19 patients (50%) were found to be GH-deficient. The GH-deficient patients had GH peak levels that were significantly lower compared to those who passed the GHRH–arginine test (GH-sufficient patients) and healthy controls (Fig. 1). Peak GH levels were also significantly lower in the GH-sufficient patients when compared to healthy controls (Fig. 1). Detailed characteristics and other relevant demographic and hormonal data of the patients are presented in Table 1.
Fig. 1.
Comparison between peak GH responses of GH-deficient and GH-sufficient patients vs. healthy controls. Symbol represents mean, and whiskers represent 95% confidence intervals.
Table 1.
Detailed profile of the 38 patients with pituitary microadenomas and normal serum IGF-1 levels
| Patient number |
Sex | Age (years) |
BMI (kg/m2) |
MRI pituitary tumour size (mm) |
IGF-1 (nmol/l) |
IGF-1 SDS |
GH peak after GHRH–arginine (µg/l) |
Pituitary hormone deficits |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 39 | 34·1 | 6 | 12·8 | −1·79 | 3·9 | TSH |
| 2 | F | 45 | 50·0 | 6 | 20·5 | −0·80 | 2·3 | TSH |
| 3 | F | 39 | 23·0 | 6 | 15·6 | −1·49 | 3·6 | TSH, ACTH, FSH, LH |
| 4 | F | 50 | 30·1 | 3 | 19·0 | −1·00 | 4·1 | None |
| 5 | F | 50 | 35·8 | 9 | 12·2 | −1·87 | 1·5 | TSH |
| 6 | F | 51 | 40·0 | 3 | 8·4 | −1·96 | 1·5 | TSH |
| 7 | F | 38 | 24·2 | 2·5 | 15·9 | −1·45 | 8·0 | TSH, FSH, LH |
| 8 | F | 53 | 23·8 | 2 | 11·0 | −1·60 | 16·0 | None |
| 9 | F | 59 | 32·5 | 3 | 13·1 | −1·31 | 6·0 | None |
| 10 | F | 35 | 23·2 | 4 | 16·2 | −1·42 | 32·0 | None |
| 11 | F | 38 | 31·6 | 4 | 20·8 | −0·93 | 5·5 | TSH, FSH, LH |
| 12 | F | 38 | 35·3 | 3 | 15·4 | −1·51 | 5·0 | None |
| 13 | F | 35 | 23·5 | 5 | 15·4 | −1·51 | 14·0 | TSH |
| 14 | F | 51 | 36·5 | 5 | 22·5 | 0 | 22·5 | None |
| 15 | F | 52 | 40·2 | 3 | 8·4 | −1·96 | 1·5 | None |
| 16 | F | 30 | 37·7 | 2 | 12·3 | −1·93 | 28·0 | TSH |
| 17 | F | 50 | 30·6 | 2·5 | 14·8 | −1·53 | 7·0 | None |
| 18 | F | 51 | 36·5 | 5 | 22·5 | 0 | 18·3 | None |
| 19 | F | 39 | 40·1 | 2 | 12·4 | −1·85 | 4·0 | None |
| 20 | F | 54 | 32·2 | 6 | 10·3 | −1·72 | 2·3 | None |
| 21 | F | 47 | 49·5 | 3 | 11·4 | −1·70 | 1·5 | TSH |
| 22 | F | 43 | 31·3 | 3 | 14·3 | −1·33 | 23·0 | TSH, FSH, LH |
| 23 | F | 52 | 51·3 | 5 | 10·5 | −1·69 | 3·6 | None |
| 24 | F | 54 | 34·5 | 2 | 13·1 | −1·16 | 4·9 | None |
| 25 | F | 56 | 32·7 | 5 | 11·1 | −1·39 | 14·3 | TSH |
| 26 | F | 53 | 38·4 | 2 | 16·5 | −0·48 | 3·7 | TSH |
| 27 | F | 50 | 39·3 | 2 | 14·0 | −1·19 | 3·0 | None |
| 28 | F | 30 | 28·1 | 6 | 19·9 | −0·83 | 21·6 | None |
| 29 | F | 57 | 22·7 | 2 | 12·3 | −1·14 | 52·5 | None |
| 30 | F | 44 | 26·0 | 4 | 14·8 | −1·23 | 36·3 | None |
| 31 | M | 39 | 24·1 | 4 | 14·4 | −1·87 | 2·3 | TSH, FSH, LH |
| 32 | M | 45 | 29·2 | 5 | 16·9 | −0·74 | 4·1 | TSH, FSH, LH |
| 33 | M | 50 | 19·7 | 3 | 11·8 | −1·67 | 5·4 | None |
| 34 | M | 42 | 36·3 | 7 | 12·0 | −1·73 | 7·1 | FSH, LH |
| 35 | M | 56 | 32·1 | 5 | 12·8 | −1·03 | 2·4 | FSH, LH |
| 36 | M | 33 | 42·1 | 6 | 14·0 | −1·69 | 2·7 | FSH, LH |
| 37 | M | 63 | 27·3 | 9 | 12·6 | −0·58 | 10 | FSH, LH |
| 38 | M | 30 | 42·1 | 4 | 12·4 | −1·91 | 1·8 | None |
| Mean ± SD | 45·8 ± 8·8 | 33·4 ± 7·9 | 4·2 ± 1·9 | 14·3 ± 3·5 | −1·34 ± 0·51 | 10·2 ± 11·6 |
TSH, thyroid-stimulating hormone; ACTH, adrenocorticotrophic hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; F, female; M, male. Peak GH responses in GH-deficient patients and partial GH-deficient patients are noted in bold and italics, respectively.
The GH-deficient patients had higher BMIs than the GH-sufficient patients and healthy controls (Table 2). Despite the serum IGF-1 levels and IGF-1 SDS of the GH-deficient patients being within the age-appropriate reference range, these levels were still significantly lower compared to the GH-sufficient patients and controls (Table 2). However, the serum IGF-1 levels and IGF-1 SDS of the GH-sufficient patients were similar to the controls. Based on criteria proposed by previous investigators for the GHRH–arginine test,25,26,29 patient numbers 8 and 13 could be classified as having partial GH deficiency (Table 1).
Table 2.
Comparison between patients and healthy controls
| Patients (n = 38) | |||
|---|---|---|---|
| GH-deficient (50%) | GH-sufficient (50%) | Controls (n = 22) | |
| Gender | 6 males/13 females | 2 males/17 females | 5 males/17 females |
| Age (years) | 45·4 ± 7·6 | 46·3 ± 10·0 | 45·6 ± 12·4 |
| BMI (kg/m2) | 36·2 ± 9·2 | 30·5 ± 5·0* | 28·8 ± 7·3** |
| Tumour size (mm) | 4·3 ± 1·9 | 4·0 ± 1·9 | – |
| Serum IGF-1 (nmol/ l) | 13·2 ± 3·0 | 15·4 ± 3·7 | 15·7 ± 4·6 |
| IGF-1 SDS | −1·52 ± 0·45 | −1·16 ± 0·52* | −0·93 ± 0·53*** |
| No. of patients with other pituitary hormone deficits | |||
| 1/TSH | 10 | 5 | |
| 2/ACTH | 1 | 0 | |
| 3/LH/FSH | 7 | 3 | |
Data are presented as mean ± SD.
P < 0·05 vs. GH-deficient patients;
P < 0·01 vs. GH-deficient patients;
P < 0·001 vs. GH-deficient patients.
Other pituitary hormone deficiencies were found in 19 patients (50%) (15 patients were TSH-deficient, 10 patients were FSH/LH-deficient and 1 patient was ACTH-deficient), while the remaining 19 patients had no detectable basal pituitary hormone deficiencies (Tables 1 and 2). All patients had normal PRL levels, and we did not specifically examine for hypoandrogenism in women in this study. The number of other defects in GH-deficient and GH-sufficient subjects is listed in Table 2.
A negative correlation (r = −0·42, P < 0·01) between peak GH levels and BMI was also identified; however, no significant correlations were found between IGF-1 and peak GH levels (r = 0·17, P = 0·30), and between tumour size and serum IGF-1 levels (r = 0·11, P = 0·52), IGF-1 SDS (r = 0·13, P = 0·44) and peak GH levels after GHRH–arginine test (r = − 0·17, P = 0·31). There was no difference in peak GH levels or serum IGF-1 levels between the group who had tumours found on MRI performed by their referring physicians and those who had an MRI performed after clinical and biochemical assessment at OHSU and the CDU.
Discussion
To our knowledge, this is the first study to evaluate the GH response rates to GHRH–arginine testing in patients with pituitary microadenomas and normal serum IGF-1 levels. Our study had two primary objectives. First, this study was aimed at determining the prevalence of patients with microadenomas and normal serum IGF-1 levels that demonstrated biochemical evidence of GH deficiency using stringent criteria based on the patient’s BMI and other pituitary hormone deficiencies. Second, this study simultaneously examined whether pituitary microadenomas per se could be associated with reduced GH response rates to GHRH–arginine stimulation.
Our results have shown that, despite normal serum IGF-1 levels, 50% of patients failed the GHRH–arginine test. We found that the prevalence of reduced hormonal secretion in subjects with microadenomas was most frequent for somatotrophs, followed by thyrotrophs, followed by gonadotrophs and rarely of corticotrophs; data that are comparable to previously published studies.30,31 Furthermore, we found that peak GH levels in the GH-sufficient individuals with underlying microadenomas were significantly lower compared to the age- and BMI-matched controls despite having normal serum IGF-1 levels, suggesting that perhaps the secretory capacity of endogenous GH secretion decreased first before endogenous IGF-1 secretion started to decline. In support of this notion, the lack of correlation of IGF-1 and peak GH levels found in our study suggests that IGF-1 is probably not a reliable marker to help guide clinicians in deciding whether or not to evaluate the GH reserve more thoroughly in patients with nonsecreting microadenomas, particularly in the absence of relevant clinical symptoms. In contrast, as the diagnosis of the adult GH deficiency syndrome requires biochemical evidence with GH stimulation testing in the appropriate clinical context,17,18 our study has also highlighted the importance to undertake long-term follow-up and periodic biochemical evaluation of GH and other pituitary hormone axes in these patients.
In many previous studies, the GH reserve was not assessed despite the fact that it is well known that somatotroph function is disturbed very early in cases of damage or irradiation to the pituitary gland. However, there is now an increasing body of evidence that nonsecreting small lesions within the pituitary gland may be more clinically significant than originally thought. For instance, hypopituitarism has been reported to occur in an estimated frequency of 4·21 cases/100 000 (95% CI, 2·95–5·47) in the absence of large pituitary lesions.32 In a recent study by Colao et al. evaluating the peak GH responses in a group of patients with partial GH deficiency and predominantly clinically nonsecreting pituitary adenomas, they reported that a peak GH response of 11·5 µg/l or less and IGF-1 SDS of −0·28 or less was highly predictive of delayed deterioration of GH secretion.25 In recognizing that the impact of impaired GH secretion should not be underestimated, the recently published GH Research Society Consensus Guidelines17 are now recommending that ongoing clinical evaluation and repeat endocrine testing should be undertaken in patients with partial GH deficiency regardless of the underlying pituitary aetiology. Reassuringly, many series describing nonsecreting pituitary microadenomas have confirmed the low probability of enlargement of this type of tumour,5,33,34 suggesting that these tumours probably have a benign course over time.
In line with previous studies,21,23 we have also found that stimulated GH secretion to the GHRH–arginine test negatively correlated with BMI in GH-deficient adults. However, it remains unclear if this relationship holds true in cases of severe obesity (BMI > 40 kg/m2) such as the seven subjects in our study (patient numbers 2, 6, 15, 19, 21, 23, 36 and 38), where the BMI effect could theoretically have more of an impact on stimulated GH secretion compared to patients with BMIs between 30 and 40 kg/m2. Nevertheless, those seven patients would still be deemed GH-deficient with currently available data,21,23 and current guidelines.17,18 Therefore, unless more data becomes available in the future to demonstrate that even lower cut-offs should be used in patients with severe obesity following GHRH–arginine testing, using the set cut-off level of 4·0 µg/l will continue to fuel the debate as to how to interpret the GH responses to the GHRH–arginine test in these patients.
Some of the caveats of this study include the fact that as the two participating centres were referral centres from other specialists, it is conceivable that the data might be more representative of patients with more complex diseases and not to the overall population of patients with microadenomas. Another limitation of this study is that, despite all of the GH analyses were performed by Esoterix Laboratory Services, two different assays were used (i.e. ICMAs for the patients recruited from OHSU and RIAs for the patients recruited from the CDU). Serum GH levels measured using ICMAs are reported to be approximately half of those measured using a standard double antibody RIA (Chandler D.W., Esoterix Laboratory Services Inc., personal communication).21,28 Nonetheless, even without halving the GH values obtained using RIAs, we still found a substantial number (37%) of GH-deficient patients in our patient population. Furthermore, the prevalence of GH deficiency were comparable between the patients that had their GH levels analysed using the ICMA methodology (52·9%) compared to those that had their GH levels analysed using the RIA methodology (47·6%), suggesting that assay methodology was not a confounder in the analysis of this data. Finally, as obesity can potentially affect serum testosterone in part due to low sex hormone binding globulin levels, and may act as a confounding factor in diagnosing ‘true’ central hypogonadism, it is worth noting that four obese patients in our study population had concurrently low gonadotrophin levels (Table 1). However, on further questioning at presentation, we found that these patients had varying degrees of erectile dysfunction and decreased libido to support their biochemical evidence of central hypogonadism.
In conclusion, our data have demonstrated that a high percentage of patients with nonsecreting pituitary microadenomas were GH-deficient, as defined by an impaired response to GHRH–arginine testing, despite normal serum IGF-1 levels, and had at least one other anterior pituitary hormone deficit. We therefore recommend that in all patients presenting with nonsecreting pituitary microadenomas, regardless of their presenting serum IGF-1 levels, long-term follow-up with periodic biochemical evaluation of basal pituitary function should be undertaken and to consider dynamic pituitary testing of the GH and ACTH axes if relevant clinical symptoms arise as this will provide an opportunity for clinicians to decide whether these patients should be started on hormone replacement therapies should hormonal deficiencies be detected during the course of follow-up.
Acknowledgements
K.C.J.Y. is supported by research grants from Pfizer Ltd., and the GH and IGF Research Society Fellowship. T.C.F. was supported in part by the following grants: U54 RR14616, S06 G068510, U54 HD41748 and R25 RR019488.
References
- 1.Auer RN, Alakija P, Sutherland GR. Asymptomatic large pituitary adenomas discovered at autopsy. Surgical Neurology. 1996;46:28–31. doi: 10.1016/0090-3019(96)00085-7. [DOI] [PubMed] [Google Scholar]
- 2.Camaris C, Balleine R, Little D. Microadenomas of the human pituitary. Pathology. 1995;27:8–11. doi: 10.1080/00313029500169382. [DOI] [PubMed] [Google Scholar]
- 3.Kontogeorgos G, Kovacs K, Horvath E, Scheithauer BW. Multiple adenomas of the human pituitary. A retrospective autopsy study with clinical implications. Journal of Neurosurgery. 1991;74:243–247. doi: 10.3171/jns.1991.74.2.0243. [DOI] [PubMed] [Google Scholar]
- 4.Teramoto A, Hirakawa K, Sanno N, Osamura Y. Incidental pituitary lesions in 1000 unselected autopsy specimens. Radiology. 1994;193:161–164. doi: 10.1148/radiology.193.1.8090885. [DOI] [PubMed] [Google Scholar]
- 5.Donovan LE, Corenblum B. The natural history of the pituitary incidentaloma. Archives of Internal Medicine. 1995;155:181–183. [PubMed] [Google Scholar]
- 6.Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Annals of Internal Medicine. 1994;120:817–820. doi: 10.7326/0003-4819-120-10-199405150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Mehta A, Hindmarsh PC, Stanhope RG, Brain CE, Preece MA, Dattani MT. Is the thyrotropin-releasing hormone test necessary in the diagnosis of central hypothyroidism in children. Journal of Clinical Endocrinology and Metabolism. 2003;88:5696–5703. doi: 10.1210/jc.2003-030943. [DOI] [PubMed] [Google Scholar]
- 8.Dobs AS, El-Deiry S, Wand G, Wiederkehr M. Central hypogonadism: distinguishing idiopathic low testosterone from pituitary tumors. Endocrine Practice. 1998;4:355–359. doi: 10.4158/EP.4.6.355. [DOI] [PubMed] [Google Scholar]
- 9.Molitch ME. Clinical review 65. Evaluation and treatment of the patient with a pituitary incidentaloma. Journal of Clinical Endocrinology and Metabolism. 1995;80:3–6. doi: 10.1210/jcem.80.1.7829630. [DOI] [PubMed] [Google Scholar]
- 10.Reincke M, Allolio B, Saeger W, Menzel J, Winkelmann W. The ‘incidentaloma’ of the pituitary gland. Is neurosurgery required? Journal of the American Medical Association. 1990;263:2772–2776. [PubMed] [Google Scholar]
- 11.Shalet SM, Toogood A, Rahim A, Brennan BM. The diagnosis of growth hormone deficiency in children and adults. Endocrine Reviews. 1998;19:203–223. doi: 10.1210/edrv.19.2.0329. [DOI] [PubMed] [Google Scholar]
- 12.Lamberton RP, Jackson IM. Investigation of hypothalamic-pituitary disease. Clinics of Endocrinology and Metabolism. 1983;12:509–534. doi: 10.1016/s0300-595x(83)80054-1. [DOI] [PubMed] [Google Scholar]
- 13.Cuneo RC, Salomon F, McGauley GA, Sonksen PH. The growth hormone deficiency syndrome in adults. Clinical Endocrinology. 1992;37:387–397. doi: 10.1111/j.1365-2265.1992.tb02347.x. [DOI] [PubMed] [Google Scholar]
- 14.Carroll PV, Christ ER, Bengtsson BA, Carlsson L, Christiansen JS, Clemmons D, Hintz R, Ho K, Laron Z, Sizonenko P, Sonksen PH, Tanaka T, Thorne M. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. Journal of Clinical Endocrinology and Metabolism. 1998;83:382–395. doi: 10.1210/jcem.83.2.4594. [DOI] [PubMed] [Google Scholar]
- 15.Simpson H, Savine R, Sonksen P, Bengtsson BA, Carlsson L, Christiansen JS, Clemmons D, Cohen P, Hintz R, Ho K, Mullis P, Robinson I, Strasburger C, Tanaka T, Thorner M. Growth hormone replacement therapy for adults: into the new millennium. Growth Hormone and IGF Research. 2002;12:1–33. doi: 10.1054/ghir.2001.0263. [DOI] [PubMed] [Google Scholar]
- 16.Feldt-Rasmussen U, Abstract R, Bengtsson BA, Bennmarker H, Bramnert M, Hernberg-Stahl E, Monson JP, Westberg B, Wilton P, Wuster C. Growth hormone deficiency and replacement in hypopituitary patients previously treated for acromegaly or Cushing’s disease. European Journal of Endocrinology. 2002;146:67–74. doi: 10.1530/eje.0.1460067. [DOI] [PubMed] [Google Scholar]
- 17.Ho KK. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. European Journal of Endocrinology. 2007;157:695–700. doi: 10.1530/EJE-07-0631. [DOI] [PubMed] [Google Scholar]
- 18.Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, Vance ML, Stephens PA. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. Journal of Clinical Endocrinology and Metabolism. 2006;91:1621–1634. doi: 10.1210/jc.2005-2227. [DOI] [PubMed] [Google Scholar]
- 19.Valetto MR, Bellone J, Baffoni C, Savio P, Aimaretti G, Gianotti L, Arvat E, Camanni F, Ghigo E. Reproducibility of the growth hormone response to stimulation with growth hormone-releasing hormone plus arginine during lifespan. European Journal of Endocrinology. 1996;135:568–572. doi: 10.1530/eje.0.1350568. [DOI] [PubMed] [Google Scholar]
- 20.Aimaretti G, Corneli G, Razzore P, Bellone S, Baffoni C, Arvat E, Camanni F, Ghigo E. Comparison between insulin-induced hypoglycemia and growth hormone (GH)-releasing hormone + arginine as provocative tests for the diagnosis of GH deficiency in adults. Journal of Clinical Endocrinology and Metabolism. 1998;83:1615–1618. doi: 10.1210/jcem.83.5.4837. [DOI] [PubMed] [Google Scholar]
- 21.Biller BM, Samuels MH, Zagar A, Cook DM, Arafah BM, Bonert V, Stavrou S, Kleinberg DL, Chipman JJ, Hartman ML. Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. Journal of Clinical Endocrinology and Metabolism. 2002;87:2067–2079. doi: 10.1210/jcem.87.5.8509. [DOI] [PubMed] [Google Scholar]
- 22.Bonert VS, Elashoff JD, Barnett P, Melmed S. Body mass index determines evoked growth hormone (GH) responsiveness in normal healthy male subjects: diagnostic caveat for adult GH deficiency. Journal of Clinical Endocrinology and Metabolism. 2004;89:3397–3401. doi: 10.1210/jc.2003-032213. [DOI] [PubMed] [Google Scholar]
- 23.Corneli G, Di Somma C, Baldelli R, Rovere S, Gasco V, Croce CG, Grottoli S, Maccario M, Colao A, Lombardi G, Ghigo E, Camanni F, Aimaretti G. The cut-off limits of the GH response to GH-releasing hormone–arginine test related to body mass index. European Journal of Endocrinology. 2005;153:257–264. doi: 10.1530/eje.1.01967. [DOI] [PubMed] [Google Scholar]
- 24.Qu X-D, Gaw Gonzalo IT, Al Sayed MY, Cohan P, Christenson PD, Swerdloff RS, Kelly DF, Wang C. Influence of body mass index and gender on growth hormone (GH) responses to GH-releasing hormone plus arginine and insulin tolerance tests. Journal of Clinical Endocrinology and Metabolism. 2005;90:1563–1569. doi: 10.1210/jc.2004-1450. [DOI] [PubMed] [Google Scholar]
- 25.Colao A, Di Somma C, Spiezia S, Rota F, Pivonello R, Savastano S, Lombardi G. The natural history of partial growth hormone deficiency in adults: a prospective study on the cardiovascular risk and atherosclerosis. Journal of Clinical Endocrinology and Metabolism. 2006;91:2191–2200. doi: 10.1210/jc.2005-2566. [DOI] [PubMed] [Google Scholar]
- 26.Murray RD, Adams JE, Shalet SM. Adults with partial growth hormone deficiency have an adverse body composition. Journal of Clinical Endocrinology and Metabolism. 2004;89:1586–1591. doi: 10.1210/jc.2003-030761. [DOI] [PubMed] [Google Scholar]
- 27.Blum WF, Albertsson-Wikland K, Rosberg S, Ranke MB. Serum levels of insulin-like growth factor I (IGF-I) and IGF binding protein 3 reflect spontaneous growth hormone secretion. Journal of Clinical Endocrinology and Metabolism. 1993;76:1610–1616. doi: 10.1210/jcem.76.6.7684744. [DOI] [PubMed] [Google Scholar]
- 28.Hartman ML, Crowe BJ, Biller BM, Ho KK, Clemmons DR, Chipman JJ. Which patients do not require a GH stimulation test for the diagnosis of adult GH deficiency? Journal of Clinical Endocrinology and Metabolism. 2002;87:477–485. doi: 10.1210/jcem.87.2.8216. [DOI] [PubMed] [Google Scholar]
- 29.Murray RD, Adams JE, Shalet SM. A densitometric and morphometric analysis of the skeleton in adults with varying degrees of growth hormone deficiency. Journal of Clinical Endocrinology and Metabolism. 2006;91:432–438. doi: 10.1210/jc.2005-0897. [DOI] [PubMed] [Google Scholar]
- 30.Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101:613–619. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 31.Ferrante E, Ferraroni M, Castrignano T, Menicatti L, Anagni M, Reimondo G, Del Monte P, Bernasconi D, Loli P, Faustini-Fustini M, Borretta G, Terzolo M, Losa M, Morabito A, Spada A, Beck-Peccoz P, Lania AG. Non-functioning pituitary adenoma database: a useful resource to improve the clinical management of pituitary tumors. European Journal of Endocrinology. 2006;155:823–829. doi: 10.1530/eje.1.02298. [DOI] [PubMed] [Google Scholar]
- 32.Regal M, Paramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clinical Endocrinology. 2001;55:735–740. doi: 10.1046/j.1365-2265.2001.01406.x. [DOI] [PubMed] [Google Scholar]
- 33.Karavitaki N, Collison K, Halliday J, Byrne JV, Price P, Cudlip S, Wass JA. What is the natural history of nonoperated nonfunctioning pituitary adenomas? Clinical Endocrinology. 2007;67:938–943. doi: 10.1111/j.1365-2265.2007.02990.x. [DOI] [PubMed] [Google Scholar]
- 34.Sanno N, Oyama K, Tahara S, Teramoto A, Kato Y. A survey of pituitary incidentaloma in Japan. European Journal of Endocrinology. 2003;149:123–127. doi: 10.1530/eje.0.1490123. [DOI] [PubMed] [Google Scholar]