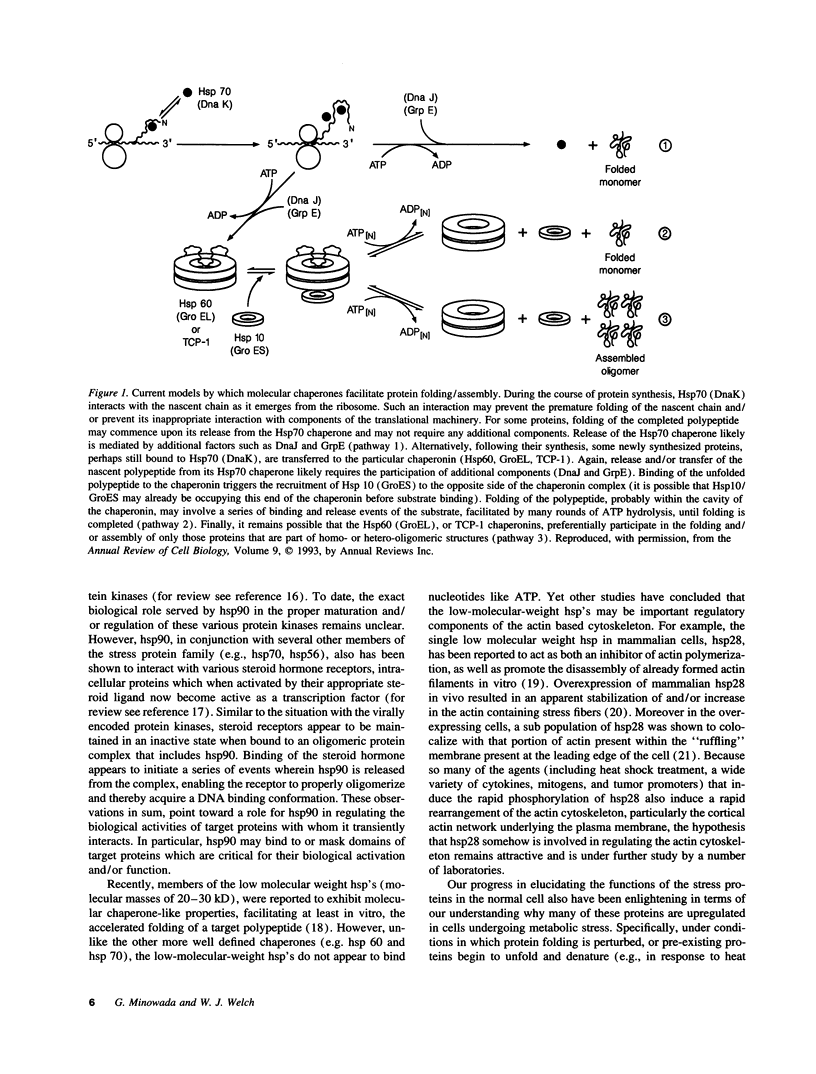
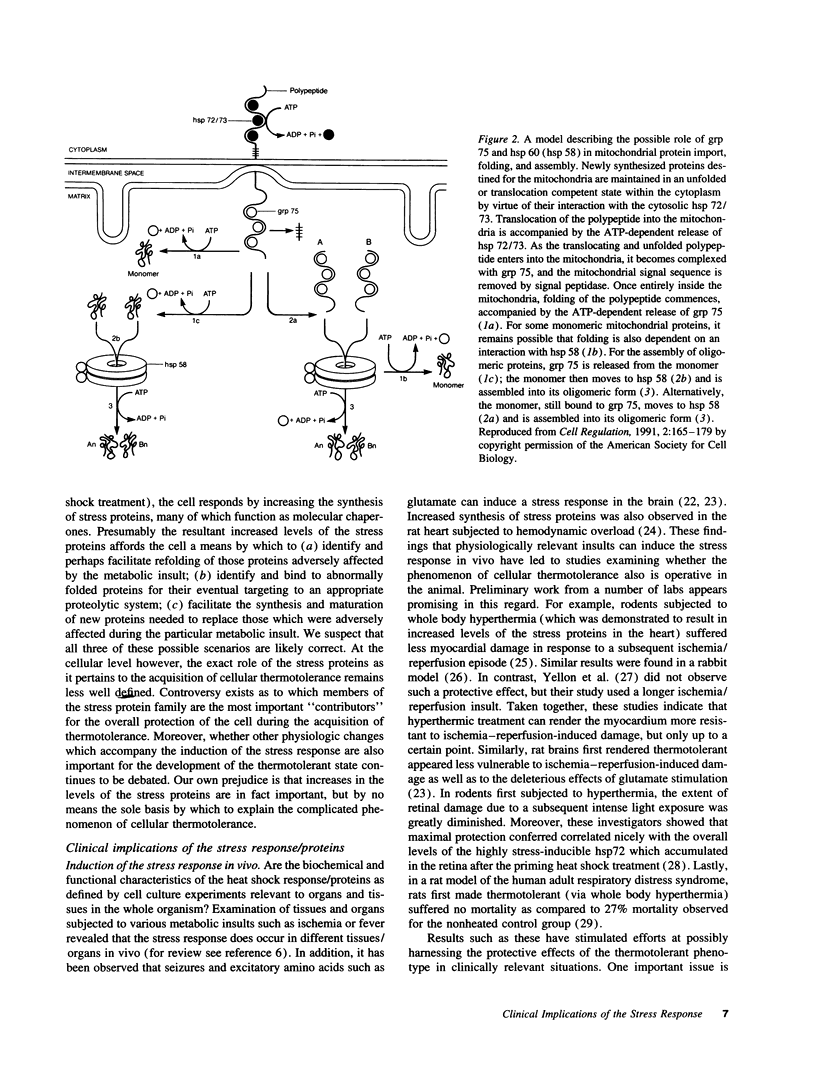
Abstract
A field of research that began with a curious observation in Drosophila has resulted in a new understanding of how cells respond to sudden and adverse changes in their environment. In addition through the study of the structure/function of the stress proteins, especially those which function as molecular chaperones, new insights into the details by which proteins are synthesized and acquire their final biologically active conformation have been realized. Equally exciting is the progress being made as it relates the potential diagnostic and therapeutic applications of the stress-response proteins. The use of stress proteins as the next generation of vaccines and/or their use as potentially powerful adjuvants, capable of stimulating both T and B cell responses to a particular antigen of interest appear close to becoming a reality. One wonders how many more surprises are in store for us as we continue to explore this evolutionally conserved cellular stress response.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinsen C. B. Principles that govern the folding of protein chains. Science. 1973 Jul 20;181(4096):223–230. doi: 10.1126/science.181.4096.223. [DOI] [PubMed] [Google Scholar]
- Barbe M. F., Tytell M., Gower D. J., Welch W. J. Hyperthermia protects against light damage in the rat retina. Science. 1988 Sep 30;241(4874):1817–1820. doi: 10.1126/science.3175623. [DOI] [PubMed] [Google Scholar]
- Barrios C., Lussow A. R., Van Embden J., Van der Zee R., Rappuoli R., Costantino P., Louis J. A., Lambert P. H., Del Giudice G. Mycobacterial heat-shock proteins as carrier molecules. II: The use of the 70-kDa mycobacterial heat-shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette Guérin priming. Eur J Immunol. 1992 Jun;22(6):1365–1372. doi: 10.1002/eji.1830220606. [DOI] [PubMed] [Google Scholar]
- Blake M. J., Buckley D. J., Buckley A. R. Dopaminergic regulation of heat shock protein-70 expression in adrenal gland and aorta. Endocrinology. 1993 Mar;132(3):1063–1070. doi: 10.1210/endo.132.3.8095012. [DOI] [PubMed] [Google Scholar]
- Blake M. J., Udelsman R., Feulner G. J., Norton D. D., Holbrook N. J. Stress-induced heat shock protein 70 expression in adrenal cortex: an adrenocorticotropic hormone-sensitive, age-dependent response. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9873–9877. doi: 10.1073/pnas.88.21.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander S. J., Horwitz M. A. Major cytoplasmic membrane protein of Legionella pneumophila, a genus common antigen and member of the hsp 60 family of heat shock proteins, induces protective immunity in a guinea pig model of Legionnaires' disease. J Clin Invest. 1993 Feb;91(2):717–723. doi: 10.1172/JCI116253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. doi: 10.1007/978-3-642-70810-7_1. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Cohen I. R. Autoimmunity to chaperonins in the pathogenesis of arthritis and diabetes. Annu Rev Immunol. 1991;9:567–589. doi: 10.1146/annurev.iy.09.040191.003031. [DOI] [PubMed] [Google Scholar]
- Currie R. W., Tanguay R. M., Kingma J. G., Jr Heat-shock response and limitation of tissue necrosis during occlusion/reperfusion in rabbit hearts. Circulation. 1993 Mar;87(3):963–971. doi: 10.1161/01.cir.87.3.963. [DOI] [PubMed] [Google Scholar]
- De Graeff-Meeder E. R., van der Zee R., Rijkers G. T., Schuurman H. J., Kuis W., Bijlsma J. W., Zegers B. J., van Eden W. Recognition of human 60 kD heat shock protein by mononuclear cells from patients with juvenile chronic arthritis. Lancet. 1991 Jun 8;337(8754):1368–1372. doi: 10.1016/0140-6736(91)93057-g. [DOI] [PubMed] [Google Scholar]
- DeNagel D. C., Pierce S. K. Heat shock proteins in immune responses. Crit Rev Immunol. 1993;13(1):71–81. [PubMed] [Google Scholar]
- Delcayre C., Samuel J. L., Marotte F., Best-Belpomme M., Mercadier J. J., Rappaport L. Synthesis of stress proteins in rat cardiac myocytes 2-4 days after imposition of hemodynamic overload. J Clin Invest. 1988 Aug;82(2):460–468. doi: 10.1172/JCI113619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly T. J., Sievers R. E., Vissern F. L., Welch W. J., Wolfe C. L. Heat shock protein induction in rat hearts. A role for improved myocardial salvage after ischemia and reperfusion? Circulation. 1992 Feb;85(2):769–778. doi: 10.1161/01.cir.85.2.769. [DOI] [PubMed] [Google Scholar]
- Dubois P., Dedet J. P., Fandeur T., Roussilhon C., Jendoubi M., Pauillac S., Mercereau-Puijalon O., Pereira Da Silva L. Protective immunization of the squirrel monkey against asexual blood stages of Plasmodium falciparum by use of parasite protein fractions. Proc Natl Acad Sci U S A. 1984 Jan;81(1):229–232. doi: 10.1073/pnas.81.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., van der Vies S. M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Ensgraber M., Loos M. A 66-kilodalton heat shock protein of Salmonella typhimurium is responsible for binding of the bacterium to intestinal mucus. Infect Immun. 1992 Aug;60(8):3072–3078. doi: 10.1128/iai.60.8.3072-3078.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A. Mucosal immunology. Immunol Today. 1990 Jan;11(1):1–3. doi: 10.1016/0167-5699(90)90002-q. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C., Welch W. J. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Gomez F. J., Gomez A. M., Deepe G. S., Jr An 80-kilodalton antigen from Histoplasma capsulatum that has homology to heat shock protein 70 induces cell-mediated immune responses and protection in mice. Infect Immun. 1992 Jul;60(7):2565–2571. doi: 10.1128/iai.60.7.2565-2571.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. The multidrug transporter, a double-edged sword. J Biol Chem. 1988 Sep 5;263(25):12163–12166. [PubMed] [Google Scholar]
- Hartl F. U., Martin J., Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. 1992;21:293–322. doi: 10.1146/annurev.bb.21.060192.001453. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980 Mar;102(3):407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- Hosokawa N., Hirayoshi K., Nakai A., Hosokawa Y., Marui N., Yoshida M., Sakai T., Nishino H., Aoike A., Kawai K. Flavonoids inhibit the expression of heat shock proteins. Cell Struct Funct. 1990 Dec;15(6):393–401. doi: 10.1247/csf.15.393. [DOI] [PubMed] [Google Scholar]
- Jakob U., Gaestel M., Engel K., Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993 Jan 25;268(3):1517–1520. [PubMed] [Google Scholar]
- Kaufman D. L., Clare-Salzler M., Tian J., Forsthuber T., Ting G. S., Robinson P., Atkinson M. A., Sercarz E. E., Tobin A. J., Lehmann P. V. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993 Nov 4;366(6450):69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and autoimmunity: facts or fiction? Curr Biol. 1991 Dec;1(6):359–361. doi: 10.1016/0960-9822(91)90192-y. [DOI] [PubMed] [Google Scholar]
- Koenig W. J., Lohner R. A., Perdrizet G. A., Lohner M. E., Schweitzer R. T., Lewis V. L., Jr Improving acute skin-flap survival through stress conditioning using heat shock and recovery. Plast Reconstr Surg. 1992 Oct;90(4):659–664. doi: 10.1097/00006534-199210000-00016. [DOI] [PubMed] [Google Scholar]
- Kubota H., Hynes G., Carne A., Ashworth A., Willison K. Identification of six Tcp-1-related genes encoding divergent subunits of the TCP-1-containing chaperonin. Curr Biol. 1994 Feb 1;4(2):89–99. doi: 10.1016/s0960-9822(94)00024-2. [DOI] [PubMed] [Google Scholar]
- Langer T., Pfeifer G., Martin J., Baumeister W., Hartl F. U. Chaperonin-mediated protein folding: GroES binds to one end of the GroEL cylinder, which accommodates the protein substrate within its central cavity. EMBO J. 1992 Dec;11(13):4757–4765. doi: 10.1002/j.1460-2075.1992.tb05581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie J. N., Gingras-Breton G., Tanguay R. M., Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993 Feb 15;268(5):3420–3429. [PubMed] [Google Scholar]
- Lavoie J. N., Hickey E., Weber L. A., Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993 Nov 15;268(32):24210–24214. [PubMed] [Google Scholar]
- Li Z., Srivastava P. K. Tumor rejection antigen gp96/grp94 is an ATPase: implications for protein folding and antigen presentation. EMBO J. 1993 Aug;12(8):3143–3151. doi: 10.1002/j.1460-2075.1993.tb05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lis J., Wu C. Protein traffic on the heat shock promoter: parking, stalling, and trucking along. Cell. 1993 Jul 16;74(1):1–4. doi: 10.1016/0092-8674(93)90286-y. [DOI] [PubMed] [Google Scholar]
- Lussow A. R., Barrios C., van Embden J., Van der Zee R., Verdini A. S., Pessi A., Louis J. A., Lambert P. H., Del Giudice G. Mycobacterial heat-shock proteins as carrier molecules. Eur J Immunol. 1991 Oct;21(10):2297–2302. doi: 10.1002/eji.1830211002. [DOI] [PubMed] [Google Scholar]
- Miron T., Vancompernolle K., Vandekerckhove J., Wilchek M., Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991 Jul;114(2):255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler S. G., Tepper M. A., Schacter B., Mazzucco C. E. Interaction of the immunosuppressant deoxyspergualin with a member of the Hsp70 family of heat shock proteins. Science. 1992 Oct 16;258(5081):484–486. doi: 10.1126/science.1411548. [DOI] [PubMed] [Google Scholar]
- Pratt W. B. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993 Oct 15;268(29):21455–21458. [PubMed] [Google Scholar]
- Rénia L., Mattei D., Goma J., Pied S., Dubois P., Miltgen F., Nüssler A., Matile H., Menégaux F., Gentilini M. A malaria heat-shock-like determinant expressed on the infected hepatocyte surface is the target of antibody-dependent cell-mediated cytotoxic mechanisms by nonparenchymal liver cells. Eur J Immunol. 1990 Jul;20(7):1445–1449. doi: 10.1002/eji.1830200706. [DOI] [PubMed] [Google Scholar]
- Sanchez E. R. Hsp56: a novel heat shock protein associated with untransformed steroid receptor complexes. J Biol Chem. 1990 Dec 25;265(36):22067–22070. [PubMed] [Google Scholar]
- Shanafelt M. C., Hindersson P., Soderberg C., Mensi N., Turck C. W., Webb D., Yssel H., Peltz G. T cell and antibody reactivity with the Borrelia burgdorferi 60-kDa heat shock protein in Lyme arthritis. J Immunol. 1991 Jun 1;146(11):3985–3992. [PubMed] [Google Scholar]
- Sistonen L., Sarge K. D., Phillips B., Abravaya K., Morimoto R. I. Activation of heat shock factor 2 during hemin-induced differentiation of human erythroleukemia cells. Mol Cell Biol. 1992 Sep;12(9):4104–4111. doi: 10.1128/mcb.12.9.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter R. S., Lowenstein D. H. Heat shock protein expression in vulnerable cells of the rat hippocampus as an indicator of excitation-induced neuronal stress. J Neurosci. 1992 Aug;12(8):3004–3009. doi: 10.1523/JNEUROSCI.12-08-03004.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. F., Toft D. O. Steroid receptors and their associated proteins. Mol Endocrinol. 1993 Jan;7(1):4–11. doi: 10.1210/mend.7.1.8446107. [DOI] [PubMed] [Google Scholar]
- Tisch R., Yang X. D., Singer S. M., Liblau R. S., Fugger L., McDevitt H. O. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993 Nov 4;366(6450):72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- Udelsman R., Blake M. J., Stagg C. A., Li D. G., Putney D. J., Holbrook N. J. Vascular heat shock protein expression in response to stress. Endocrine and autonomic regulation of this age-dependent response. J Clin Invest. 1993 Feb;91(2):465–473. doi: 10.1172/JCI116224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udono H., Srivastava P. K. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993 Oct 1;178(4):1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass K., Berger M. L., Nowak T. S., Jr, Welch W. J., Lassmann H. Induction of stress protein HSP70 in nerve cells after status epilepticus in the rat. Neurosci Lett. 1989 May 22;100(1-3):259–264. doi: 10.1016/0304-3940(89)90695-2. [DOI] [PubMed] [Google Scholar]
- Villar J., Edelson J. D., Post M., Mullen J. B., Slutsky A. S. Induction of heat stress proteins is associated with decreased mortality in an animal model of acute lung injury. Am Rev Respir Dis. 1993 Jan;147(1):177–181. doi: 10.1164/ajrccm/147.1.177. [DOI] [PubMed] [Google Scholar]
- Welch W. J. How cells respond to stress. Sci Am. 1993 May;268(5):56–64. doi: 10.1038/scientificamerican0593-56. [DOI] [PubMed] [Google Scholar]
- Welch W. J. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992 Oct;72(4):1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- Yellon D. M., Iliodromitis E., Latchman D. S., Van Winkle D. M., Downey J. M., Williams F. M., Williams T. J. Whole body heat stress fails to limit infarct size in the reperfused rabbit heart. Cardiovasc Res. 1992 Apr;26(4):342–346. doi: 10.1093/cvr/26.4.342. [DOI] [PubMed] [Google Scholar]
- Yem A. W., Tomasselli A. G., Heinrikson R. L., Zurcher-Neely H., Ruff V. A., Johnson R. A., Deibel M. R., Jr The Hsp56 component of steroid receptor complexes binds to immobilized FK506 and shows homology to FKBP-12 and FKBP-13. J Biol Chem. 1992 Feb 15;267(5):2868–2871. [PubMed] [Google Scholar]
- Young D. B. Heat-shock proteins: immunity and autoimmunity. Curr Opin Immunol. 1992 Aug;4(4):396–400. doi: 10.1016/s0952-7915(06)80029-4. [DOI] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]



