Abstract
The mechanisms by which the intracellular pathogen Francisella tularensis evades innate immunity are not well defined. We have identified a gene with homology to Escherichia coli mviN, a putative lipid II flippase, which F. tularensis uses to evade activation of innate immune pathways. Infection of mice with a F. tularensis mviN mutant resulted in improved survival and decreased bacterial burdens compared to infection with wild-type F. tularensis. The mviN mutant also induced increased AIM2 inflammasome-dependent IL-1β secretion and cytotoxicity in macrophages. The compromised in vivo virulence of the mviN mutant depended upon inflammasome activation, as caspase-1- and ASC-deficient mice did not exhibit preferential survival following infection. This study demonstrates that mviN limits F. tularensis-induced AIM2 inflammasome activation which is critical for its virulence in vivo.
The ability of F. tularensis to disrupt phagocyte function plays a key role in its virulence. Following phagocytosis, F. tularensis escapes the phagosome prior to phagosome/lysosome fusion and subsequently replicates within the cytosol (1, 2). Intracellular survival of F. tularensis is also associated with its ability to inhibit NADPH oxidase activity as well as subsequent NF-κ B activation (3, 4). In activated macrophages (Mφ) the escape of F. tularensis from the phagosome into the cytosol triggers activation of the AIM2 inflammasome (5–7), a multiprotein complex containing AIM2 (absent in melanoma 2; an interferon-inducible HIN-200 family member), ASC (apoptosis-associated speck-like protein containing a CARD) and the cysteine protease caspase-1 (8–11). Activation of the AIM2 inflammasome results in autocatalytic cleavage of caspase-1, resulting in the processing and secretion of IL-1β and IL-18. Inflammasome activation plays a crucial role in innate immune responses against F. tularensis, as AIM2-, caspase-1- and ASC-deficient mice have markedly increased bacterial burdens and mortality following infection with F. tularensis (5, 6).
In this study we demonstrate that F. tularensis live vaccine strain (LVS) avoids efficient AIM2 inflammasome activation. We show that mviN is required to inhibit F. tularensis-induced caspase-1-dependent processing and secretion of IL-1β and IL-18 as well as to limit F. tularensis-mediated cytotoxicity. Mutation of mviN resulted in a marked change in the morphology of the bacterium and was required for full virulence of F. tularensis LVS in an in vivo model of infection.
Materials and Methods
Bacterial strains, plasmid construction and growth conditions
F. tularensis ssp. holarctica LVS was obtained from ATCC (ATCC 29684). The LVS strain containing a chromosomal mutation in mviN was transformed with the Tn5 delivery plasmid pBB109 as previously described (12). Complementation of the mviN::Tn5 strain with wild-type mviN containing the ribosomal binding site in the plasmid pBB103 was accomplished by cryotransformation as previously described (13). For in vitro studies LVS strains were grown on Difco cysteine heart agar supplemented with 9% SRBC for 48 h at 37° C. 25 μg/ml spectinomycin was added to plates for growth of the mviN::Tn5 + mviN strain. For in vivo studies bacteria were grown overnight in modified Mueller-Hinton (MMH) broth (Becton Dickinson) supplemented with 1% (wt/vol) glucose, 0.025% ferric pyrophosphate, and 2% IsoVitaleX.
Scanning electron microscopy
Bacterial strains were grown in MMH broth for 6 h to an OD600 between 0.2 and 0.4. Samples were placed on silicon wafers and fixed in 2.5% glutaraldehyde and dehydrated using a standard graded ethanol series, with a final clearance in hexa-methyl-disilazane. Samples were coated with gold/palladium and viewed on a Hitachi S-4800 scanning electron microscope.
Mice and F. tularensis LVS infections
The generation of ASC-, caspase-1-, NLRP3-, NLRC4- and AIM2-deficient mice have been described previously (6, 14–16). The University of Iowa Institutional Animal Care and Use Committee approved all protocols used in this study. Mice (6–8 wk old) were injected i.p. withthe indicated dose of F. tularensis LVS or the mviN mutant. Mice were monitored every 12 h for lethality; mice found to be in a moribund state for more than 4 h were considered terminal and euthanized.
Mφ infections
Bone marrow-derived Mφ were generated as previously described (14). Unless indicated, Mφ were activated by stimulating with 50 ng/ml LPS from E. coli serotype 0111:B4 (Invivogen) for 3–6 h prior to infection. Mφ were infected with F. tularensis LVS or the mviN mutant at an MOI of 50:1 unless otherwise indicated. 9 h later, or at the indicated time, supernatants were collected and assayed for IL–1β , IL-18, TNFα and IL-6 by ELISA (14). Mφ cell death wasdetermined by measuring lactate dehydrogenase (LDH) release usinga cytotoxicity detection kit (Promega). Western blotting was performed as previously described (14).
Statistical analysis
Two-tailed Mann-Whitney U test were performed using Prism software. Values of p < 0.05 were considered statistically significant. Unless stated otherwise, determinations are expressed as the mean ± SEM.
Results and Discussion
MviN is required for F. tularensis LVS virulence in vivo
Peptidoglycan, a major component of bacterial cell walls, is composed of glycan chains that are cross-linked by peptide bridges. A lipid-linked peptidoglycan precursor (Lipid II) is generated in the cytoplasm by a series of highly conserved enzymatic steps. MviN was recently identified as being responsible for flipping the lipid II across the cytoplasmic membrane (17, 18); once on the periplasmic side, penicillin-binding proteins use lipid II to generate mature peptidoglycan. F. tularensis LVS possesses an mviN gene (FTL_1305) that shares homology to that of E. coli (30% identity, 54% similarity) as well as Salmonella typhimurium (28% identity, 52% similarity) and Legionella pneumophila (31% identity, 54% similarity), two pathogens that upon infecting Mφ can also activate caspase-1 (19–21). mviN is also highly conserved amongst other F. tularensis subspecies with homology to the mviN genes of F. tularensis ssp. novicida (98% identity 99% similarity) and F. tularensis ssp. tularensis (98% identity 99% similarity).
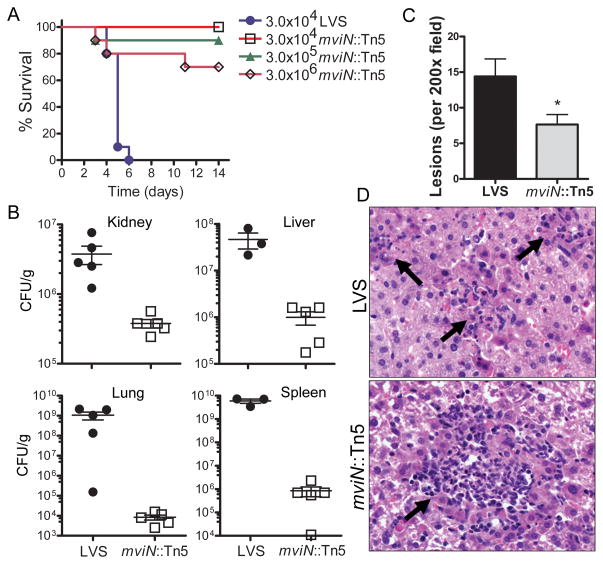
To determine if mviN is important for F. tularensis LVS virulence in vivo, we utilized an mviN mutant, mviN::Tn5, isolated from a F. tularensis LVS transposon library. WT mice were challenged i.p. with either LVS or the mviN mutant, and survival monitored. Infection with 3 × 104 CFU of LVS resulted in 100% mortality by day 6 (Fig. 1A). In contrast, all mice infected with 3 × 104 CFU of the mviN mutant survived until day 14 (Fig. 1A). Infection with 3 × 105 or 3 × 106 CFU of mviN mutant resulted in a 90% and 70% survival rate respectively (Fig. 1A). The increased survival of mice infected with the mviN mutant relative to wild-type LVS was also reflected in the 10–100,000 fold lower bacterial burdens in infected organs 3 d post-infection (Fig. 1B). Histology of liver 3 d after infection revealed fewer hepatic lesions in mice infected with the mviN mutant compared to LVS (Fig. 1C and D). Although fewer in number, the necropurulent foci seen in mviN mutant infected mice trended towards being larger than those of LVS infected mice (Fig. 1D). Similarly, histology of spleen 3 d after infection demonstrated occasional necropurulent foci in mviN mutant infected mice, whereas LVS infected mice had extensive coalescing necropurulent foci in the splenic red pulp that extended into the white pulp (Suppl. Fig. 1A and B). Collectively, these data suggest that the F. tularensis LVS mviN mutant was highly attenuated in vivo.
Figure 1.
F. tularensis LVS mviN mutant is attenuated in vivo. (A) WT (n=10 per group) mice were injected i.p. with the indicated amount of F. tularensis LVS (LVS) or the mviN mutant (mviN::Tn5) and survival monitored. Data are representative of 5 independent experiments at the 3 × 104 infective dose, each involving a minimum of 5 mice per group. (B) 3 d p.i. with 3 × 104 CFU of LVS or the mviN mutant organs were harvested, homogenized and dilutions plated for enumeration of CFU (n=3–5 mice per group). (C) Number of lesions per 200 × field of H&E-stained liver sections from WT mice 3 d p.i. with either LVS or the mviN mutant were scored; n=5 mice per group (3 random fields were examined per mouse); *, p = 0.016. (D) Representative H&E-stained sections of liver from WT mice 3 d p.i. with either F. tularensis LVS or the mviN mutant. Arrows indicate necropurulent foci.
MviN is dispensable for F. tularensis LVS growth in vitro
Mutant E. coli strains lacking functional mviN fail to grow and undergo lysis (17, 18); similarly, mviN was essential for the viability of Sinorhizobium meliloti and Burkholderia pseudomallei (22, 23). However, the F. tularensis LVS mviN mutant grew normally in broth (Suppl. Fig. 2A), consistent with the findings that mviN mutations in S. typhimurium and mutations of mviN homologs in Bacillus subtilis do not exhibit defective growth (24, 25). These results suggest that mviN is dispensable for the growth of F. tularensis LVS and it is possible that LVS may possess redundant pathways that compensate for the lack of mviN.
Since Mφ support Francisella replication in the host during natural infection, we examined the intracellular replication of the mviN mutant in vitro. Both LVS and the mviN mutant replicated with similar kinetics within unprimed Mφ (Suppl. Fig. 2B), suggesting that the attenuation of infection by the mviN mutant in mice was not simply due to an inability of the intracellular organism to replicate in this cell type.
Although we did not detect a defect in bacterial replication of the mviN mutant in vitro, scanning electron microscopy revealed a striking change in cellular morphology of the mviN mutant; mviN mutants appeared round and lacked the pleomorphic structure typical of LVS (Suppl. Fig. 2C). These morphological results suggest that mviN serves an important role in the synthesis of Francisella cell wall structures, although additional studies are required assess the full impact of mviN on F. tularensis peptidoglycan synthesis.
MviN restricts Francisella-induced caspase-1 activation
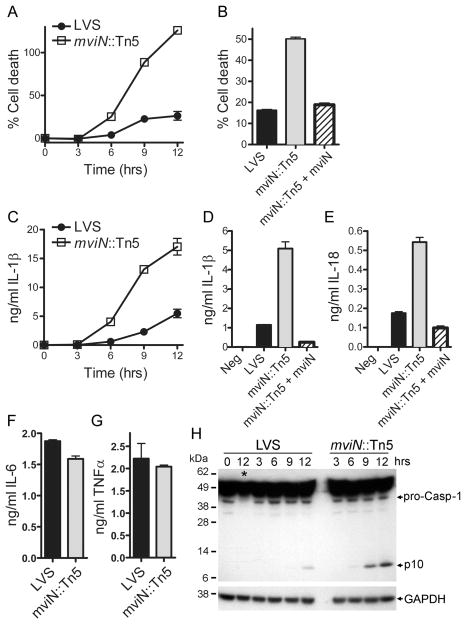
Since Francisella induces cell death in primed Mφ in a caspase-1-dependent manner (5), we tested whether mviN was necessary for Francisella-mediated cytotoxicity. LPS-primed WT Mφ infected with LVS underwent celllysis with 25% cytotoxicity 12 h after infection. However, infection of LPS-primed WT Mφ infected with the mviN mutant resulted in more extensive cell death compared to LVS, with all cells lysed at 12 h (Fig. 2A). Complementation of the mviN mutant induced Mφ death at levels similar to wild-type LVS (Fig. 2B) confirming the role of mviN in this process. The Mφ death induced by the mviN mutant was dependent on caspase-1, as caspase-1-deficient Mφ infected with the mviN mutant had a delayed cell death compared to WT Mφ (Suppl. Fig. 3A). We also examined the intracellular replication of the mviN mutant within LPS-primed Mφ and found decreased growth of the mviN mutant compared to LVS at 24 h (Suppl. Fig. 3B) consistent with the increased Mφ death induced by the mviN mutant.
Figure 2.
Mutation of F. tularensis LVS mviN results in enhanced Mφ cytotoxicity and caspase-1 activation. (A–E) LPS-primed WT Mφ were infected with F. tularensis LVS (LVS), the mviN mutant (mviN::Tn5) or the complemented mviN mutant (mviN::Tn5 + mviN) at an MOI of 50:1. Supernatants were collected 9 h after infection (B, D, and E) or as indicated (A and C). Cytotoxicity was measured by LDH release and expressed as a percentage of LDH release by Triton X-100 detergent (A and B). IL-1β secreted into supernatants was measured by ELISA (C-E). (F, G) Unprimed WT Mφ were infected with LVS or the mviN mutant for 9 h; IL-6 and TNFα released into the supernatant was measured by ELISA. (H) Lysates from LPS-primed WT and caspase-1-deficient Mφ (lane with caspase-1-/- Mφ lysate is marked *) infected with LVS or the mviN mutant (50:1 MOI) for the indicated times were immunoblotted with antibodies against the p10 subunit of caspase-1 or GAPDH. Results are representative of two (B, F, G) and three (A, C-E, H) separate experiments.
In addition to its role in mediating F. tularensis-induced Mφ death, caspase-1 is required for the processing and secretion of IL-1β and IL-18 by infected cells (5). The mviN mutant induced greater secretion of IL-1β and IL-18 from LPS-primed Mφ compared to LVS (Fig. 2C–E), at all multiplicities of infection tested (Supp. Fig. 3C). Mutant bacteria complemented with mviN failed to induce increased IL-1β and IL-18 secretion (Fig. 2D, E). In contrast to the augmented IL-1β and IL-18 release, the secretion of IL-6 and TNFα by unprimed Mφ infected with F. tularensis LVS was unaffected by the absence of mviN (Fig. 2F, G).
Caspase-1 activation is a two-step process culminating in the autocatalytic processing of the45-kD pro-caspase-1 to generate two subunits, p20 and p10. In the first step, treatment with an agent such as LPS not only results in the generation of pro-IL-1β but also primes the inflammasome for subsequent activation (26). In the case of AIM2 inflammasome activation, phagosomal escape of F. tularensis into the Mφ cytoplasm, likely with concurrent release of bacterial DNA into the cytosol, serves as the second signal (6). The mviN mutant did not enhance IL-1β secretion by bypassing the need for a priming step, as neither wild-type LVS nor the mviN mutant was capable of inducing IL-1β secretion from unprimed Mφ (Suppl. Fig. 3D). Whereas primed Mφ infected with LVS activated caspase-1by 9 h post infection, as judged by Westernblot detection of the p10 cleavage product (Fig. 2H), those infected with the mviN mutant induced caspase-1 activation more rapidly (by 6 h post infection) and to a greater extent compared to wild-type LVS (Fig. 2H). Taken together, these data suggest that F. tularensis LVS expression of mviN was required to limit caspase-1 activation and subsequent cytotoxicity and secretion of IL-1β and IL-18; and although the mviN mutant was able to replicate within unprimed Mφ in vitro, attenuation of infection by the mviN mutant in vivo was most probably due to increased death of F. tularensis-infected Mφ and increased proinflammatory cytokine production.
MviN limits Francisella-induced AIM2 inflammasome activation
In addition to AIM2, the NLR family members NLRP3 and NLRC4 can activate caspase-1 in response to bacterial pathogens (27). To determine if NLRP3 or NLRC4 was responsible for the increased IL-1β secretion induced by infection with the mviN mutant, Mφ from WT, caspase-1-/-, ASC-/-, NLRP3-/- and NLRC4-/- mice were infected with either LVS or the mviN mutant (Fig. 3A). Secretion of IL-1β in response both to wild-type LVS and to the mviN mutant was dependent on caspase-1 and ASC but independent of NLRP3 and NLRC4 (Fig. 3A). In contrast, caspase-1 activation and IL-1β secretion in response to infection both with wild-type LVS and with the mviN mutant were completely dependent on AIM2 (Fig. 3B, C). As expected, AIM2-deficient Mφ were fully capable of secreting IL-1β in response to the NLRP3 agonist alum (Fig. 3B). Taken together, these data demonstrate that the augmented caspase-1 activation induced by the mviN mutant did not reflect the mviN mutant engaging a different inflammasome pathway, such as NLRP3 or NLRC4, but rather increasing activation of the AIM2-dependent pathway. Consistent with our in vitro findings, ASC-/- and caspase-1-/- mice infected i.p. with the mviN mutant succumbed to infection at similar rates as did mice infected with wild-type LVS (Fig. 3D, E). In contrast to WT mice, no difference in bacterial burdens was observed in the spleen and liver of caspase-1-/- mice infected with either LVS or the mviN mutant (Supp. Fig. 4). Similarly, no difference in bacterial burdens was observed in the liver of ASC-/- mice (Supp. Fig. 4). Although ASC-/-spleens did have statistically lower burdens of the mviN mutant compared to LVS (Supp. Fig. 4) this difference was dramatically smaller than that observed in WT mice (3 fold vs 150,000 fold respectively). These observations suggest that the attenuation of survival of the mviN mutant in vivo was due to its increased activation of the AIM2 inflammasome.
Figure 3.
MviN limits Francisella-induced AIM2 inflammasome activation. (A, B) LPS-primed Mφ from WT, caspase-1-, ASC-, NLRP3-, NLRC4, and AIM2-deficient mice were infected with either F. tularensis LVS (LVS) or the mviN mutant (mviN::Tn5) at a 50:1 MOI, or challenged with Alum (500 μg/ml). Supernatants were collected at 9 h and IL-1β release measured by ELISA. Results are representative of two (A) and five (B) separate experiments. (C) Lysates from LPS-primed WT and AIM2-deficient Mφ infected with LVS or the mviN mutant for 9 h were immunoblotted with antibodies against the p10 subunit of caspase-1 or GAPDH. Results are representative of two separate experiments. (D, E) WT, caspase-1- or ASC-deficient mice (n=5 per group) were infected i.p. with 1 × 105 CFUs of LVS or the mviN mutant and survival monitored.
Given the importance of the AIM2 inflammasome in host defense against F. tularensis (6), it is not unexpected that a successful pathogen such as F. tularensis would evolve mechanisms to limit activation of this important innate immune pathway. Our results indicate that mviN was a critical for virulence of F. tularensis by virtue of its ability to restrict inflammasome activation. In fact, two genes have been identified in the related organism F. novicida, FTT_0748 and FTT_0584, that also inhibit Mφ cytotoxicity and IL-1β secretion (28). Although the role of F. tularensis LVS genes FTL_1364 and FTL_1327, homologues of FTT_0748 and FTT_0584 respectively, remain to be elucidated, they do not appear to compensate for a deficiency in mviN. It appears that Francisella possesses multiple genes that contribute to evading inflammasome activation, raising the possibility that strains that elicit more severe systemic disease, such as the type A strain F. tularensis ssp. tularensis, may also more efficiently evade AIM2 inflammasome activation.
It is postulated that acidification of the Mφ phagosome results in lysis of some ingested F. tularensis with the subsequent release of bacterial DNA (6). This DNA enters the cytoplasm, possibly through F. tularensis-induced phagosomal membrane damage, and activates the AIM2 inflammasome, resulting in the pyroptotic death of the Mφ along with processing and secretion of IL-1β and IL-18. The aberrant morphology of the mviN mutant may leave the bacteria more susceptible to lysis, with increased release of bacterial DNA. Alternatively, bacteriolysis may be occurring within the cytosol once the bacteria have escaped the phagosome. Similar to our findings here, a recent study by Sauer et al. identified an lmo2473 mutant of Listeria monocytogenes that induces hyperactivation of the AIM2 inflammasome following bacteriolysis in the macrophage cytosol (29). In conclusion, our data demonstrate that mviN played a critical role in limiting F. tularensis LVS-induced AIM2 inflammasome activation and in doing so subverted an important innate immune defense pathway.
Supplementary Material
Acknowledgments
We thank Richard Flavell, Anthony Coyle, Ethan Grant, and John Bertin for providing knockout mice, Leobaldo Solorzano and Vickie Knepper-Adrian for technical assistance and Jian Shao for assistance with electron microscopy. We thank Lee-Ann Allen for critical review of this manuscript. A Veterans Administration Merit Review Grant IBX000167A (F.S.S.), NIH grants K08 AI065517 (F.S.S.), P01 AI44642 (W.M.N.), AG14357 (E.S.A), AR055398 (E.S.A.) and MRCE project grant U54 AI057160 (B.D.J.) supported this work.
References
- 1.Clemens DL, Lee BY, Horwitz MA. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect Immun. 2004;72:3204–3217. doi: 10.1128/IAI.72.6.3204-3217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc Natl Acad Sci U S A. 2006;103:14578–14583. doi: 10.1073/pnas.0601838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Telepnev M, Golovliov I, Grundstrom T, Tarnvik A, Sjostedt A. Francisella tularensis inhibits Toll-like receptor-mediated activation of intracellular signalling and secretion of TNFα and IL-1 from murine macrophages. Cell Microbiol. 2003;5:41–51. doi: 10.1046/j.1462-5822.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- 4.McCaffrey RL, Allen LA. Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J Leukoc Biol. 2006;80:1224–1230. doi: 10.1189/jlb.0406287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariathasan S, Weiss DS, Dixit VM, Monack DM. Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis. J Exp Med. 2005;202:1043–1049. doi: 10.1084/jem.20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E, Eisenlohr L, Landel CP, Alnemri ES. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 11.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, Hume DA, Stacey KJ. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 12.Buchan BW, McLendon MK, Jones BD. Identification of differentially regulated Francisella tularensis genes by use of a newly developed Tn5-based transposon delivery system. Appl Environ Microbiol. 2008;74:2637–2645. doi: 10.1128/AEM.02882-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchan BW, McCaffrey RL, Lindemann SR, Allen LA, Jones BD. Identification of migR, a regulatory element of the Francisella tularensis live vaccine strain iglABCD virulence operon required for normal replication and trafficking in macrophages. Infect Immun. 2009;77:2517–2529. doi: 10.1128/IAI.00229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galan JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, Galan JE. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MS, Flavell RA. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz N. Bioinformatics identification of MurJ (MviN) as the peptidoglycan lipid II flippase in Escherichia coli. Proc Natl Acad Sci U S A. 2008;105:15553–15557. doi: 10.1073/pnas.0808352105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue A, Murata Y, Takahashi H, Tsuji N, Fujisaki S, Kato J. Involvement of an essential gene, mviN, in murein synthesis in Escherichia coli. J Bacteriol. 2008;190:7298–7301. doi: 10.1128/JB.00551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 21.Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, Dietrich WF, Roy CR. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 22.Rudnick PA, Arcondeguy T, Kennedy CK, Kahn D. glnD and mviN are genes of an essential operon in Sinorhizobium meliloti. J Bacteriol. 2001;183:2682–2685. doi: 10.1128/JB.183.8.2682-2685.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ling JM, Moore RA, Surette MG, Woods DE. The mviN homolog in Burkholderia pseudomallei is essential for viability and virulence. Can J Microbiol. 2006;52:831–842. doi: 10.1139/w06-042. [DOI] [PubMed] [Google Scholar]
- 24.Fay A, Dworkin J. Bacillus subtilis homologs of MviN (MurJ), the putative Escherichia coli lipid II flippase, are not essential for growth. J Bacteriol. 2009;191:6020–6028. doi: 10.1128/JB.00605-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carsiotis M, Stocker BA, Weinstein DL, O'Brien AD. A Salmonella typhimurium virulence gene linked to flg. Infect Immun. 1989;57:3276–3280. doi: 10.1128/iai.57.11.3276-3280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol. 40:620–623. doi: 10.1002/eji.200940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedra JH, Cassel SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol. 2009;21:10–16. doi: 10.1016/j.coi.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss DS, Brotcke A, Henry T, Margolis JJ, Chan K, Monack DM. In vivo negative selection screen identifies genes required for Francisella virulence. Proc Natl Acad Sci U S A. 2007;104:6037–6042. doi: 10.1073/pnas.0609675104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauer JD, Witte CE, Zemansky J, Hanson B, Lauer P, Portnoy DA. Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe. 2010;7:412–419. doi: 10.1016/j.chom.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.