Abstract
Infantile spasms (IS), the most common of the early epileptic encephalopathies, afflicts thousands of children each year and results in significant disability. Also known as West syndrome, IS is characterized by intractable stereotyped seizures, poor developmental outcome and a characteristic electroencephalogram (EEG) pattern. IS often progresses into another epileptic encephalopathy known as Lennox-Gastaut syndrome, and continues with the patient being burdened by lifelong epilepsy and varying degrees of mental retardation. Little is known about the biological basis of IS. As the etiologies of IS are diverse, the multiple causes must converge into a final common pathway that results in this specific epilepsy phenotype. Finding a model or models to test this final pathway is necessary both to understand why the greatest susceptibility to seizure development occurs during infancy and early childhood, and what underlies the decreased cognitive potential associated with IS. Furthermore, appropriate models would permit better testing of potential therapies directed specifically at IS. This review will describe the clinical features and etiologies of IS; the ideal features that IS models should contain; and the IS models that exist currently. Finally, we will discuss the limitations of these models and the potential avenues for future research on IS.
Infantile spasms syndrome: a clinical overview
The early epileptic encephalopathies are a group of conditions that all manifest with three major diagnostic criteria: medically refractory seizures, diffuse encephalopathy and a poor developmental outcome. These syndromes include early infantile epileptic encephalopathy (EEIE, also known as Otahara’s syndrome), severe myoclonic epilepsy of infancy (Dravet’s syndrome), infantile spasms (IS, also known as West syndrome), Lennox-Gastaut syndrome and a few less common syndromes (for a review, see Korff and Nordli, 2006). These syndromes make up the continuum of the developmental epileptic encephalopathies and often proceed from one syndrome to another. IS syndrome, the most common developmental epileptic encephalopathy, has an estimated incidence of 1 in 2000–6000 live births (Hurst, 1994). Issues regarding the modeling of IS are common and applicable to any of the early epileptic encephalopathies.
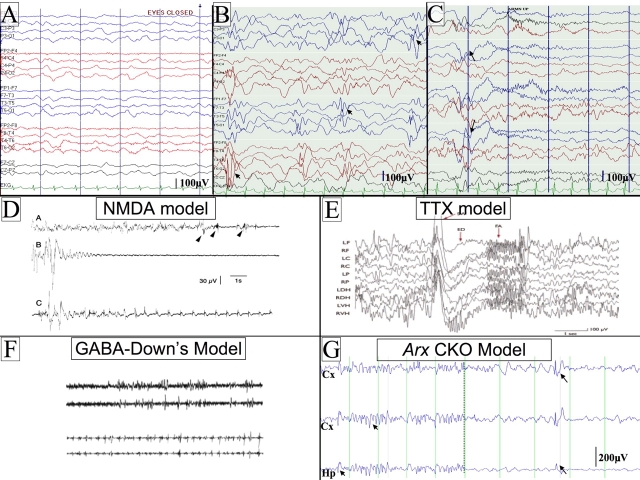
The characteristic features of IS are the intractable seizures, a specific electroencephalogram (EEG) finding of hypsarrythmia (Fig. 1), and a poor developmental outcome (Zupanc, 2003). The classic seizures consist of flexor or extensor truncal movements with abduction or adduction of the arms, usually in clusters, and the seizures often occur at sleep-wake transitions. Electrographically, each seizure is typically accompanied by a flattening of the EEG, termed an electrodecrement. Along with the classic spasms, IS patients often have focal, myoclonic, and/or generalized seizures. At the onset of the syndrome, normally between 3 and 12 months of age, the child can be developmentally normal or already delayed. Typically, the spasms begin subtly and can be misdiagnosed as gastroesophageal reflux, an exaggerated moro reflex, or as ‘funny baby movements’.
Fig. 1.
Examples of human and mouse hypsarrythmic EEGs and electrodecrements. (A) An example of a background EEG from a normal 6-month-old child. (B) An EEG from an infant with infantile spasms showing the classic EEG pattern of hypsarrythmia. The EEG is high voltage, disorganized and has frequent multifocal sharps (arrowheads). (C) An example of a clinical seizure with a flexor spasm and electrodecrement on an EEG (see arrows for the onset of the seizure). Scale bars for the human tracings (100 microvolts) are presented in the lower right hand corner of each panel. The intervals between the vertical lines represent a duration of 1 second. (D–G) Rodent EEGs from four of the current models of IS. (D) NMDA model. The upper trace shows an abnormal EEG with onset of seizures (arrowheads). The middle trace shows an expanded scale of one of the seizures from the upper trace, showing electrodecrement. The lower trace shows a clustering of seizures. Figure reproduced with permission from John Wiley & Sons, Inc. (Velísek et al., 2007). (E) TTX model. An example of an abnormal EEG and electrodecrement with seizures. A large spike and wave is presented, followed by fast activity; the EEG is abnormal preceding the seizure. Figure reproduced with permission from Wiley-Blackwell (Lee et al., 2008). (F) GABA in Down’s syndrome mouse model. Two pairs of tracings are shown. The upper pair represents the abnormal EEG from the Down’s syndrome mouse. The lower pair shows seizures that occur in clusters after injection of GABA agonist. Figure reproduced with permission from The International Pediatric Research Foundation (Cortez et al., 2009). (G) Arx CKO mouse model (Marsh et al., 2009). The traces on the left of the panel show an EEG from an awake Arx CKO adult male mouse. Notice that the EEG is high voltage with frequent sharps (arrowheads). The traces on the right show an example of a clinical seizure with a flexor spasm and electrodecrement on the EEG (the arrows show the onset of the seizure) in the Arx CKO mouse. Scale bars are given for each rodent EEG recording. In G, the distance between vertical lines represents a duration of 1 second. Abbreviations: Hp, hippocampal electrode; Cx, cortical electrode; μV, microvolts; SW, spike wave; ED, electrodecrement; FA, fast activity. The montage names on the left side of A,B are the same for the tracings in C.
The etiology of IS is variable. Potential etiologies for IS have characteristically been classified as cryptogenic, symptomatic and idiopathic, but a more intuitive approach is to divide the cases into two main groups: acquired and congenital/developmental. The acquired causes include, but are not limited to: sequelae from central nervous system (CNS) infections, hypoxic-ischemic damage and post-traumatic injury. The congenital/developmental conditions include: malformations of cortical development, channelopathies, metabolic disorders, known genetic disorders such as tuberous sclerosis or Rett’s syndrome, and chromosomal anomalies (trisomies 13, 18 and 21). In addition, an increasing number of single gene mutations, contiguous gene deletions and duplication syndromes are being identified as the underlying cause of these early onset developmental epilepsies. Recent work has shown that mutations in ARX, CDKL5, MUNC18-1 (also known as STXBP1), SLC25A22 and MAGI2 have each been linked to an IS or EIEE-like phenotype (Kitamura et al., 2002; Weaving et al., 2004; Molinari et al., 2005; Marshall et al., 2008; Saitsu et al., 2008). Inexplicably, each of the proteins encoded by these genes has a very different function: MUNC18 and MAGI2 are involved in synaptic development, ARX is a transcription factor, SLC25A22 is a mitochondrial glutamate/H+ symporter, and CDKL5 is a serine/threonine protein kinase with undefined phosphorylation targets. The fact that such variability in gene function leads to similar phenotypes suggests that these genes are all within a broad developmental pathway, or within a number of pathways that interact to guide the normal developmental processes. This variability in etiologies has confounded the research into the biological basis of IS.
The primary goal of treatment for IS is to both stop the seizures and normalize the EEG, with the ultimate goal being to improve developmental outcome. There is currently a debate as to whether the clinical outcome is influenced by the duration of the spasms and/or the time required to normalize the EEG (Kivity et al., 2004; Lux et al., 2004; Lux et al., 2005). In the USA, first-line treatment is with adrenocorticotropin hormone (ACTH). Depending on the clinical scenario, vigabatrin (for tuberous sclerosis), topiramate, zonisamide and prednisone are variably effective and can also be used as an initial treatment (for reviews, see Haines and Casto, 1994; Hancock et al., 2008). A number of case series’, randomized case-control studies and single-blinded studies have reported that up to 80% of patients respond to medical treatment, with the highest efficacy observed with ACTH, prednisone and then vigabatrin (Lux et al., 2005). In addition to pharmacotherapy, the ketogenic diet and surgical interventions have been attempted, both with variable success.
Even if therapy is successful, seizures are controlled and hypsarrythmia abolished, the outcome of many cases of IS is dependent on the underlying etiology. The most successful outcomes are typically in children who are considered to be idiopathic with normal early development. However, even in these children, only up to 20% have a normal developmental outcome and most develop epilepsy later in life. As this clinical information shows, there are a number of etiologies and, in many cases, the disease origin is never identified. Such variability in etiology leads to an overriding question: is there a ‘final common pathway’ by which the various etiologies develop a similar clinical picture, or are there multiple pathophysiological mechanisms that generate an early epileptic encephalopathy (i.e. a phenotypic convergence)?
Does a unifying pathophysiology exist for IS?
Prior to advances brought about from genetic studies, a few theories had been proposed to explain the phenotypic convergence in IS. The early theories included: (1) a brainstem origin of the spasms owing to alterations in the cholinergic and serotonergic systems, (2) immune system dysfunction and (3) abnormalities in corticalsubcortical interactions (Dulac et al., 1994; Lado and Moshe, 2002; Frost and Hrachovy, 2005). With a lack of supporting data for these early theories, two other hypotheses gained ground. One theory is that stress during development leads to increases in corticotropin-releasing hormone (CRH) production, which in turn leads to increased neuronal excitability and seizures (Baram et al., 1992; Baram and Schultz, 1995; Brunson et al., 2001). This theory originated from data demonstrating decreased ACTH levels in the cerebrospinal fluid (CSF) of IS patients (Baram et al., 1992; Baram and Schultz, 1995; Brunson et al., 2001) and the effectiveness of steroids for IS treatment. The other hypothesis, which attempts to synthesize the brainstem-onset theory with the various other etiologies, suggests that a disruption in one of many normal developmental processes (e.g. synaptogenesis, myelination, migration) causes a network problem that leads to diffuse brain dysfunction and IS (Frost and Hrachovy, 2005). How both these mechanisms actually lead to the timing, semiology, EEG findings and outcome of IS is incompletely understood.
Case study.
Patient E.M. was born at term to a prima gravida mother. The pregnancy, labor and delivery were uncomplicated. Development during the first 3 months was normal. At 3 months of age, the parents noticed stereotyped events that lasted several seconds, characterized by his body flexing and arms extending. The events were observed initially upon falling asleep and would occur in a cluster over 10–15 minutes. There often was a cry associated with the first few movements. Reflux was suspected, but the episodes did not resolve after a trial on ranitidine and he was referred to a child neurologist. On exam, the neurologist found E.M. to be a comfortable, well-appearing, non-dysmorphic child. A general medical exam was unremarkable and did not show evidence of neurocutaneous stigmata or abnormal genitalia. The patient’s neurological examination was significant for poor visual fixation and tracking for his age; diffuse low tone; and mild weakness for his age. There were no asymmetries or focal findings on examination. An EEG was obtained (Fig. 1) and showed a high-voltage, disorganized background with multifocal sharps, and captured a typical seizure. A diagnosis of infantile spasms was made. A variety of tests were performed to determine the etiology of the spasms. Magnetic resonance imaging (MRI) of the brain revealed partial agenesis of the corpus collosum and large, presumed Virchow-Robin spaces in the basal ganglia. A single nucleotide polymorphism genomewide array was normal. Metabolic testing, including plasma amino acids, organic acids, acylcarnitine profile, lactate and pyruvate, was unremarkable. Given that ARX (aristaless-related homeobox) mutations are known to affect male patients and cause basal ganglia cysts, sequencing of the ARX gene was performed and showed an expansion of the second alanine repeat. Prior to the diagnosis of the ARX mutation, the patient was treated with ACTH, resulting in a partial reduction in seizure frequency but no change in the hypsarrythmia. Topiramate, zonisamide and valproic acid were also tried without success. The ketogenic diet was initiated and had partial efficacy. Lorazepam was added to the patient’s drug regimen with good effect but seizures persisted so he was placed on vigabatrin. This combined therapy mostly stopped the patient’s seizures. The patient is non-mobile; wheelchair-bound with hypotonic cerebral palsy; non-verbal with profound mental retardation; and has rare breakthrough seizures.
The Hrachovy developmental desynchronization theory is very general, but serves as an excellent springboard to further analyze a unifying hypothesis for IS. Recently, Dobyns and colleagues put forth a new hypothesis that attempts to unify the multiple etiologies in children with IS (Kato and Dobyns, 2005). As mentioned above, one of the genetic etiologies of IS is mutations in the gene ARX. ARX encodes a transcription factor with a putative role in cortical development. In 2002, ARX mutations were simultaneously linked to three different pediatric neurological conditions (Kitamura et al., 2002; Stromme et al., 2002a; Stromme et al., 2002b), each with seizures and spasms as a major component. Since these first reports, ARX mutations have been found to be associated with a variety of neurological phenotypes in humans (Bienvenu et al., 2002; Stromme et al., 2002a; Stromme et al., 2002b; Turner et al., 2002; Hirose and Mitsudome, 2003; Guerrini et al., 2007), from non-syndromic mental retardation to lissencephaly with ambiguous genitalia. An apparent phenotype-genotype correlation exists, with mutations that cause a loss of the DNA-binding function (interruption of the homeodomain) causing the more ‘severe’ phenotype, where the children have brain malformations and often die at a young age. The clinical pictures of ARX mutations are unique in two aspects: the broad extent of the phenotypic spectrum and the particularly intractable nature of the seizures. Dobyns et al. argue that the severe epilepsy phenotype observed in children with ARX mutations results primarily from cerebral cortical interneuron dysfunction, which they termed an ‘interneuropathy’. This hypothesis could be expanded to other non-ARX disorders, suggesting that the specific loss of interneurons at a crucial time during development leads to a hyperexcitable network and a spasm-like phenotype. With these theories to explain the pathogenesis of IS in mind, we can discuss what makes a good IS model.
What should constitute a model of IS?
A few authors and a National Institute of Neurological Disorders and Stroke (NINDS)/National Institutes of Health (NIH) workshop have comprehensively addressed the key constituent factors of an IS model from a clinical perspective (Stafstrom and Holmes, 2002; Stafstrom et al., 2006; Baram, 2007), arguing that the important features to mimic are those that are found in infants but that are difficult to address in human studies. These authors proposed minimal criteria (age dependency, response to ACTH, cognitive problems), as well as a set of optimal features (spontaneous clinical spasms, sleep-wake clustering, hypsarrythmia, cognitive decline), that are both required in a valid IS model. The authors argue that a model with all of these features could be used to design novel therapeutics and to understand the pathophysiological basis of IS.
Clinical terms.
Breakthrough seizures – unexpected seizure or bout of seizures experienced in a patient with otherwise reliably controlled epilepsy
EEG electrodecrement – attenuation or ‘flattening’ of the observed EEG trace
Hypsarrythmia – abnormal EEG pattern characterized by an irregular, disorganized high-voltage background with frequent and diffuse spikes and slow waves
Moro reflex – a normal reflex in infants up to 5 months old, characterized by a startled expression and spreading of the arms in response to falling backwards
Prima gravida – first pregnancy
Virchow-Robin spaces – perivascular spaces surrounding the blood vessels that enter the brain
We agree with the criteria proposed by Stafstrom and Holmes (Stafstrom and Holmes, 2002) but would suggest that the primary goal of a model would be to elucidate the pathophysiological process and address how the different etiologies lead to this fixed set of clinical symptoms, in essence to prove or disprove the theories on pathogenesis. To understand whether there is a ‘final common pathway’ versus a ‘phenotypic convergence’, the models should, at minimum, have an abnormal EEG, spontaneous seizures that begin in the postnatal period, and cognitive deficits. As the EEG is a major physiological indicator of the underlying pathology, changes in the EEG are essential. In humans, a hypsarrythmic EEG consists of a high-voltage, disorganized tracing with multifocal epileptiform discharges. At least two reasons exist to make it difficult to document hypsarrythmia in rodents. First, the regional cortical organization in rodents is different than in humans. Second, the small brain/skull size of rodents will make it difficult to show electrographic organization. Therefore, the criteria for a rodent EEG to be hypsarrythmic should not include disorganization, but rather be significantly different than that of a control animal (e.g. a higher amplitude and with frequent epileptiform discharges).
Ideally, the seizures in any model should be spontaneous because seizures in patients are spontaneous and not provoked. However, many rodent epilepsy models have provoked seizures. A particular problem with this type of model is that there could be a difference between the network that generates/initiates the seizure and the network that propagates or determines the semiology of a seizure. In a provoked seizure model, the latter network could be dissected, but the former initiation network would still not be known. Therefore, models with provoked seizures are likely to be useful to look at the necessary circuitry for a spasm to occur, but will still miss what generates the spasm.
The issue of age parallels in IS rodent models is an interesting problem. Although there are parallels to many neural developmental milestones (i.e. the increase in synaptogenesis in postnatal humans at 2–3 months of age and the last few days in utero in a rodent), a direct age parallel is difficult to define. A primary reason for this difficulty is that adult rodent brains and adult human brains are very different. The rodent brain is lissencephalic and the archicortex areas of the hippocampus, pyriform and cingulate gyri comprise a larger proportion of the rodent brain than the human brain. These differences may make the adult rodent brain more equivalent to an immature human brain. Hence, the age parallel is difficult to model and should not be a prerequisite criteria for any IS model. That said, an ideal model would have an onset of spasms or myoclonic seizures in the newborn period, and would have seizures that change throughout the life of the animal.
Finally, the epileptic spasms of IS are believed to be a generalized seizure type. Increasing evidence from surgical case series’ and the existing models have suggested that single or multiple focal areas of network dysfunction can lead to IS. How a focal abnormality leads to a more global dysfunction and a ‘generalized’ pattern on an EEG is not known. The fact that patients have significant cognitive disabilities suggests a global dysfunction, but whether this is a primary problem or secondary to a focal abnormality is unclear. By requiring cognitive changes in an animal model, there must be some aspect of generalized dysfunction and this could give an insight into the two important questions: (1) what are the pathophysiological changes in IS that lead to cognitive problems, and (2) can a focal abnormality lead to a ‘generalized’ EEG and seizure presentation.
What is required to develop an ideal model for these early epilepsies can be debated, and the differences between species will invariably result in models that successfully mimic the human condition for some features and not others. The next question is what models currently exist and how do they compare with our minimal criteria or the previously published criteria.
Current models of IS
Currently, no model for IS encompasses all of the desired components. Based on the theoretical mechanisms described above, investigators have proposed five models: the intracortical tetrodotoxin (TTX) model (Lee et al., 2008), intraperitoneal (IP) injections of N-methyl-D-aspartate (NMDA) (Velísek et al., 2007), injection of a lipopolysaccharide (LPS) into the cortex (Scantlebury and Moshe, 2006), administration of γ-aminobutyric acid (GABA) agonists in a Down’s syndrome mouse (Cortez et al., 2009), and an Arx conditional knockout (CKO) mouse model (Marsh et al., 2009) (see Table 1 for a summary of the models). Although these models are all useful, each has inherent limitations. Since all of these models may be useful, but each has limitations, one possibility would be to use multiple models to dissect different aspects of the underlying pathophysiological issues in IS. This may well be the direction that research by child neurologists, epileptologists and neuroscientists would take, but this would not answer the fundamental question: does a single, final common pathway exist for IS and, if so, what is the neurophsysiological correlate?
Table 1.
Summary of current IS models
| Model | Method | Result | Positives | Negatives | Reference |
|---|---|---|---|---|---|
| Developmental desynchronization | Intracortical or hippocampal infusion of TTX at P14 | Spontaneous spasms at P24; abnormal EEG | Tests hypothesis of a ‘final common pathway’ | Late age for spasms | Lee et al., 2008 |
| Down’s inhibition | Down’s syndrome mouse model with IP injections of GABA | Provoked spasms from 1 week to 2 months of age; abnormal EEG | Down’s syndrome is a known genetic cause of IS; tests role of inhibitory networks in IS | Provoked spasms | Cortez et al., 2009 |
| Remote symptomatic causes | P3 injection of LPS and doxorubicin into cortex, followed by IP injection of PCPA | Spontaneous spasms at P10; abnormal EEG | Models the most common group of causes of IS; correct age of spasms | Only available in abstract form; severe damage to the cortex | Scantlebury and Moshe, 2006 |
| Directed therapies model | IP injection of NMDA | Provoked spasms at P15; abnormal EEG | Developed as a way to test therapies to stop spasms; correct age | Provoked spasms; does not test mechanism or hypothesis of spasm generation | Kabova et al., 1999; Velísek et al., 2007 |
| Interneuronopathy | Conditional Arx knockout | Convulsive seizures that evolve to spasms in adult mice; abnormal EEG | Seizures that evolve; spasms present; abnormal EEG; tests the hypothesis of interneuronal dysfunction | Late age for spasms; Arx mutations rarely cause IS | Marsh et al., 2009 |
All five models described in the text are listed with the model, method and concise findings. The positive and negative features of each model are also listed. Abbreviations: P, postnatal day; TTX, tetrodotoxin; IP, intraperitoneal; IS, infantile spasms; LPS, lipopolysaccharide; PCPA, p-chlorophenylalanine; Arx, aristaless-related homeobox gene.
We will now review the different models, including a discussion of the limitations of each. The TTX model of Hrachovy and colleagues (Lee et al., 2008) was used to test the developmental desynchronization hypothesis. TTX is infused for 10–30 days into the cortex and/or hippocampus of a rat, starting at postnatal day (P)14. After 10 days, approximately 30% of the animals develop flexor or extensor seizures, frequently in clusters, with an associated electrodecrement on the EEG. The model reports a hypsarrythmic EEG, but it is unclear where the background EEG differs from that of the control animals because the control data is not shown (Lee et al., 2008). This model includes spontaneous seizures, a spasm phenotype with an electrodecrement on the EEG, and potentially hypsarrythmic EEG as features that meet the criteria for an animal model of IS. However, the model has some shortcomings; the animals do not develop seizures until they are 1-month old, which is the age of late adolescent rats, and it is unclear what happens to the seizures over time and how different the background EEG is from normal. In addition, the authors need to report on the cognitive problems and the response to treatment.
Another interesting model mimics the situation seen in an infant with diffuse cortical damage, as can be seen following perinatal hypoxic-ischemia, intraventricular hemorrhage or perinatal infection (Scantlebury and Moshe, 2006). This model utilizes doxorubicin, LPS and p-chlorophenylalanine (PCPA). Doxorubicin and LPS are injected into the cortex at P3 and PCPA is injected intraperitoneally at P5. This model induces widespread damage into the cortex of perinatal animals, with spontaneous spasm-like seizures occurring a few days after the IP injection of PCPA. The details of this model have yet to be published outside of abstract form, but the authors claim that this is a model of symptomatic spasms. This is an interesting model because it replicates a symptomatic etiology and the spasms develop at the appropriate ages. Further assessment can be made once more data is published on this model.
Two models with acutely symptomatic seizures are the IP NMDA and GABA models. The NMDA model tests both the CRH and the brainstem serotonin/cholinergic hypotheses by IP administration of NMDA after prenatal treatment of betamethasone (Kabova et al., 1999; Velísek et al., 2007). These animals develop flexion seizures after injection of IP NMDA, with possible electrodecrements on EEGs. The authors also show that the activity is blocked by pre-treatment with ACTH. The c-fos expression and 2-deoxyglucose uptake data from the two studies suggest that limbic areas, the hypothalamus and the serotonin pathways in the brainstem are all involved in the generation of spasms in the model. A major limitation of this model is that the seizures stop when the injections are discontinued. The fact that the spasms of this model are induced by injection and do not occur spontaneously suggests that the underlying pathogenesis will be different from patients with IS. The major use of this model will be to test whether drugs can stop acute spasms. Whether this will lead to novel treatments for IS has not yet been tested.
The other acute administration model utilizes IP injections of GABA agonists in a Down’s syndrome mouse model (Cortez et al., 2009). The Ts65Dn Down’s syndrome mouse model was used at different ages. Injection of two different GABA agonists resulted in brief flexor spasms associated with an electrodecrement on the EEG. This model is interesting because a subset of children with Down’s syndrome have IS, but the model is limited because the acute symptomatic seizures do not occur in the absence of the injections.
Finally, our laboratory has recently published an IS-like model by generating a mouse with conditional loss of the gene Arx. With the original clinical descriptions of ARX-related disorders, described above, Kitamura and colleagues generated an engineered mutation in mouse Arx (Kitamura et al., 2002; Collombat et al., 2003). These mice recapitulate some aspects of the human pathology of ARX IS cases, including basal ganglia with an abnormal appearance, an anomalous corpus callosum, cortical layer abnormalities and, most notably, a profound interneuron migratory defect (Kitamura et al., 2002; Collombat et al., 2003). Unfortunately, these mice die at birth, limiting postnatal physiological and behavioral analyses. As the complete gene knockout was perinatal lethal, our lab generated a mouse line carrying loxP sites in introns 1 and 2 of one allele of Arx (Fulp et al., 2008), to allow for conditional deletion of Arx. These CKO mice show complex partial-like seizures that begin by P12 in the perinatal period and evolve to spasms. The animals have an abnormal EEG background and electrodecrements with the seizures (Fig. 1). The CKO mimics the heterozygous condition in females as well. The mice show a subtype-specific loss of interneurons as the pathology behind the phenotype. One possible advantage of the Arx model is the potential to generalize the mechanisms that are uncovered so that they apply to all cases of IS. Many different conditions could lead to a predilection for loss or alteration of interneuronal function, including hypoxic-ischemic damage, other genetic conditions, and cortical developmental abnormalities. The limitations of this model are that we have not yet shown cognitive dysfunction; spasms occur at a later age than in humans; and ARX mutations may only be found in a small percentage of patients with IS.
Of these models, the Arx, TTX and LPS models are the most promising as they have spontaneous spasm-like seizures, which develop either on a genetic basis or after an acute insult to the brain. Work on these models has yet to show how they lead to spasms and has yet to answer the question: does a ‘final common pathway’ exist or do multiple mechanisms lead to a phenotypic convergence?
Future directions
These models all have the potential to uncover the pathophysiological basis of IS and allow researchers to design and test novel therapeutics. Using these models, the role of the seizures, the underlying pathology of the seizures, and medications can all be investigated as causes of the poor developmental outcome. The next step for these models should be to perform mechanistic studies on how the insult(s) lead to the network changes that are the cause of the spasms and hypsarrythmia. Once one or more of these models begin to prove their utility in uncovering the pathophysiology of IS, then an ideal framework can be determined and used to design models for all the other early epileptic encephalopathies.
Ultimately, for any model to be truly useful, the insights from the experiments performed in the lab, whether uncovering the pathobiology or testing treatments, has to be transferable to the clinic. Any rodent model for IS may be limited in its scope because of the major species difference between rats, mice and humans. One major goal of any early rodent model may be to decide how to implement the insult or genetic changes in an additional animal system, such as a piglet or dog, that more closely follows the early developmental course of human infants when IS occurs.
Clinical and basic research opportunities.
Investigating phenotypic convergence or the possibility of IS dependence on a final pathway, regardless of etiology
Using existing models to determine how each mechanism results in global brain signaling changes
Exploring animal models of IS in organisms with a more similar developmental timeline to humans
Using animal models to design and test novel therapeutics to control IS symptoms
Over the last decade, there has been steady progress in attempting to understand the underlying mechanisms of IS, as well as in developing models such as those described in this Clinical Puzzle. One reason for this advancing progress is consensus in the epilepsy community that this is an important avenue for research. Hopefully, exposure of the problem beyond the epilepsy community will allow for further progress to be made.
Acknowledgments
We would like to thank Dennis Dlugos for his thoughtful and careful reading of the manuscript.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Baram TZ. (2007). Models for infantile spasms: an arduous journey to the Holy Grail. Ann Neurol. 61, 89–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Schultz L. (1995). ACTH does not control neonatal seizures induced by administration of exogenous corticotropin-releasing hormone. Epilepsia 36, 174–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baram TZ, Mitchell WG, Snead OC, 3rd, Horton EJ, Saito M. (1992). Brain-adrenal axis hormones are altered in the CSF of infants with massive infantile spasms. Neurology 42, 1171–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu T, Poirier K, Friocourt G, Bahi N, Beaumont D, Fauchereau F, Ben Jeema L, Zemni R, Vinet MC, Francis F, et al. (2002). ARX, a novel Prd-class-homeobox gene highly expressed in the telencephalon, is mutated in X-linked mental retardation. Hum Mol Genet. 11, 981–991 [DOI] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Baram TZ. (2001). How do the many etiologies of West syndrome lead to excitability and seizures? The corticotropin releasing hormone excess hypothesis. Brain Dev. 23, 533–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. (2003). Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 17, 2591–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez MA, Shen L, Wu Y, Aleem IS, Trepanier CH, Sadeghnia HR, Ashraf A, Kanawaty A, Liu CC, Stewart L, et al. (2009). Infantile Spasms and Down syndrome: a new animal model. Pediatr Res. 65, 499–503 [DOI] [PubMed] [Google Scholar]
- Dulac O, Chiron C, Robain O, Plouin P, Jambaque II, Pinard JM. (1994). Infantile spasms: a pathophysiological hypothesis. Semin Pediatr Neurol. 1, 83–89 [PubMed] [Google Scholar]
- Frost JD, Jr, Hrachovy RA. (2005). Pathogenesis of infantile spasms: a model based on developmental desynchronization. J Clin Neurophysiol. 22, 25–36 [DOI] [PubMed] [Google Scholar]
- Fulp CT, Cho G, Marsh ED, Nasrallah IM, Labowski PA, Golden JA. (2008). Identification of Arx transcriptional targets in the developing basal forebrain. Hum Mol Genet. 17, 3740–3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini R, Moro F, Kato M, Barkovich AJ, Shiihara T, McShane MA, Hurst J, Loi M, Tohyama J, Norci V, et al. (2007). Expansion of the first PolyA tract of ARX causes infantile spasms and status dystonicus. Neurology 69, 427–433 [DOI] [PubMed] [Google Scholar]
- Haines ST, Casto DT. (1994). Treatment of infantile spasms. Annals Pharmacother. 28, 779–791 [DOI] [PubMed] [Google Scholar]
- Hancock EC, Osborne JP, Edwards SW. (2008. Treatment of infantile spasms. Cochrane Database Syst. Rev. CD001770. [DOI] [PubMed] [Google Scholar]
- Hirose S, Mitsudome A. (2003). X-linked mental retardation and epilepsy: pathogenetic significance of ARX mutations. Brain Dev. 25, 161–165 [DOI] [PubMed] [Google Scholar]
- Hurst DL. (1994). Epidemilogy. In Infantile Spasms and West Syndrome (ed. Dulac O, Bernardina BD, Chugani HT.). London: W. B. Saunders [Google Scholar]
- Kabova R, Liptakova S, Slamberova R, Pometlova M, Velisek L. (1999). Age-specific N-methyl-D-aspartate-induced seizures: perspectives for the West syndrome model. Epilepsia 40, 1357–1369 [DOI] [PubMed] [Google Scholar]
- Kato M, Dobyns WB. (2005). X-linked lissencephaly with abnormal genitalia as a tangential migration disorder causing intractable epilepsy: proposal for a new term, “interneuronopathy”. J Child Neurol. 20, 392–397 [DOI] [PubMed] [Google Scholar]
- Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka-Kogo A, Kusaka M, Omichi K, Suzuki R, Kato-Fukui Y, Kamiirisa K, et al. (2002). Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 32, 359–369 [DOI] [PubMed] [Google Scholar]
- Kivity S, Lerman P, Ariel R, Danziger Y, Mimouni M, Shinnar S. (2004). Long-term cognitive outcomes of a cohort of children with cryptogenic infantile spasms treated with high-dose adrenocorticotropic hormone. Epilepsia 45, 255–262 [DOI] [PubMed] [Google Scholar]
- Korff CM, Nordli DR., Jr (2006). Epilepsy syndromes in infancy. Pediatr Neurol. 34, 253–263 [DOI] [PubMed] [Google Scholar]
- Lado FA, Moshe SL. (2002). Role of subcortical structures in the pathogenesis of infantile spasms: what are possible subcortical mediators? Int Rev Neurobiol. 49, 115–140 [DOI] [PubMed] [Google Scholar]
- Lee CL, Frost JD, Jr, Swann JW, Hrachovy RA. (2008). A new animal model of infantile spasms with unprovoked persistent seizures. Epilepsia 49, 298–307 [DOI] [PubMed] [Google Scholar]
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, O’Callaghan FJ, Verity CM, Osborne JP. (2004). The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet 364, 1773–1778 [DOI] [PubMed] [Google Scholar]
- Lux AL, Edwards SW, Hancock E, Johnson AL, Kennedy CR, Newton RW, O’Callaghan FJ, Verity CM, Osborne JP. (2005). The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 4, 712–717 [DOI] [PubMed] [Google Scholar]
- Marsh E, Fulp C, Gomez E, Nasrallah I, Minarcik J, Sudi J, Christian SL, Mancini G, Labosky P, Dobyns W, et al. (2009). Targeted loss of Arx results in a developmental epilepsy mouse model and recapitulates the human phenotype in heterozygous females. Brain 132, 1563–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Young EJ, Pani AM, Freckmann ML, Lacassie Y, Howald C, Fitzgerald KK, Peippo M, Morris CA, Shane K, et al. (2008). Infantile spasms is associated with deletion of the MAGI2 gene on chromosome 7q11.23–q21.11. Am J Hum Genet. 83, 106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari F, Raas-Rothschild A, Rio M, Fiermonte G, Encha-Razavi F, Palmieri L, Palmieri F, Ben-Neriah Z, Kadhom N, Vekemans M, et al. (2005). Impaired mitochondrial glutamate transport in autosomal recessive neonatal myoclonic epilepsy. Am J Hum Genet. 76, 334–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitsu H, Kato M, Mizuguchi T, Hamada K, Osaka H, Tohyama J, Uruno K, Kumada S, Nishiyama K, Nishimura A, et al. (2008). De novo mutations in the gene encoding STXBP1 (MUNC18-1) cause early infantile epileptic encephalopathy. Nat Genet. 40, 782–788 [DOI] [PubMed] [Google Scholar]
- Scantlebury MH, Moshe SL. (2006. A new animal model of infantile spasms. Epilepsia 47 Suppl. 4, 1917105454 [Google Scholar]
- Stafstrom CE, Holmes GL. (2002). Infantile spasms: criteria for an animal model. Int Rev Neurobiol. 49, 391–411 [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Moshe SL, Swann JW, Nehlig A, Jacobs MP, Schwartzkroin PA. (2006). Models of pediatric epilepsies: strategies and opportunities. Epilepsia 47, 1407–1414 [DOI] [PubMed] [Google Scholar]
- Stromme P, Mangelsdorf ME, Scheffer IE, Gecz J. (2002a). Infantile spasms, dystonia, and other X-linked phenotypes caused by mutations in Aristaless related homeobox gene, ARX. Brain Dev. 24, 266–268 [DOI] [PubMed] [Google Scholar]
- Stromme P, Mangelsdorf ME, Shaw MA, Lower KM, Lewis SM, Bruyere H, Lutcherath V, Gedeon AK, Wallace RH, Scheffer IE, et al. (2002b). Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nat Genet. 30, 441–445 [DOI] [PubMed] [Google Scholar]
- Turner G, Partington M, Kerr B, Mangelsdorf M, Gecz J. (2002). Variable expression of mental retardation, autism, seizures, and dystonic hand movements in two families with an identical ARX gene mutation. Am J Med Genet. 112, 405–411 [DOI] [PubMed] [Google Scholar]
- Velísek L, Jehle K, Asche S, Velísková J. (2007). Model of infantile spasms induced by N-methyl-D-aspartic acid in prenatally impaired brain. Ann Neurol. 61, 109–119 [DOI] [PubMed] [Google Scholar]
- Weaving LS, Christodoulou J, Williamson SL, Friend KL, McKenzie OL, Archer H, Evans J, Clarke A, Pelka GJ, Tam PP, et al. (2004). Mutations of CDKL5 cause a severe neurodevelopmental disorder with infantile spasms and mental retardation. Am J Hum Genet. 75, 1079–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupanc ML. (2003). Infantile spasms. Expert Opin Pharmacother. 4, 2039–2048 [DOI] [PubMed] [Google Scholar]