Abstract
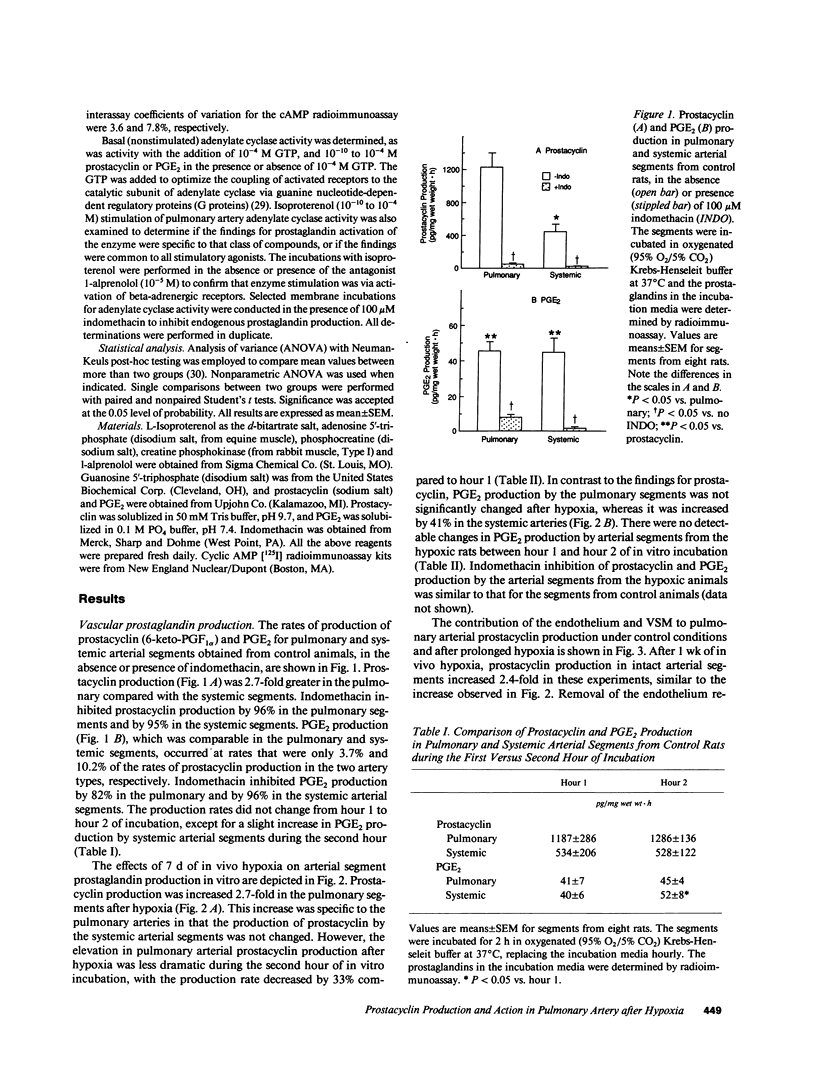
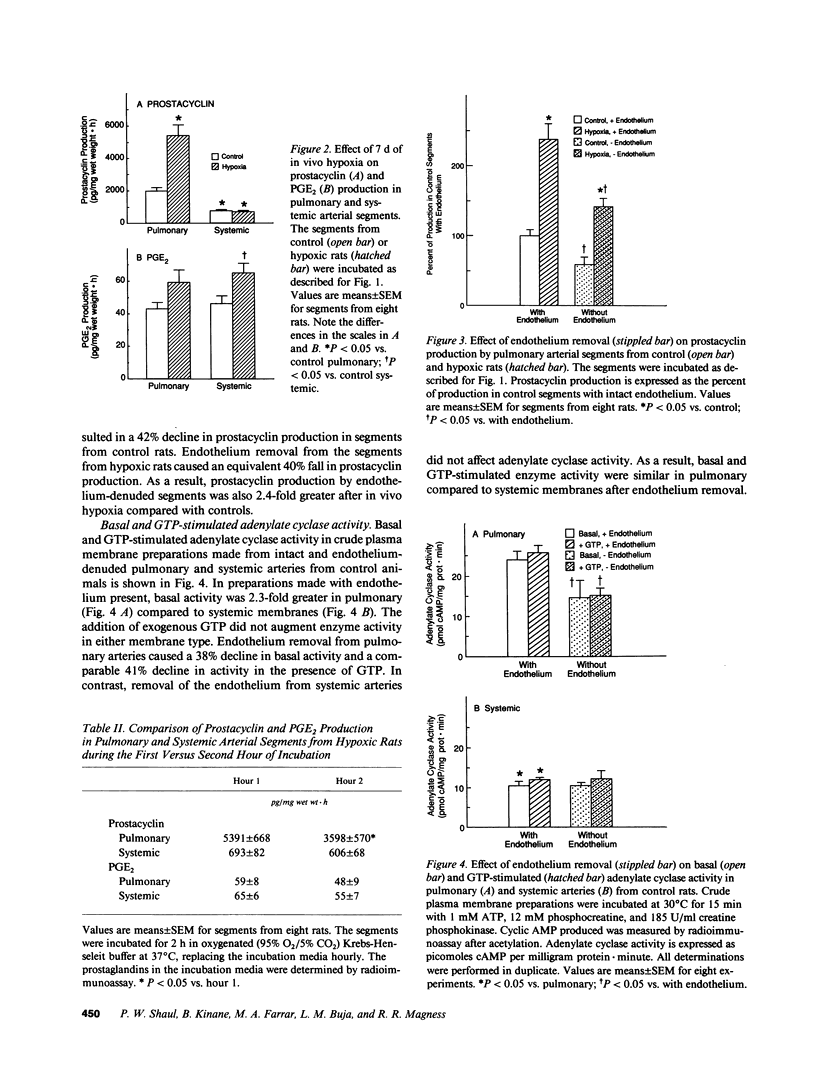
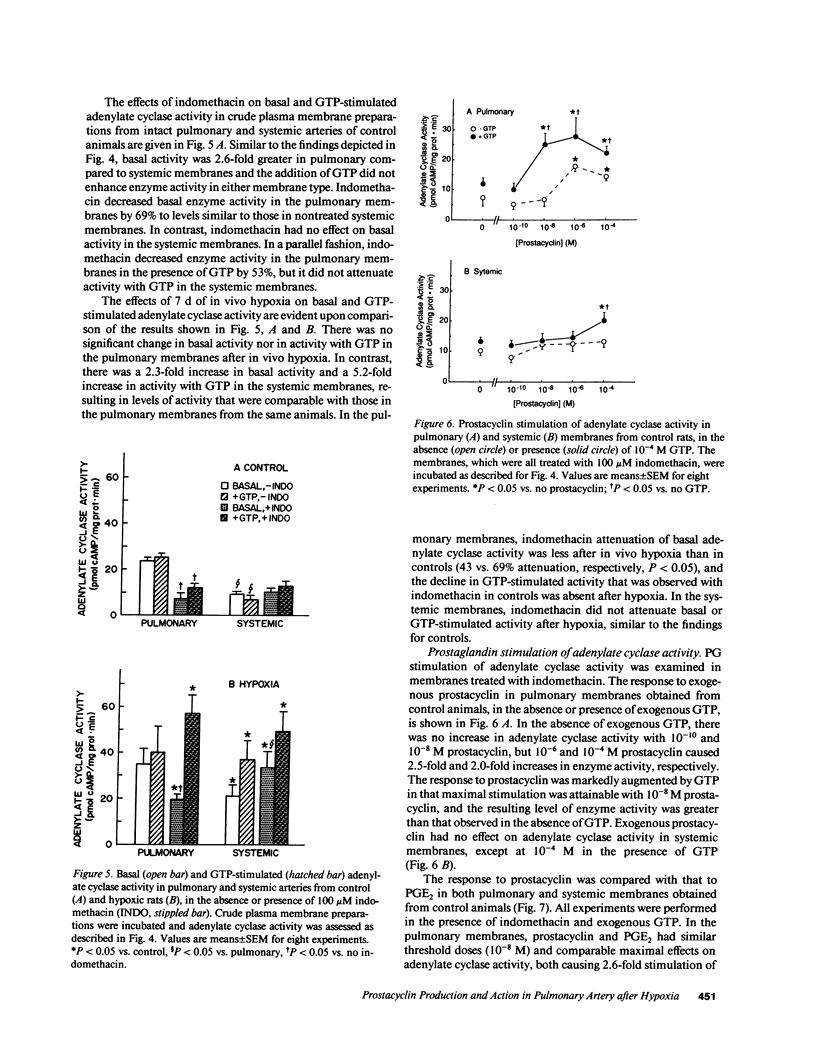
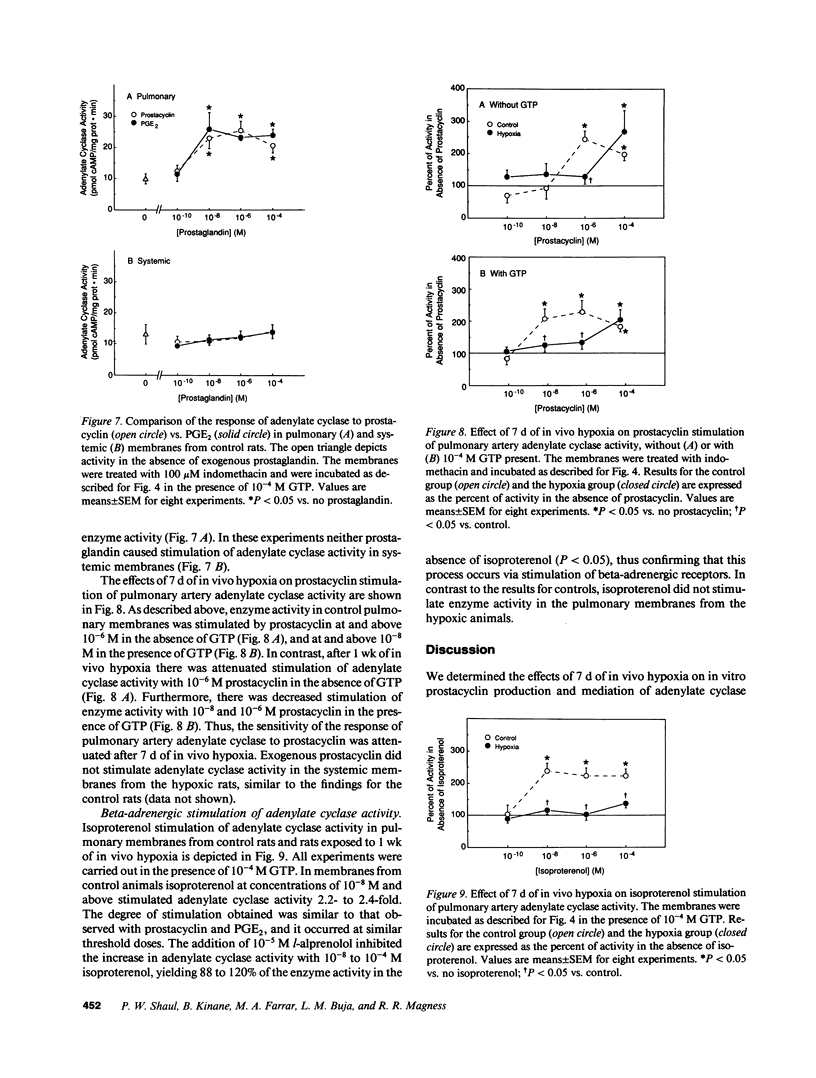
Prostacyclin is a critical mediator of structure and function in the pulmonary circulation, causing both the inhibition of vascular smooth muscle growth and vasodilation via the stimulation of adenylate cyclase. To examine the potential role of alterations in prostacyclin production or mechanism of action in chronic hypoxic pulmonary hypertension, we determined the effects of prolonged (7 d) in vivo hypoxia on in vitro prostacyclin synthesis and mediation of adenylate cyclase activity in rat main pulmonary arteries. In control arteries prostacyclin production exceeded that of prostaglandin (PG) E2 by 25-fold, with 42% originating from the endothelium. Studies utilizing indomethacin revealed that endogenous prostaglandins mediate at least 69% of basal adenylate cyclase activity. Prostacyclin-stimulated enzyme activity was enhanced by exogenous GTP, indicating that this is a receptor-mediated process involving G protein amplification. Comparable dose-related responses to prostacyclin and PGE2 suggest that these agents may activate a common receptor. After 7 d of in vivo hypoxia there was a 2.7-fold increase in in vitro prostacyclin production, with equivalent increases in synthesis in the endothelium and vascular smooth muscle. However, despite this increase there was no change in basal adenylate cyclase activity, and this was associated with attenuated sensitivity of the enzyme to prostacyclin stimulation. Concomitant diminution of the response to beta-adrenergic stimulation, with previously-demonstrated beta receptor downregulation and unaltered postreceptor-mediated activity, suggests that the blunted response to prostacyclin is due to receptor downregulation. Parallel studies of the thoracic aorta indicated that these changes are specific to the pulmonary artery. It is postulated that attenuation of the response of adenylate cyclase to prostacyclin may contribute to the structural changes and hypertension observed in the pulmonary vasculature of the rat with chronic hypoxia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badesch D. B., Orton E. C., Zapp L. M., Westcott J. Y., Hester J., Voelkel N. F., Stenmark K. R. Decreased arterial wall prostaglandin production in neonatal calves with severe chronic pulmonary hypertension. Am J Respir Cell Mol Biol. 1989 Dec;1(6):489–498. doi: 10.1165/ajrcmb/1.6.489. [DOI] [PubMed] [Google Scholar]
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- Dembinska-Kiec A., Rücker W., Schönhöfer P. S. Effects of PGI2 and PGI analogues on cAMP levels in cultured endothelial and smooth muscle cells derived from bovine arteries. Naunyn Schmiedebergs Arch Pharmacol. 1980 Feb;311(1):67–70. doi: 10.1007/BF00500304. [DOI] [PubMed] [Google Scholar]
- Fox W. W., Duara S. Persistent pulmonary hypertension in the neonate: diagnosis and management. J Pediatr. 1983 Oct;103(4):505–514. doi: 10.1016/s0022-3476(83)80573-3. [DOI] [PubMed] [Google Scholar]
- Fried R., Reid L. M. The effect of isoproterenol on the development and recovery of hypoxic pulmonary hypertension. A structural and hemodynamic study. Am J Pathol. 1985 Oct;121(1):102–111. [PMC free article] [PubMed] [Google Scholar]
- Green R. S., Leffler C. W. Hypoxia stimulates prostacyclin synthesis by neonatal lungs. Pediatr Res. 1984 Sep;18(9):832–835. doi: 10.1203/00006450-198409000-00005. [DOI] [PubMed] [Google Scholar]
- Hamasaki Y., Tai H. H., Said S. I. Hypoxia stimulates prostacyclin generation by dog lung in vitro. Prostaglandins Leukot Med. 1982 Apr;8(4):311–316. doi: 10.1016/0262-1746(82)90054-3. [DOI] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Hislop A., Reid L. New findings in pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. Br J Exp Pathol. 1976 Oct;57(5):542–554. [PMC free article] [PubMed] [Google Scholar]
- Holzmann S., Kukovetz W. R., Schmidt K. Mode of action of coronary arterial relaxation by prostacyclin. J Cyclic Nucleotide Res. 1980;6(6):451–460. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Madden M. C., Vender R. L., Friedman M. Effect of hypoxia on prostacyclin production in cultured pulmonary artery endothelium. Prostaglandins. 1986 Jun;31(6):1049–1062. doi: 10.1016/0090-6980(86)90208-x. [DOI] [PubMed] [Google Scholar]
- Magness R. R., Osei-Boaten K., Mitchell M. D., Rosenfeld C. R. In vitro prostacyclin production by ovine uterine and systemic arteries. Effects of angiotensin II. J Clin Invest. 1985 Dec;76(6):2206–2212. doi: 10.1172/JCI112229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Niedermeyer M. E., Ogletree M. L., Brigham K. L. Pulmonary hypertension and increased vasoreactivity caused by repeated indomethacin in sheep. J Appl Physiol (1985) 1985 Aug;59(2):443–452. doi: 10.1152/jappl.1985.59.2.443. [DOI] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Morganroth M. L., Stenmark K. R., Morris K. G., Murphy R. C., Mathias M., Reeves J. T., Voelkel N. F. Diethylcarbamazine inhibits acute and chronic hypoxic pulmonary hypertension in awake rats. Am Rev Respir Dis. 1985 Apr;131(4):488–492. doi: 10.1164/arrd.1985.131.4.488. [DOI] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. D., Rabinovitch M., Goldstein J. D., Reid L. M. The structural basis of persistent pulmonary hypertension of the newborn infant. J Pediatr. 1981 Jun;98(6):962–967. doi: 10.1016/s0022-3476(81)80605-1. [DOI] [PubMed] [Google Scholar]
- Oliva D., Bernini F., Corsini A., Nicosia S. 6-Keto-prostaglandin E1-sensitive adenylate cyclase and binding sites in membranes from platelets and cultured smooth muscle cells. Biochem Pharmacol. 1984 Dec 1;33(23):3755–3758. doi: 10.1016/0006-2952(84)90036-4. [DOI] [PubMed] [Google Scholar]
- Oliva D., Noè A., Nicosia S., Bernini F., Fumagalli R., Whittle B. J., Moncada S., Vane J. R. Prostacyclin-sensitive adenylate cyclase in cultured myocytes: differences between rabbit aorta and mesenteric artery. Eur J Pharmacol. 1984 Oct 15;105(3-4):207–213. doi: 10.1016/0014-2999(84)90611-3. [DOI] [PubMed] [Google Scholar]
- Perkin R. M., Anas N. G. Pulmonary hypertension in pediatric patients. J Pediatr. 1984 Oct;105(4):511–522. doi: 10.1016/s0022-3476(84)80413-8. [DOI] [PubMed] [Google Scholar]
- Popovich K. J., Hiller C., Hough A., Norris J. S., Cornett L. E. Characterization of a beta-adrenergic receptor in porcine trachealis muscle. Am J Physiol. 1984 Nov;247(5 Pt 1):C342–C349. doi: 10.1152/ajpcell.1984.247.5.C342. [DOI] [PubMed] [Google Scholar]
- Porcelli R. J., Bergman M. J. Effects of chronic hypoxia on pulmonary vascular responses to biogenic amines. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):534–540. doi: 10.1152/jappl.1983.55.2.534. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., Boudreau N., Vella G., Coceani F., Olley P. M. Oxygen-related prostaglandin synthesis in ductus arteriosus and other vascular cells. Pediatr Res. 1989 Oct;26(4):330–335. doi: 10.1203/00006450-198910000-00009. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., Gamble W., Nadas A. S., Miettinen O. S., Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am J Physiol. 1979 Jun;236(6):H818–H827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., Konstam M. A., Gamble W. J., Papanicolaou N., Aronovitz M. J., Treves S., Reid L. Changes in pulmonary blood flow affect vascular response to chronic hypoxia in rats. Circ Res. 1983 Apr;52(4):432–441. doi: 10.1161/01.res.52.4.432. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M., Mullen M., Rosenberg H. C., Maruyama K., O'Brodovich H., Olley P. M. Angiotensin II prevents hypoxic pulmonary hypertension and vascular changes in rat. Am J Physiol. 1988 Mar;254(3 Pt 2):H500–H508. doi: 10.1152/ajpheart.1988.254.3.H500. [DOI] [PubMed] [Google Scholar]
- Rodman D. M., Yamaguchi T., O'Brien R. F., McMurtry I. F. Hypoxic contraction of isolated rat pulmonary artery. J Pharmacol Exp Ther. 1989 Mar;248(3):952–959. [PubMed] [Google Scholar]
- Samuelsson B., Goldyne M., Granström E., Hamberg M., Hammarström S., Malmsten C. Prostaglandins and thromboxanes. Annu Rev Biochem. 1978;47:997–1029. doi: 10.1146/annurev.bi.47.070178.005025. [DOI] [PubMed] [Google Scholar]
- Shaul P. W., Muntz K. H., Buja L. M. Comparison of beta adrenergic receptor binding characteristics and coupling to adenylate cyclase in rat pulmonary artery versus aorta. J Pharmacol Exp Ther. 1990 Jan;252(1):86–92. [PubMed] [Google Scholar]
- Shaul P. W., Muntz K. H., DeBeltz D., Buja L. M. Effects of prolonged hypoxia on adenylate cyclase activity and beta-adrenergic receptors in pulmonary and systemic arteries of the rat. Circ Res. 1990 Jun;66(6):1526–1534. doi: 10.1161/01.res.66.6.1526. [DOI] [PubMed] [Google Scholar]
- Spitz B., Magness R. R., Cox S. M., Brown C. E., Rosenfeld C. R., Gant N. F. Low-dose aspirin. I. Effect on angiotensin II pressor responses and blood prostaglandin concentrations in pregnant women sensitive to angiotensin II. Am J Obstet Gynecol. 1988 Nov;159(5):1035–1043. doi: 10.1016/0002-9378(88)90406-1. [DOI] [PubMed] [Google Scholar]
- Terragno N. A., Terragno A. Prostaglandin metabolism in the fetal and maternal vasculature. Fed Proc. 1979 Jan;38(1):75–77. [PubMed] [Google Scholar]
- Tozzi C. A., Poiani G. J., Edelman N. H., Riley D. J. Vascular collagen affects reactivity of hypertensive pulmonary arteries of the rat. J Appl Physiol (1985) 1989 Apr;66(4):1730–1735. doi: 10.1152/jappl.1989.66.4.1730. [DOI] [PubMed] [Google Scholar]
- Voelkel N. F., Gerber J. G., McMurtry I. F., Nies A. S., Reeves J. T. Release of vasodilator prostaglandin, PGI2, from isolated rat lung during vasoconstriction. Circ Res. 1981 Feb;48(2):207–213. doi: 10.1161/01.res.48.2.207. [DOI] [PubMed] [Google Scholar]
- Voelkel N. F. Mechanisms of hypoxic pulmonary vasoconstriction. Am Rev Respir Dis. 1986 Jun;133(6):1186–1195. doi: 10.1164/arrd.1986.133.6.1186. [DOI] [PubMed] [Google Scholar]
- van Grondelle A., Worthen G. S., Ellis D., Mathias M. M., Murphy R. C., Strife R. J., Reeves J. T., Voelkel N. F. Altering hydrodynamic variables influences PGI2 production by isolated lungs and endothelial cells. J Appl Physiol Respir Environ Exerc Physiol. 1984 Aug;57(2):388–395. doi: 10.1152/jappl.1984.57.2.388. [DOI] [PubMed] [Google Scholar]



