Abstract
Introduction
Pseudoangiomatous stromal hyperplasia (PASH) is a benign localized fibrotic lesion in which clusters of spindle cells form cleft-like spaces, resembling ectatic vessels. Its relationship to breast cancer risk has not been characterized.
Methods
Histological presence of PASH was evaluated by review of archival slides in a single institution cohort of women who underwent benign excisional breast biopsy from 1967-1991. Relative risks for subsequent breast cancer were estimated using standardized incidence ratios (SIR), comparing the observed number of cancers with those expected based on Iowa SEER data (mean follow-up 18.5 years).
Results
PASH was identified in 579/9065 biopsies (6.4%). Women with PASH were younger, more likely to have a palpable mass as indication for biopsy, and had less lobular involution compared to those without PASH (all p<0.001), while they did not differ by family history of breast cancer or degree of epithelial proliferation. Breast cancers occurred in 34 women with PASH (5.9%) and 789 without (8.8%). Women with PASH had lower risk of breast cancer (SIR 1.03, 95% CI 0.71 to 1.44) than those without PASH (SIR 1.54, 95% CI 1.43 to 1.65), p=0.01. Lower levels of breast cancer risk for the PASH group persisted in analyses stratified by age, family history, epithelial proliferation, and involution. The cancers in the PASH group occurred predominantly in the ipsilateral breast greater than five years after biopsy.
Conclusions
Despite clinical concern generated by palpable density often associated with PASH, this relatively uncommon histological finding does not connote increased risk of subsequent breast cancer.
Introduction
Pseudoangiomatous stromal hyperplasia (PASH) is a benign stromal lesion of the breast characterized by dense collagenous stroma forming capillary-like spaces lined by slender spindle cells. First described by Vuitch et al in 1986[1], PASH is a relatively rare clinical problem. Since its original description, most information on PASH has consisted primarily of case reports [2-5] with a few moderate size case series more recently [6-8]. There are two primary clinical presentations- either distinct nodular growth or diffuse enlargement of the breast.[3, 9] Despite the relative rarity of PASH as symptomatic breast disease, it can also present as a mammographic density at screening,[8] and microscopic PASH may be much more common as a histologic finding in breast tissue.
In one retrospective histologic review of 200 consecutive benign or malignant breast specimens, Ibrahim et al reported microscopic PASH in 23% of specimens, with multifocality in 60% of cases [10]. In contrast, a later report indicated a frequency of clinical PASH in only 7 of 1661 breast biopsies (0.4%) [11]. This discrepancy raises questions about the true frequency of PASH and its possible association with breast cancer. In this report, we describe the frequency of histologic PASH in a large cohort of women with benign surgical breast biopsy and the relationship of PASH to subsequent breast cancer risk.
Methods
Study population
The Mayo Benign Breast Disease Cohort consists of 9087 women age 18 to 85 years who underwent surgical excisional biopsy of a benign breast lesion at the Mayo Clinic between January 1967 and December 1991 for whom follow-up information could be obtained; this cohort previously has been described in detail.[12, 13] Information on family history and other possible risk factors for breast cancer was obtained from a study specific questionnaire. A family history of breast cancer was categorized as follows: 1) Strong- at least one first-degree relative with breast cancer before the age of 50 years or two or more relatives with breast cancer (at least one a first degree relative), 2) Weak- any family history lesser than the definition of strong, or 3) None. Follow-up for breast cancer events was obtained through questionnaire data as well as the inpatient and outpatient medical record. All study procedures were reviewed and approved by the Mayo Clinic institutional review board.
Histologic review
Archived hematoxylin-and-eosin stained sections from each case were secured and reviewed by a single breast pathologist who was blinded to the initial histologic diagnoses and subsequent patient outcomes. Biopsy findings were classified into customary major histologic categories of benign breast disease according to the criteria of Dupont and Page [14] as nonproliferative changes (NP), proliferative changes without atypia (PDWA), and proliferative changes with atypical hyperplasia (AH). In addition, the degree of lobular involution in the background breast lobules was characterized as none, partial (1-74% of terminal duct lobular units [TDLUs]), or complete (75% or more).[15]
PASH
PASH cases were defined based upon the microscopic review that was performed by the dedicated study pathologist for all 9087 benign biopsies. PASH status could be determined for 9065 of the 9087 eligible subjects (22 cases had no background breast tissue to assess). PASH was defined as a well demarcated nodule comprised of dense collagen with increased numbers of cytologically benign spindle shaped cells arranged around clefted spaces, resembling ectatic vessels (Figure 1). The lesions contained epithelial lobules having a simplified (unfolded) architecture, with enlarged microcystic acini and edematous stroma. To be defined as PASH in the cohort we required that the lesion incorporate at least three lobules and comprise the majority of a low magnification field (4X objective).
Figure 1.
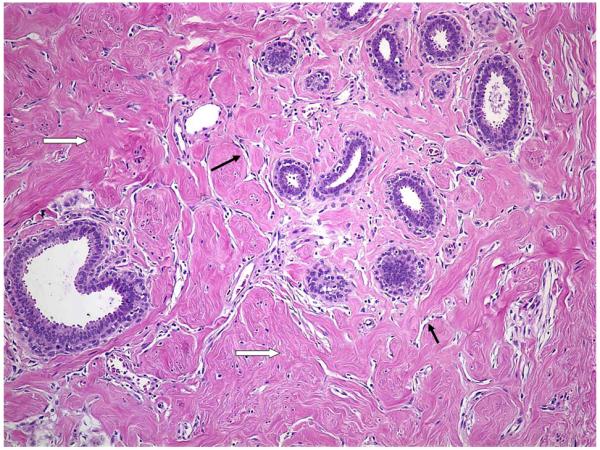
Photomicrograph of PASH at 100X magnification. Defining histologic features include dense collagen (marked with ⇨) with increased numbers of cytologically benign spindle shaped cells arranged around clefted spaces, resembling ectatic vessels (marked with →). The epithelial lobules have a simplified (unfolded) architecture, with enlarged microcystic acini and edematous stroma.
Statistical analysis
Data were descriptively summarized using frequencies and percents for categorical variables and means and standard deviation for continuous variables. We compared presence versus absence of PASH across levels of categorical variables (including age at biopsy, year of biopsy, indication for biopsy, category of epithelial proliferation, extent of lobular involution, family history of breast cancer, and presence of concomitant fibroadenoma) using chi-square tests of significance. All variables found to be univariately associated with PASH status were then included in a multivariate logistic regression analysis to assess associations independent of other effects.
The duration of follow-up was calculated as the number of days from biopsy of the benign lesion to the date of the diagnosis of breast cancer, death, or last contact. Additional censoring events included prophylactic mastectomy and lobular carcinoma in situ (LCIS). We estimated relative risks (RR) using standardized incidence ratios (SIRs) and corresponding 95% confidence intervals (CI), dividing the observed numbers of incident breast cancers by population-based expected counts. We calculated these expected counts by apportioning each woman’s follow-up into 5-year age and calendar period categories, thereby accounting for differences associated with these variables. We used the Iowa Surveillance, Epidemiology, and End Results (SEER) registry as the reference population because of its geographic proximity and its demographic similarities to the Mayo Clinic population (80% of cohort members reside in the upper Midwest).[12] Over 95% of our cohort was Caucasian, equivalent to that reported in Iowa census data during the study period. SIRs were calculated for the presence/absence of PASH in general and also for subgroups defined by PASH with other demographic and histologic variables. We assessed potential heterogeneity in SIRs across subgroups using Poisson regression analysis, with the log transformed expected event rate for each individual modeled as the offset term. Statistical tests were two-sided and analyses were conducted using SAS (SAS Institute, Inc., Cary, NC) software.
Results
Clinical characteristics
Among 9065 benign surgical breast biopsies, 579 (6.4%) had histologic PASH. Compared to women with other benign breast disease, women whose biopsies showed PASH were significantly younger and more frequently had a palpable lump as the indication for biopsy (Table 1). A family history of breast cancer was present at similar frequency among women with and without PASH. Data on indication for biopsy were missing in 558 women, but there was no significant difference in the frequency of missingness between those with PASH (8%) and those without (6%).
Table 1.
Demographic and Clinical Characteristics by PASH Status
| NO (N=8486) | YES (N=579) | p value1 | p value2 | |
|---|---|---|---|---|
| Age at Benign Biopsy | <0.001 | <0.001 | ||
| <45 | 2662 (31.4) | 309 (53.4) | ||
| 45 - 54 | 2360 (27.8) | 203 (35.1) | ||
| 55+ | 3464 (40.8) | 67 (11.6) | ||
| Family History of Breast Cancer | 0.116 | |||
| (missing, n=39) | 37 | 2 | ||
| None | 5625 (66.6) | 360 (62.4) | ||
| Weak | 2014 (23.8) | 153 (26.5) | ||
| Strong | 810 (9.6) | 64 (11.1) | ||
| Indication for Biopsy | <0.001 | 0.005 | ||
| (missing, n=558) | 513 | 45 | ||
| Lump | 4722 (59.2) | 379 (71) | ||
| Mammogram | 3251 (40.8) | 155 (29) | ||
| Epithelial Proliferation | 0.606 | |||
| Non-proliferative | 5643 (66.5) | 394 (68) | ||
| Proliferative without atypia | 2538 (29.9) | 168 (29) | ||
| Atypical hyperplasia | 305 (3.6) | 17 (2.9) | ||
| Lobular Involution | <0.001 | <0.001 | ||
| (missing, n=609) | 604 | 5 | ||
| None | 1481 (18.8) | 98 (17.1) | ||
| Partial (1-74% TDLU) | 4595 (58.3) | 455 (79.3) | ||
| Complete (>75% TDLU) | 1806 (22.9) | 21 (3.7) | ||
| Fibroadenoma | <0.001 | <0.001 | ||
| (missing, n=65) | 62 | 3 | ||
| NO | 6325 (75.1) | 527 (91.5) | ||
| YES | 2099 (24.9) | 49 (8.5) |
Values displayed as number (percent). Numbers may not always sum to column totals due to missing values for some subjects. P-values based on chi-square test of significance.
P-value from multivariate logistic regression adjusting for age, year of biopsy, indication for biopsy, number of breast biopsies, lobular involution, and fibroadenoma. Only variables univariately significant were included in the multivariate analysis.
Histologic findings
The overall histologic category of benign breast disease findings (reflecting the degree of epithelial hyperplasia) was not significantly different between women with and without PASH, with approximately two-thirds nonproliferative disease, one third proliferative disease without atypia, and ~3% atypical hyperplasia (Table 1). Complete lobular involution was significantly less common in women with PASH (only 3.7% with >75% involution compared to 22.9% in those without PASH, p<0.001) and this remained significant with multivariate adjustment. Findings on lobular involution were unavailable for 609 women (less than 1% of the PASH group and 7% of the remaining cohort) due to lack of adequate background breast tissue in these samples to assess this histologic feature. Fibroadenoma was present less frequently in biopsies with PASH (8.5%) compared to those without PASH (24.9%, p<0.001).
Breast cancer risk
Mean follow up time for the cohort is 18.5 years. Despite longer mean follow up among those with histologic PASH than those without (19.8 years versus 18.4 years, p=0.0005), the proportion of women that developed a subsequent breast cancer was lower for those with PASH (34/579=5.9%) compared to those without PASH (789/8486= 9.3%, Table 2). When breast cancer risk was standardized to the baseline cancer incidence in the comparison Iowa SEER population (which also accounted for length of follow up), women with histologic PASH did not have a higher risk of breast cancer than the general population (SIR 1.03, 95% confidence interval 0.71-1.44), while the remaining women with benign breast disease did (SIR 1.54, 95% CI 1.43-1.65, test for heterogeneity in SIRs p=0.01). Lower levels of breast cancer risk for women with PASH persisted in subgroup analyses stratified by age, family history, epithelial proliferation, and lobular involution (Table 2 and Figure 2). In the subgroup with both PASH and atypical hyperplasia, breast cancer risk was not lower, but the risk estimate has wide confidence intervals due to small subsample size (n=17). Similarly, there were only 21 patients with both PASH and complete involution, limiting results in this subgroup. The overall general trend in subgroup analyses was toward lower risk in women with PASH, although confidence intervals overlapped in many subgroups due to small numbers of subjects and breast cancer events in subsets.
Table 2.
PASH and the Risk of Breast Cancer
| Variable | N | Person Years |
Observed Events |
Expected Events |
Risk Ratio (95% CI) |
|---|---|---|---|---|---|
| PASH | |||||
| No | 8486 | 156375 | 789 | 512.1 | 1.54(1.43 - 1.65) |
| Yes | 579 | 11485 | 34 | 33.1 | 1.03(0.71 - 1.44) |
| Age by PASH Status | |||||
| Age <45 | |||||
| No | 2662 | 57323 | 194 | 120.1 | 1.62(1.40 - 1.86) |
| Yes | 309 | 6421 | 13 | 14.2 | 0.91(0.48 - 1.56) |
| Age 45-54 | |||||
| No | 2360 | 48625 | 274 | 170.7 | 1.61(1.42 - 1.81) |
| Yes | 203 | 4070 | 15 | 14.3 | 1.05(0.59 - 1.73) |
| Age 55+ | |||||
| No | 3464 | 50427 | 321 | 221.4 | 1.45(1.30 - 1.62) |
| Yes | 67 | 994 | 6 | 4.5 | 1.33(0.49 - 2.89) |
|
Family History by PASH
Status | |||||
| No Family History | |||||
| No | 5625 | 98782 | 437 | 326.9 | 1.34(1.21 - 1.47) |
| Yes | 360 | 7116 | 16 | 20.7 | 0.77(0.44 - 1.25) |
| Weak Family History | |||||
| No | 2014 | 40066 | 236 | 127.0 | 1.86(1.63 - 2.11) |
| Yes | 153 | 3095 | 12 | 8.9 | 1.35(0.70 - 2.36) |
| Strong Family History | |||||
| No | 810 | 16979 | 114 | 56.7 | 2.01(1.66 - 2.41) |
| Yes | 64 | 1266 | 6 | 3.5 | 1.72(0.63 - 3.73) |
|
Epithelial Proliferation by
PASH Status | |||||
| Non-proliferative disease | |||||
| No | 5643 | 106460 | 412 | 336.0 | 1.23(1.11 - 1.35) |
| Yes | 394 | 8234 | 17 | 22.3 | 0.76(0.44 - 1.22) |
| Proliferative without atypia | |||||
| No | 2538 | 45540 | 309 | 159.8 | 1.93(1.72 - 2.16) |
| Yes | 168 | 3002 | 12 | 9.9 | 1.21(0.63 - 2.11) |
| Atypical hyperplasia | |||||
| No | 305 | 4375 | 68 | 16.4 | 4.14(3.21 - 5.25) |
| Yes | 17 | 249 | 5 | 0.8 | 6.02(1.96 - 14.02) |
| Involution by PASH Status | |||||
| None | |||||
| No | 1481 | 31952 | 159 | 81.2 | 1.96(1.67 - 2.29) |
| Yes | 98 | 2061 | 5 | 5.2 | 0.95(0.31 - 2.22) |
| Partial | |||||
| No | 4595 | 84937 | 451 | 288.5 | 1.56(1.42 - 1.71) |
| Yes | 455 | 9022 | 28 | 26.4 | 1.06(0.70 - 1.53) |
| Complete | |||||
| No | 1806 | 27476 | 116 | 112.6 | 1.03(0.85 - 1.24) |
| Yes | 21 | 318 | 0 | 1.2 | 0.00 (NA) |
|
Indication for Biopsy by
PASH Status | |||||
| Lump | |||||
| No | 4722 | 94428 | 489 | 287.4 | 1.70(1.55 - 1.86) |
| Yes | 379 | 7808 | 19 | 21.6 | 0.88(0.53 - 1.38) |
| Mammogram | |||||
| No | 3251 | 50687 | 279 | 197.5 | 1.41(1.25 - 1.59) |
| Yes | 155 | 2621 | 15 | 8.9 | 1.69(0.95 - 2.79) |
|
Fibroadenoma by PASH
Status | |||||
| Fibroadenoma | |||||
| No | 2099 | 39160 | 177 | 110.8 | 1.60(1.37 - 1.85) |
| Yes | 49 | 902 | 4 | 2.3 | 1.71(0.47 - 4.38) |
| No Fibroadenoma | |||||
| No | 6325 | 116003 | 607 | 397.2 | 1.53(1.41 - 1.65) |
| Yes | 527 | 10521 | 30 | 30.5 | 0.98(0.66 - 1.40) |
SIRs compare the observed number of breast cancer events with the number expected on the basis of Iowa SEER Data. All analyses account for the effects of age and calendar period.
Figure 2.
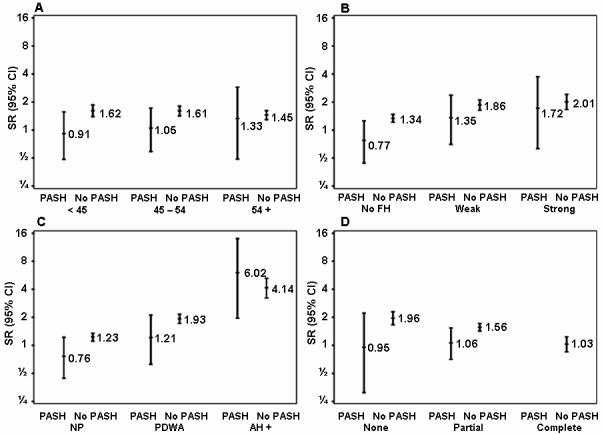
Risk of breast cancer by combinations of PASH status and other demographic and clinical characteristics. Relative risks are calculated using standardized incidence ratios (SIRs), comparing the observed number of breast cancer events with the number expected on the basis of Iowa SEER data. Panel A examines age; Panel B, family history of breast cancer; Panel C, degree of epithelial proliferation; and Panel D, extent of lobular involution.
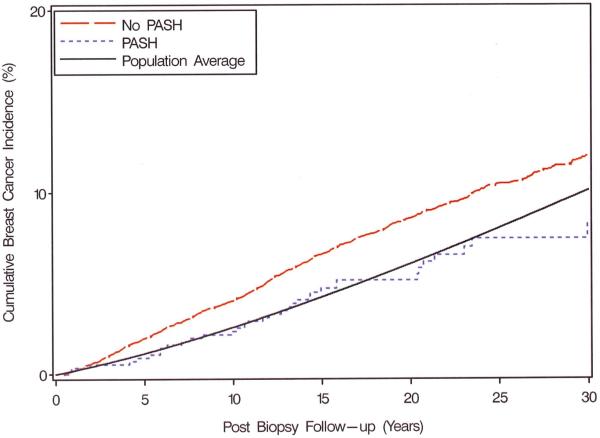
Among the 34 breast cancers that developed in women with PASH, 8 were DCIS, 23 were invasive, and 3 were unknown. The proportions of DCIS and invasive cancer were not significantly different in women with and without PASH (Table 3). The time interval between benign biopsy and subsequent breast cancer was similar regardless of the presence of PASH, with 85% diagnosed more than five years after benign biopsy, showing strong similarity to the time course of cancers developing in the SEER comparison general population (Figure 3). The few cancers that occurred within the first five years after biopsy showed no predilection for occurrence in the ipsilateral breast that had undergone biopsy. However, there was a striking difference in the laterality of later breast cancers between women with and without PASH. Among the women with PASH, 84.6% of the cancers diagnosed after five years occurred in the ipsilateral breast compared to 50.3% in the ipsilateral breast for women whose benign biopsies did not show PASH (p=0.001).
Table 3.
Tumor attributes by PASH Status among those developing cancer
| NO (N=789) |
YES (N=34) |
p value | |
|---|---|---|---|
| Breast Cancer Type | 0.292 | ||
| Invasive | 581 (81.7) | 23 (74.2) | |
| In situ | 130 (18.3) | 8 (25.8) | |
| Tumor Type, Invasive Cancers | 0.521 | ||
| Ductal | 301 (61.2) | 16 (76.2) | |
| Lobular | 59 (12) | 2 (9.5) | |
| Mixed Ductal and Lobular | 64 (13) | 2 (9.5) | |
| Other | 68 (13.8) | 1 (4.8) | |
| Time to Breast Cancer | 0.436 | ||
| <5 Years | 159 (20.2) | 5 (14.7) | |
| 5+ Years | 630 (79.8) | 29 (85.3) | |
| Laterality of Cancer | 0.003 | ||
| Ipsilateral | 372 (53.2) | 25 (80.6) | |
| Contralateral | 327 (46.8) | 6 (19.4) | |
| Laterality of Cancer (Cancer <5 Years after Biopsy) | 0.833 | ||
| Ipsilateral | 93 (64.6) | 3 (60.0) | |
| Contralateral | 51 (35.4) | 2 (40.0) | |
| Laterality of Cancer (Cancer 5+ Years after Biopsy) | |||
| Ipsilateral | 279 (50.3) | 22 (84.6) | 0.001 |
| Contralateral | 276 (49.7) | 4 (15.4) |
Values displayed as number (percent). P-values based on chi-square test of significance. Numbers may not always sum to column totals due to missing values for some subjects.
Figure 3.
Observed and expected (population average) cumulative breast cancer incidence, by levels of PASH status. Expected events calculated by applying age- and calendar period-stratified person years of observation among all women in the cohort to corresponding Iowa SEER breast cancer incidence rates. Observed and expected events cumulated after accounting for death as a competing risk.
Discussion
We have evaluated the histological presence of PASH in a large retrospective cohort of women who underwent surgical breast biopsy, for whom long term follow-up on cancer events is known. The primary findings of our study are that 1) PASH is an infrequent but not rare major pathologic finding, present in ~6% of benign surgical breast biopsies, and 2) PASH appears to indicate a lower than average risk of subsequent breast cancer among benign breast disease findings and thus should not be considered a component or manifestation of proliferative breast disease (in which risk is related to epithelial proliferation).
Our study confirms the predominantly palpable presentation of PASH in premenopausal/perimenopausal women during our study timeframe. Nearly all of our patients were less than 55 years of age (88%) and the majority (71%) had a palpable abnormality as indication for their biopsy. Women with PASH were significantly younger than the remainder with non-PASH benign breast disease. In this limited sense, PASH is clinically similar to other fibroepithelial lesions, especially fibroadenoma, that develop in younger age populations.
Other clinical manifestations of PASH remain incompletely studied. Initial reports described cases presenting in premenopausal women as a palpable mass which were treated with mastectomy occasionally [3, 5]; among cases treated with excision the disease recurred infrequently [1]. The size of individual lesions can vary from small masses near one centimeter to bulky and rapidly enlarging masses that can simulate locally advanced malignancy [10, 11]. More recent reports have described the imaging findings of PASH cases, including some that present clinically as a non-palpable mammographic abnormality [8, 16, 17].
There are a few reports describing clinical management of PASH in small- to moderate-sized series of patients with PASH. Ferreira et al reported 26 cases of PASH, with one case (4%) harboring concomitant cancer- this patient had a 4 cm mass with a 1.4 cm hypoechoic suspicious area within the larger mass.(7) In addition, they described 8 patients diagnosed with core biopsy who were followed successfully without excision who had stable disease at 2 years follow-up. In another series of 22 patients, no cancers were associated with PASH, and five women who did not undergo excision had no growth or recurrence at 4 years of follow-up.(6) The largest series on clinical management of PASH cases was presented by Hargaden et al with 149 patients, some who presented with a clinically palpable concern (n=90) and some mammographically detected (n=59).(8) Cancer was associated with PASH in 4% of the cases; all of these had mammographic calcifications. The majority (89%) of lesions were surgically excised, with only 16 patients who had a core biopsy and clinical surveillance. With four years of follow-up, they reported no subsequent cancers detected at the site of PASH. In summary, the published experience on patients with unresected PASH is limited, but the available evidence supports a clinical recommendation for core needle biopsy for initial diagnosis and proceeding to surgical excision for lesions that are symptomatic, enlarging clinically, or have suspicious imaging findings (BIRADS 4 or 5).
Frequency of PASH has been evaluated in relatively small series of cases that were highly selected by sample type or detection method which partly accounts for widely discrepant reported frequencies of PASH. Another factor in variable reported frequencies is the lack of consensus opinion about minimal diagnostic criteria for PASH in pathologic specimens. Ibrahim et al included foci as small as individual lobules in their definition, which probably accounts for the high observed incidence in their series of specimens - 23%.[10] Our study employed an arbitrary size threshold for diagnosis that required involvement of multiple (at least 3) lobular units, which may explain the lower case frequency observed in our series. The size cutoff in this study (about 3mm) would likely have permitted inclusion of subclinical examples in the series. It is important to emphasize in this context that PASH clearly represents a fairly broad clinicopathologic spectrum, ranging from incidental microscopic finding to a sizeable or symptomatic mass.
Our data suggest that the pathogenesis of PASH is fundamentally different from that of proliferative breast lesions such as duct hyperplasia or adenosis. Rather than a proliferation of epithelial cells, histologically PASH is a proliferation of myofibroblastic cells.[9] For this reason, some have suggested that it should be renamed nodular myofibroblastic stromal hyperplasia.[19] In a general sense, pathologists understand myofibroblasts to be fibroblasts that have become activated by cytokines and growth factors as a component of the inflammatory and wound healing response. As opposed to fibroblasts, they have increased secretory activity (proteases and extracellular matrix proteins). They also have contractile filaments - mostly actin – which are thought to mediate motility and wound closure.[20] These changes have been considered analogous to macrophage activation. PASH may represent a situation where stromal fibroblasts have become activated, resulting in both cellular proliferation and oversecretion of collagen (which is dense and overabundant in PASH). An epithelial-stromal interaction is strongly suggested, since the lobules in PASH are not normal. They are architecturally simplified and embedded in the stromal proliferation as an integral part of the process.
There is evidence that hormones play a role in PASH, with common progesterone staining of PASH stromal cells. [9] [21] In an early characterization of estrogen and progesterone staining in five cases of PASH, all five showed patchy but intense staining of stromal cells with progesterone receptor antibodies, in contrast to normal mammary stroma which showed no progesterone receptor staining.[16] In a later sample of 14 specimens, some variability in estrogen and progesterone receptor staining was seen, with stromal nuclei negative for both in five cases, two cases positive for both estrogen and progesterone receptors, five positive for progesterone only, and two for estrogen only.[9] A hormonal etiology is also supported by the higher frequency of PASH in premenopausal women and by one reported case where PASH improved dramatically with tamoxifen treatment. [22]
Reported associations with oral contraceptives or hormonal replacement therapy and cases with rapid tumor growth all suggest that PASH may reflect exaggerated hormonal responsiveness of breast tissue in affected patients. Given the retrospective nature of our cohort, data on oral contraceptive and hormone replacement therapy use were missing for the majority of women, so we were not able to directly evaluate such associations with PASH. Our subjects with PASH were significantly younger than women with other benign breast disease, in keeping with divergent pathogenesis for PASH and epithelial lesions. Furthermore, PASH lesions did not segregate more frequently with proliferative as opposed to non-proliferative biopsies. Finally, we identified no evidence that PASH was associated with clinical markers of increased risk, such as family history of disease.
To the contrary, subjects with PASH in our cohort were significantly less likely to develop breast malignancy than those without. We identified no clinical, epidemiological or histological association with PASH that might explain this negative correlation. It should be noted, however, that there is less understanding of markers of low risk for breast cancer, such as decreased mammographic density, compared to high risk markers. Therefore further study of women and breast tissue with PASH might improve insight into factors and mechanisms that inhibit mammary tumorigenesis. On the other hand, the strong ipsilateral predilection for subsequent breast cancer more than five years after biopsy suggests that the presence of PASH may somehow represent a permissive stromal environment that facilitates breast carcinogenesis locally in epithelial cells once they have progressed along a pathway of proliferation. A growing body of literature supports the critical role of the stroma in promoting mammary neoplasia.[23, 24] Perhaps the host hormonal environment that leads to development of PASH discourages breast carcinogenesis in global ways via suppression of epithelial proliferation, while the myofibroblasts or secreted cytokines in the microenvironment of PASH promote carcinogenesis locally in the event that epithelial proliferation does occur. These paradoxical findings on PASH and its global versus local breast cancer risk deserve validation and further investigation, with potential for insight into epithelial-stromal interactive mechanisms of breast cancer development.
Synopsis.
Pseudoangiomatous stromal hyperplasia (PASH) occurs as a major histologic finding in ~6% of surgical breast biopsies. Women with PASH do not have an increased future risk of breast cancer.
Acknowledgements
Amy C. Degnim is supported by the CA90628-08 Paul Calabresi Award for Clinical-Translational Research (K12) via the Mayo Clinic Cancer Center. Lynn C. Hartmann and V. Shane Pankratz are supported in part by R01CA132879. We thank Teresa Allers, Jo Johnson, Mary Campion, Melanie Kasner, and Romayne Thompson for data collection; Joel Worra for database development; Ann Harris and the Survey Research Center for patient follow-up; and Marilyn Churchward for assistance with manuscript preparation.
References
- 1.Vuitch MF, Rosen PP, Erlandson RA. Pseudoangiomatous hyperplasia of mammary stroma. Hum Pathol. 1986;17:185–191. doi: 10.1016/s0046-8177(86)80292-1. [DOI] [PubMed] [Google Scholar]
- 2.Castro CY, Whitman GJ, Sahin AA. Pseudoangiomatous stromal hyperplasia of the breast. Am J Clin Oncol. 2002;25:213–216. doi: 10.1097/00000421-200204000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Singh KA, Lewis MM, Runge RL, Carlson GW. Pseudoangiomatous stromal hyperplasia. A case for bilateral mastectomy in a 12-year-old girl. Breast J. 2007;13:603–606. doi: 10.1111/j.1524-4741.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- 4.Prasad SN, Houserkova D, Svach I, et al. Pseudoangiomatous stromal hyperplasia of breast: a case report. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:117–120. doi: 10.5507/bp.2008.018. [DOI] [PubMed] [Google Scholar]
- 5.Zubor P, Kajo K, Dussan CA, et al. Rapidly growing nodular pseudoangiomatous stromal hyperplasia of the breast in an 18-year-old girl. Apmis. 2006;114:389–392. doi: 10.1111/j.1600-0463.2006.apm_207.x. [DOI] [PubMed] [Google Scholar]
- 6.Wieman SM, Landercasper J, Johnson JM, et al. Tumoral pseudoangiomatous stromal hyperplasia of the breast. Am Surg. 2008;74:1211–1214. [PubMed] [Google Scholar]
- 7.Ferreira M, Albarracin CT, Resetkova E. Pseudoangiomatous stromal hyperplasia tumor: a clinical, radiologic and pathologic study of 26 cases. Mod Pathol. 2008;21:201–207. doi: 10.1038/modpathol.3801003. [DOI] [PubMed] [Google Scholar]
- 8.Hargaden GC, Yeh ED, Georgian-Smith D, et al. Analysis of the mammographic and sonographic features of pseudoangiomatous stromal hyperplasia. AJR Am J Roentgenol. 2008;191:359–363. doi: 10.2214/AJR.07.2479. [DOI] [PubMed] [Google Scholar]
- 9.Powell CM, Cranor ML, Rosen PP. Pseudoangiomatous stromal hyperplasia (PASH). A mammary stromal tumor with myofibroblastic differentiation. Am J Surg Pathol. 1995;19:270–277. doi: 10.1097/00000478-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim RE, Sciotto CG, Weidner N. Pseudoangiomatous hyperplasia of mammary stroma. Some observations regarding its clinicopathologic spectrum. Cancer. 1989;63:1154–1160. doi: 10.1002/1097-0142(19890315)63:6<1154::aid-cncr2820630619>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Polger MR, Denison CM, Lester S, Meyer JE. Pseudoangiomatous stromal hyperplasia: mammographic and sonographic appearances. AJR Am J Roentgenol. 1996;166:349–352. doi: 10.2214/ajr.166.2.8553945. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–237. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 13.McKian KP, Reynolds CA, Visscher DW, et al. Novel breast tissue feature strongly associated with risk of breast cancer. J Clin Oncol. 2009;27:5893–5898. doi: 10.1200/JCO.2008.21.5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 15.Milanese TR, Hartmann LC, Sellers TA, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98:1600–1607. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 16.Choi YJ, Ko EY, Kook S. Diagnosis of pseudoangiomatous stromal hyperplasia of the breast: ultrasonography findings and different biopsy methods. Yonsei Med J. 2008;49:757–764. doi: 10.3349/ymj.2008.49.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercado CL, Naidrich SA, Hamele-Bena D, et al. Pseudoangiomatous stromal hyperplasia of the breast: sonographic features with histopathologic correlation. Breast J. 2004;10:427–432. doi: 10.1111/j.1075-122X.2004.21373.x. [DOI] [PubMed] [Google Scholar]
- 18.Sng KK, Tan SM, Mancer JF, Tay KH. The contrasting presentation and management of pseudoangiomatous stromal hyperplasia of the breast. Singapore Med J. 2008;49:e82–85. [PubMed] [Google Scholar]
- 19.Leon ME, Leon MA, Ahuja J, Garcia FU. Nodular myofibroblastic stromal hyperplasia of the mammary gland as an accurate name for pseudoangiomatous stromal hyperplasia of the mammary gland. Breast J. 2002;8:290–293. doi: 10.1046/j.1524-4741.2002.08508.x. [DOI] [PubMed] [Google Scholar]
- 20.Eyden B. The myofibroblast: an assessment of controversial issues and a definition useful in diagnosis and research. Ultrastruct Pathol. 2001;25:39–50. doi: 10.1080/019131201300004672. [DOI] [PubMed] [Google Scholar]
- 21.Anderson C, Ricci A, Jr., Pedersen CA, Cartun RW. Immunocytochemical analysis of estrogen and progesterone receptors in benign stromal lesions of the breast. Evidence for hormonal etiology in pseudoangiomatous hyperplasia of mammary stroma. Am J Surg Pathol. 1991;15:145–149. doi: 10.1097/00000478-199102000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Pruthi S, Reynolds C, Johnson RE, Gisvold JJ. Tamoxifen in the management of pseudoangiomatous stromal hyperplasia. Breast J. 2001;7:434–439. doi: 10.1046/j.1524-4741.2001.07611.x. [DOI] [PubMed] [Google Scholar]
- 23.Radisky ES, Radisky DC. Stromal induction of breast cancer: inflammation and invasion. Rev Endocr Metab Disord. 2007;8:279–287. doi: 10.1007/s11154-007-9037-1. [DOI] [PubMed] [Google Scholar]
- 24.Rozenchan PB, Carraro DM, Brentani H, et al. Reciprocal changes in gene expression profiles of cocultured breast epithelial cells and primary fibroblasts. Int J Cancer. 2009;125:2767–2777. doi: 10.1002/ijc.24646. [DOI] [PubMed] [Google Scholar]