Abstract
The adhesion receptor dystroglycan positively regulates terminal differentiation of oligodendrocytes, however the mechanism by which this occurs remains unclear. Using primary oligodendrocyte cultures, we identified and examined a connection between dystroglycan expression and the ability of insulin-like growth factor-1 (IGF-1) to promote oligodendrocyte differentiation. Consistent with previous reports, treatment with exogenous IGF-1 caused an increase in MBP protein that was preceded by activation of PI3K (AKT) and MAPK (ERK) signaling pathways. The extracellular matrix protein laminin was furthermore shown to potentiate the effect of IGF-1 on oligodendrocyte differentiation. Depletion of the laminin receptor dystroglycan using siRNA, however, blocked the ability of IGF-1 to promote oligodendrocyte differentiation of cells grown on laminin, suggesting a role for dystroglycan in IGF-1-mediated differentiation. Indeed, loss of dystroglycan led to a reduction in the ability of IGF-1 to activate MAPK, but not PI3K, signaling pathways. Pharmacological inhibition of MAPK signaling also prevented IGF-1-induced increases in MBP, indicating that MAPK signaling was necessary to drive IGF-1-mediated enhancement of oligodendrocyte differentiation. Using immunoprecipitation, we found that dystroglycan, the adaptor protein Grb2, and insulin receptor substrate-1 (IRS1), were associated in a protein complex. Taken together, our results suggest that the positive regulatory effect of laminin on oligodendrocyte differentiation may be attributed, at least in part, to dystroglycan’s ability to promote IGF-1-induced differentiation.
Keywords: Dystroglycan, IGF-1, oligodendrocyte, myelin, laminin, MAPK
Introduction
The majority of cells in the brain lack a detectable cell-associated extracellular matrix (ECM), or basement membrane. However, basement membrane proteins such as laminins have been identified outside of traditional basement membrane structures e.g. in the adult subventricular zone (SVZ) in blood vessel-associated “fractone” structures, and, in the developing brain associated with future white matter axon tracts (Colognato et al. 2002; Mercier et al. 2002; Tavazoie et al. 2008). Laminins that contain the α2-subunit are furthermore proposed to regulate CNS development as children born with laminin α2 deficiencies have white matter abnormalities, agyrias, and hypoplasias (Jones et al. 2001). In vitro, laminins have been shown to enhance the differentiation of oligodendrocytes, the myelinating glia of the CNS (Buttery and ffrench-Constant 1999). Recently, laminin-deficient mice have been shown to have myelin abnormalities (Chun et al. 2003) as well as delayed oligodendrocyte differentiation (Relucio et al. 2009), suggesting that laminins may regulate oligodendrogenesis.
While the precise role of laminins during CNS myelination is not fully understood, an examination of oligodendroglial laminin receptors (α6β1 integrin and the transmembrane glycoprotein dystroglycan) has provided insight into laminin’s potential influence on oligodendrocyte differentiation. α6β1 integrin plays a role in mediating growth factor-induced signaling through activation of PI3K/AKT (Barros et al. 2009; Colognato et al. 2002; Colognato et al. 2004). Yet, while animals with deleted or disrupted β1 integrin in the oligodendrocyte lineage exhibit CNS myelin abnormalities (Barros et al. 2009; Camara et al. 2009; Lee et al. 2006) and cell survival deficits (Benninger et al. 2006), all of these animal models exhibit relatively normal oligodendrocyte development, in contrast to the abnormal oligodendrocyte differentiation observed in laminin-deficient animals (Chun et al. 2003; Relucio et al. 2009). Dystroglycan, on the other hand, has been shown to promote the differentiation of cultured oligodendrocytes (Colognato et al. 2007). In contrast to α6β1 integrin, however, dystroglycan does not appear to affect oligodendrocyte survival, suggesting that the two receptors have distinct roles during oligodendrocyte development. Here, we aim to elucidate signaling mechanism(s) responsible for dystroglycan-mediated differentiation of oligodendrocytes.
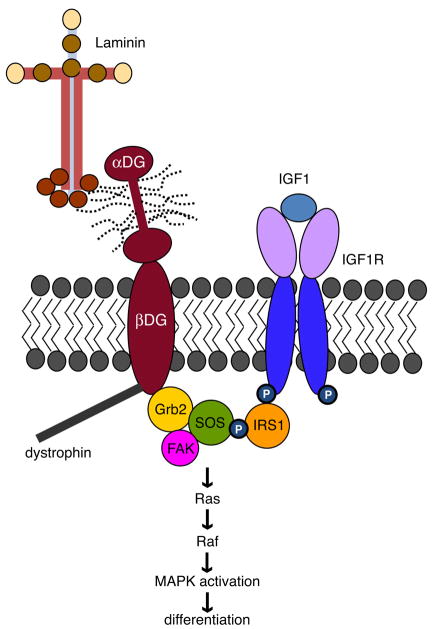
In muscle and other tissues, dystroglycan is a critical component of the dystrophin-glycoprotein complex that links the cytoskeleton with the ECM (Sunada and Campbell 1995). In addition, evidence suggests that dystroglycan may influence intracellular signaling through its association with adaptor molecules such as Grb2 (Russo et al. 2000). In both muscle and the brain, Grb2 has been shown to bind to dystroglycan (Yang et al. 1995). And, Grb2 contains SH2 and SH3 domains enabling it to bind a number of signaling molecules e.g. association between Grb2 and the guanine exchange factor SOS mediates activation of MAPK/ERK signaling through RAS/RAF (Skolnik et al. 1993b). Grb2 has also been shown to be critical in the propagation of IGF-1 signaling via its association with insulin receptor substrate-1 (IRS-1) (Holgado-Madruga et al. 1996). Given that dystroglycan and the IGF-1-receptor have each been shown to promote oligodendrocyte differentiation, and have each been linked to MAPK signaling, we examined potential connections between dystroglycan, IGF-1, and oligodendrocyte differentiation. We observed that, when cells were grown on laminin substrates, IGF-mediated effects on oligodendrocyte differentiation depended on dystroglycan, suggesting a functional connection between oligodendrocyte dystroglycan signal transduction and IGF-1 receptor signaling. Indeed, maximal activation of MAPK signaling by IGF-1, which was necessary for IGF-1-mediated enhancement of oligodendrocyte differentiation on laminin, was also found to be dystroglycan-dependent. We furthermore identified a protein complex in differentiating oligodendrocytes containing dystroglycan, Grb2, and IRS-1, and propose that this complex is a critical regulatory element that integrates extrinsic input from laminins and IGF-1 during oligodendrocyte differentation.
Methods
Cell Culture
Neonatal rat cortices were dissociated with papain and cultured on poly-D-lysine (PDL) coated flasks in progenitor maintenance media (high glucose Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS)) at 37°C in 7.5% CO2. Media was refreshed every 48-72 hours. A mixed glial culture, consisting of oligodendrocyte precursor cells (OPCs) and microglia bound to an astrocyte monolayer, was attained after approximately 10–12 days. OPCs were separated from mixed glial cultures by a procedure modified from the mechanical agitation and differential adhesion method described by McCarthy and De Vellis (Colognato et al. 2004; McCarthy and de Vellis 1980). OPCs were transferred to chamber slides or Nunclon tissue culture dishes that were pre-coated for 4 hrs with PDL or laminin-2 (following a 1 hr pre-coat with PDL), and incubated at 37°C in 7.5% CO2. To promote differentiation of oligodendrocytes, progenitor maintenance media was replaced with differentiation media (Sato with 0.5% FCS) and samples were incubated for an additional 72 hours. For experiments involving intracellular signaling, progenitor maintenance media was replaced with serum-free DMEM for 2 hours (serum starvation conditions). Growth factor was then added to appropriate samples for indicated times prior to lysis.
Protein Analysis
Cells were scraped from culture dishes into boil buffer (20mM Tris (pH 7.4), 1% Sodium dodecyl sulfate containing protease and phosphatase inhibitor cocktails (Calbiochem)) preheated to 95 °C. Lysates were transferred into microtubes and incubated at 95°C for 10 minutes. Insoluble material was separated by centrifugation at 18,000 g for 10 minutes. Protein concentration of lysates was evaluated using a detergent-compatible Bradford assay (Biorad). Lysates were combined with NuPAGER lithium dodecyl sulphate sample buffer (Invitrogen) with BME (final concentration of 3%) and boiled for 5 minutes. SDS-PAGE using 8%, 10%, 12% , or 15% mini-gels was used to separate proteins, which were then blotted onto 0.45 μm nitrocellulose. To prevent non-specific binding of antibodies, membranes were incubated for 60 minutes in blocking reagent (Tris buffered saline with 0.1% Tween20 (TBS-T) containing 4% BSA or 1% Milk). Primary antibodies were diluted in blocking reagent and incubated with membranes overnight at 4°C. Membranes were next washed with TBS-T and incubated for 60 minutes at room temperature in HRP-conjugated secondary antibodies (Amersham) diluted (1:3000) in blocking buffer. Finally, membranes were washed and developed using enhanced chemiluminescence (Amersham). All experiments were carried out at least 3 times. Representative blots are depicted.
Immunocytochemistry
Dystroglycan immunocytochemistry was performed on live cells. Media was replaced with fresh oligodendrocyte progenitor media containing 15ug/mL of IIH6 anti-α dystroglycan antibody and cells were incubated at room temperature for 45 minutes, before being washed 3 times with medium. Cells were then fixed with 100% methanol at −20°C for 5 minutes, incubated with DAPI, and coverslipped with SlowFade Gold (Molecular Probes). For dystroglycan co-immunocytochemistry, cells were fixed and processed for standard immunocytochemistry following live labeling with dystroglycan antibodies. Then, after fixation with 100% methanol at −20°C, cells were blocked for 30 minutes in PBS containing 5% donkey serum (blocking buffer), and then incubated for 60 minutes with additional primary antibodies diluted in blocking buffer. Slides were then treated for 60 minutes with FITC, Texas Red, or CY5-conjugated donkey IgG against mouse IgM (for IIH6), rabbit and/or rat IgG in blocking buffer. Washes were carried out using PBS (4 times, 5 minutes each). Slides were subjected to a final wash and mounted in Fluoromount G (Southern Biotech) or SlowFade Gold (Molecular Probes).
Microscopy and image acquisition
Cells were visualized using a Zeiss Axioplan inverted fluorescence microscope fitted with a 10X eyepiece using 20X (0.5 N.A.), 40X (0.75 N.A.), or 63X oil immersion objectives. Images were captured using a Zeiss Axiocam MRM digital camera and Zeiss Axiovision imaging software. Z-stacks were captured at 0.25 micron intervals and deconvolved using an iterative algorithm (Axiovision); maximal intensity projections obtained from the deconvolved stacked images are shown (panels B and D in Figure 5).
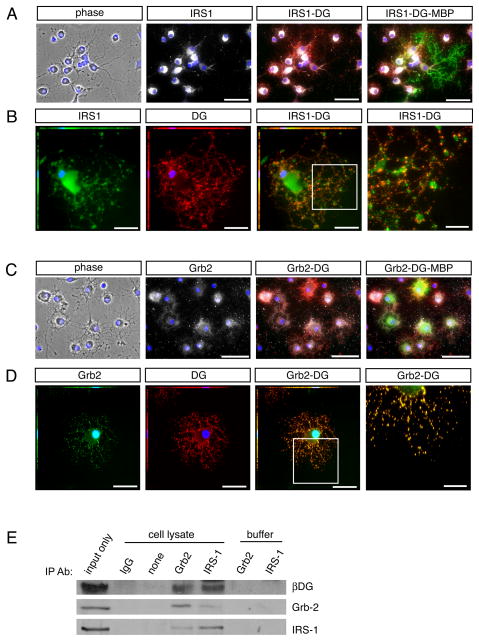
Fig 5. Dystroglycan is found in a protein complex with Grb2 and IRS1.
Oligodendrocytes were differentiated for 3 days on laminin followed by Immunocytochemistry. (A) Immunocytochemistry to visualize IRS1 (white) in conjunction with dystroglycan (red). Co-expression of the mature oligodendrocyte protein, MBP (green), was used to confirm oligodendrocyte identity. Scale bar, 50 μM. (B) Immunocytochemistry to visualize IRS1 (green) in conjunction with dystroglycan (red). Scale bar, 25 μM; 10 μM in inset, right. (C) Immunocytochemistry to visualize Grb2 (white) in conjunction with dystroglycan (red). Co-expression of the mature oligodendrocyte protein, MBP (green), was used to confirm oligodendrocyte identity. Scale bar, 50 μM. (D) Immunocytochemistry to visualize Grb2 (green) in conjunction with dystroglycan (red). Scale bar, 25 μM; 10 μM in inset, right. (E) Immunoprecipitations were performed with lysates obtained from oligodendrocytes differentiated for 3 days (input), using antibodies against Grb2, IRS1, or control rabbit IgG, in conjunction with Protein A/G beads. Immunopreciptation complexes isolated with Grb-2 antibodies contained Grb-2, β-dystroglycan (βDG), and IRS1. Immunopreciptation complexes isolated with IRS-1 antibodies contained IRS1, β-dystroglycan and Grb-2. Samples devoid of antibody (“none”), or treated with the equivalent amount of control antibody (“IgG”) did not isolate detectable levels of Grb-2, β-dystroglycan, or IRS1. Control samples containing equivalent amounts of antibody (Grb-2 or IRS1) but devoid of lysate were included to demonstrate that observed bands were not due to cross-reactivity of Western Blot secondary antibodies with IP antibodies.
Quantitation and Statistical Analysis
The relative intensity of Western Blot protein bands was determined using densitometry (ImageJTM). Values were normalized to appropriate loading controls (actin, or total protein in the case of evaluating changes in phosphorylation states) and expressed as the fold change relative to control samples (assigned a value of 100%). N values ranged from 3–4, as indicated. Error bars represent standard error of the mean. Mean relative pixel intensity was measured from fluorescent micrographs obtained using equal exposure times and acquisition settings (Axiovision). Area coverage by immunoreactivity was determined by measuring the area of immunofluorescence above a set threshold from 3 or more images per condition. This value was divided by the total number of cells in each field to give the immunoreactive-positive area per cell in a given image. Graphs depict the average fluorescent-positive area per cell of 4 experiments, with error bars depicting standard error of the mean. Statistical analysis was carried out using the 2-tailed paired student’s t-test.
RNA interference
A mixture of 4 siRNA duplexes specific for rat DG mRNA was used to substantially diminish dystroglycan protein expression (Dharmacon). Control cells were treated with a non-rat-targeting pool of siRNAs (Dharmacon). Transfection into OPCs was achieved using the Nucleofector electroporation system with the rat oligodendrocyte transfection reagent as described in the manufacturers protocol (Amaxa). Cells were transferred immediately into culture dishes or chamber slides. 16 hours later, cells were switched into either differentiation medium (Sato+ 0.5% FCS) or serum-free DMEM (signaling experiments). At 24 hours post DG siRNA transfection, we obtained an average dystroglycan protein level of 24.99 ± 12.78% that observed in cells transfected with control siRNA (n=3; p=0.01773; not shown).
Immunoprecipitation
Primary oligodendrocyte cultures were lysed at 4°C using 1% Triton X-100, 150nM NaCl, 50mM Tris (pH 7.5), 5mM EDTA, and protease/phosphatase inhibitors (Calbiochem). Cell lysates containing 150ug of total protein were pre-cleared for 30 minutes using Protein A/G agarose beads (Santa Cruz) in TBS. Antibodies were added to pre-cleared samples (input) or lysis buffer (control samples) at a final concentration of 50μg/mL and inverted overnight at 4°C. Samples were combined with 30μL of Protein A/G bead 1:1 suspension and gently inverted overnight at 4°C. Bound proteins were separated from beads by boiling in 100 μL of 1X NuPAGE® lithium dodecyl sulphate sample buffer (Invitrogen) containing 3% BME.
Pharmacological inhibition
To assess the effects of disrupting MAPK signaling, 10μM PD98059 (Sigma) or equivalent volume of vehicle (75% ETOH, 25% DMSO) was added to cells 30 minutes prior to growth factor treatment, and, for long-term differentiation experiments, again at 24 hours later followed by lysis at ~60 hours after the first inhibitor treatment. To assess the effects of endogenous IGF-1 receptor signaling in primary oligodendrocyte cultures, 10μM Tyrphostin1-OMe-AG 538 (Sigma) or the vehicle control (75% ETOH, 25% DMSO) was added to cells (Blum et al., 2000). Cytotoxicity studies were performed using this inhibitor and it was determined that 10 uM Tyrphostin1-OMe-AG 538 was not toxic to oligodendrocyte cultures (not shown). For signaling experiments, inhibitor or vehicle was added to oligodendrocytes following a 2h incubation in serum-free media. Cells were lysed after 30 minutes. For differentiation experiments, inhibitor or vehicle was added to OPCs placed in differentiation media for 3 days.
Reagents
Antibodies
The following antibodies were used for Western blotting: MBP (rat IgG; Serotec), 2’,3’-cyclic nucleotide 3’-phosphadiesterase (CNP) (rat IgG; Sigma), β-dystroglycan (mouse IgG; Novocastra), NG2 (rabbit IgG; Chemicon), β-actin (mouse IgG; Sigma), Grb2 (rabbit IgG; Abcam), Fyn (mouse IgG; BD Biosciences), Src Family Kinase phosphorylated-tyrosine 418 (rabbit IgG; Calbiochem), phosphorylated Akt Ser473 (rabbit IgG; Cell Signaling), Akt (rabbit IgG; Cell Signaling) , phosphorylated ERK1/ERK2 (T202/Y204) (rabbit IgG; R&D Systems), p44/42 MAP Kinase (total ERK1/2) (rabbit IgG; Cell Signaling), IRS1 (rabbit IgG; Abcam). The following antibodies were used in immunocyochemistry: α-DG (mouse IgM, clone IIH6; Upstate Biotechnologies); MBP (rat IgG; Serotec), 2’,3’-cyclic nucleotide 3’-phosphadiesterase (CNP) (rat IgG; Sigma); FITC-conjugated goat anti-mouse IgM (Sigma) or Texas Red-conjugated donkey anti-mouse IgM (Jackson ImmunoResearch), and FITC-, Texas Red-, or CY5-conjugated donkey IgG against mouse, rabbit or rat IgG.
Proteins
Human recombinant IGF-1 (PeproTech) was used at 50 or 100ng/mL. Unless otherwise indicated, plates or chamber slides were coated for 1 hour with 2.5ug/mL PDL (Sigma) at 37°C in 7.5% CO2. This wasfollowed by washes and 4 hour incubation with 10ug/mL laminin-2 (Millipore) under the same conditions.
Results
Laminin potentiates the ability of insulin-like growth factor-1 (IGF-1) to promote oligodendrocyte differentiation
IGF-1 has been shown previously to regulate several stages in oligodendrocyte development, including proliferation and differentiation; here we focused on the ability of IGF-1 to promote oligodendrocyte differentiation (McMorris et al. 1986; Ye et al. 1995; Ye et al. 2002b; Zeger et al. 2007). To confirm the pro-differentiation influence of exogenous IGF-1, oligodendrocytes were differentiated for 3 days in the presence or absence of 50ng/mL IGF-1 and evaluated for myelin basic protein (MBP) protein immunoreactivity. Immunoblots revealed that IGF1-treated cells contained higher levels of MBP compared to control cells (Supplementary Fig. S1A), and MBP immunocytochemistry furthermore revealed increased MBP immunoreactivity in IGF-1-treated cells (Supplementary Fig. S1B). Since the extracellular matrix protein laminin has been shown previously to enhance oligodendrocyte responses to the growth factors neuregulin-1 and PDGF (Baron et al. 2003; Colognato et al. 2002; Frost et al. 1999), we examined whether laminin could also enhance the pro-differentiation effects of IGF-1 (Fig. 1). Cells were differentiated for 3 days on PDL or laminin substrates in the presence or absence of IGF-1, and followed by CNP and MBP immunocytochemistry to visualize oligodendrocytes (CNP; Fig. 1A) and mature oligodendrocytes (MBP; Fig. 1B). Oligodendrocytes differentiated with laminin plus IGF showed an increase in cell area covered by MBP-positive, mature oligodendrocytes (Fig. 1C; in the absence of IGF-1: 46.8 ± 18.8% on PDL versus 64.7 ± 32.9% on Lm, n=3, p=0.3497; in the presence of IGF-1: 108.9±12.8% on Lm versus 70.0±17.0% on PDL, n=3, p=0.0429). In addition, average area covered by CNP-positive oligodendrocytes was also increased by laminin in IGF-1-treated cells (484.1±176.4% on Lm versus 335.6±155.0% on PDL, n=3, p=0.0268, not shown). Finally, the percentages of mature oligodendrocytes increased when cells were differentiated with laminin plus IGF, relative to those differentiated with IGF-1 on PDL (53.7±4.2% CNP(+) cells on Lm versus 41.7±7.0% on PDL, n=3, p=0.1545; 21.4±1.3% MBP(+) cells on Lm versus 14.4±1.5% on PDL, n=3, p=0.0438; not shown). Together, these data suggested that IGF-mediated effects on oligodendrocyte differentiation were amplified by the presence of laminin.
Fig. 1. Laminin potentiates IGF-mediated effects on oligodendrocyte differentiation.
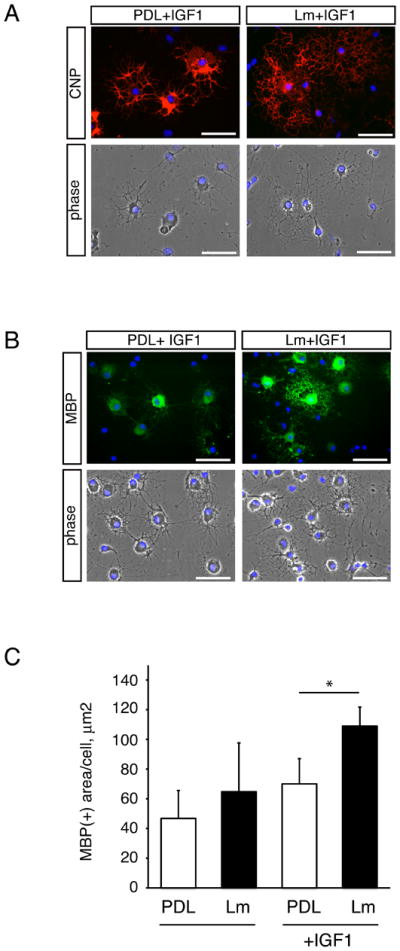
Oligodendrocytes were differentiated on PDL or laminin substrates for 3 days in the presence or absence of 50 ng/ml IGF-1. (A) Differentiated oligodendrocytes were evaluated using CNP immunocytochemistry. Representative micrographs of CNP immunoreactivity (red) and DAPI nuclear stain (blue) are shown. Scale bar, 50 μM. (B) Differentiated oligodendrocytes were evaluated using MBP immunocytochemistry. Representative micrographs of MBP immunoreactivity (green) and DAPI nuclear stain (blue) are shown. Scale bar, 50 μM. (C) Bars represent the mean (± sem) relative change in MBP(+) oligodendrocyte cell area after differentiation on PDL or laminin (Lm), in the presence or absence of 50 ng/ml IGF-1. Mean areas are normalized to mean area of control cells on PDL (*p<0.05, n=3).
Dystroglycan is required for laminin to enhance IGF-1-mediated differentiation
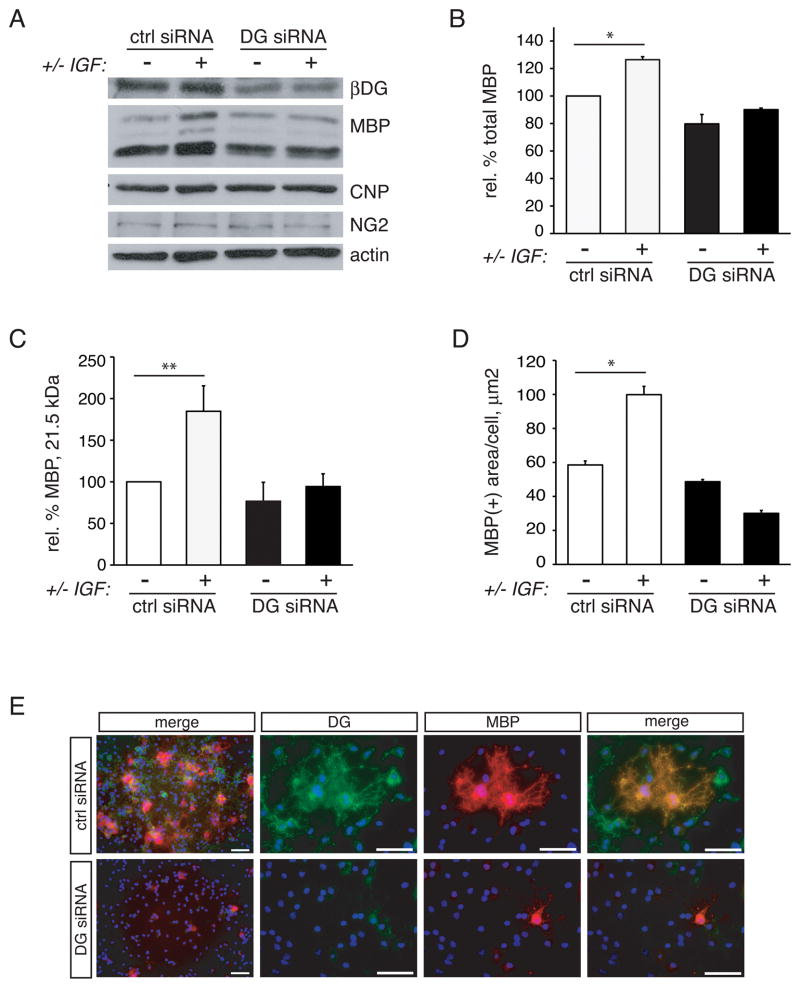
To investigate whether the laminin receptor dystroglycan was needed for laminin to enhance IGF-1-mediated increases in differentiation, we compared IGF-1-induced differentiation on laminin for 3 days in cells containing normal levels of dystroglycan (transfected with control siRNA) with that in cells containing reduced levels of dystroglycan (transfected with DAG-1 siRNA) (Fig. 2). First, we obtained protein lysates from control or dystroglycan-deficient cells to confirm effective knock-down of dystroglycan protein. As in a previous study (Colognato et al. 2007) we obtained significant depletion of oligodendrocyte dystroglycan using a pool or 4 siRNAs to rat dystroglycan mRNA (Fig. 2A; Supplementary Fig. S2). To circumvent differences in adhesion, we used laminin substrates that had been precoated with PDL, and it should be noted that control siRNA treated cells attached to laminin substrates no differently than DG siRNA treated cells: control cells attached at a density at 1.40 X10−3 ± 0.22 X 10−3 cells per micron2, while DG siRNA treated cells attached at a cell density of 1.37 X10−3 ±0.24 X10−3 cells per micron2 (n=3, p=0.859; not shown). Cell lysates were also evaluated for the levels of oligodendrocyte lineage stage-specific proteins, NG2 (OPC), CNP (oligodendrocyte), and MBP (mature oligodendrocyte) (Fig. 2A). As expected, control cells differentiated with IGF-1 showed a significant increase in total MBP protein content relative to untreated control cells (Fig. 2B; 126.4±2.2%, n=3, p=0.0084). In contrast, dystroglycan-deficient cells did not increase MBP protein levels in the presence of added IGF-1 (Fig. 2B; 79.7±6.9% of untreated control cells in the absence of IGF-1 versus 90.1±1.1% of untreated control cells in the presence of IGF-1, n=3, p=0.2975). The exon-2-containing 21.5 kDa MBP is believed to play a role in the regulating the onset of myelin synthesis and is highly associated with active myelination (Capello et al. 1997). Densitometry was therefore performed on the 21.5 kDa MBP isoform to determine whether dystroglycan loss preferentially affected this key isoform (Fig. 2C). Control cells differentiated with IGF-1 showed a significant increase in the MBP protein content relative to untreated control cells (Fig. 2C; 184.7±30.6%, n=3, p=0.0368). In contrast, dystroglycan-deficient cells did not show increased levels of the 21.5 kDa MBP isoform in the presence of IGF-1 (Fig. 2C; 76.7±22.7% of untreated control cells in the absence of IGF-1 versus 94.3±15.3% of untreated control cells in the presence of IGF-1, n=3, p=0.2782). In comparison with MBP, however, the loss of dystroglycan had little effect on the levels of NG2 or CNP, indicating that the requirement for dystroglycan in laminin’s ability to potentiate IGF-1-mediated differentiation was restricted to later stages of oligodendrocyte development i.e. acquisition of myelin proteins (Fig. 2A).
Fig 2. Dystroglycan-laminin interactions regulate the ability of IGF-1 to enhance oligodendrocyte differentiation.
Oligodendrocytes were transfected with control (ctrl) or dystroglycan-specific (DG) siRNA and differentiated on laminin for 3 days in the presence or absence of 50 ng/ml IGF-1. (A) Oligodendrocytes were evaluated by Western blot to determine MBP, CNP, and NG2 protein relative to actin loading control. β-dystroglycan (βDG) blots were additionally performed to verify siRNA-mediated dystroglycan depletion. (B) Densitometry to determine levels of MBP protein following IGF-1 treatment in dystroglycan (DG) or control (ctrl) siRNA transfected oligodendrocytes. Bars represent the mean (± sem) change in MBP protein relative to actin from 3 independent experiments (**p<0.01). (C) Densitometry to determine levels of MBP 21.5 kDa protein isoform following IGF-1 treatment in dystroglycan (DG) or control (ctrl) siRNA transfected oligodendrocytes. Bars represent the mean (± sem) change in 21.5 kDa MBP protein relative to actin from 3 independent experiments (*p<0.05). (D) Bars represent the mean (± sem) relative change in MBP(+) oligodendrocyte cell area after the differentiation of transfected cells in the presence or absence of IGF-1. Mean areas are normalized to mean area of control-transfected cells without IGF-1 (**p<0.01; ***p<0.001). (E) Differentiated oligodendrocytes expressing either DG or control (ctrl) siRNA were evaluated using MBP (red) and dystroglycan (DG, green) immunocytochemistry, in conjunction with DAPI nuclear stain (blue). Representative micrographs at two different magnifications are shown. MBP and DG immunoreactivity was reduced in oligodendrocytes expressing DG siRNA. Scale bar, 50 μM.
As oligodendrocytes expand the space covered by their processes and plasma membranes during their maturation process, an additional read-out for oligodendrocyte differentiation is the increase in the area covered by MBP immunoreactivity i.e. cell area. MBP-positive cell regions were visualized using immunocytochemistry on control, or dystroglycan-depleted, cells that were differentiated on laminin in the presence or absence of IGF-1 (Fig. 2D). Mean MBP-immunoreactive areas, as a function of total cells per field, were significantly increased in control cells that were differentiated in the presence of IGF-1 (Fig. 2E; 99.9±4.9 μm2 with IGF-1 versus 58.5±2.3 μm2 without IGF-1, n=4, p=0.0043). In contrast, dystroglycan-deficient cells showed less MBP-positive area coverage and even showed a slight decrease upon added IGF-1 (Fig. 2E; 48.7±1.3 μm2 without IGF-1 versus 30.1±1.7 μm2 with IGF-1, n=4, p=0.0177). And, when comparing both cell types in the presence of IGF-1, dystroglycan-deficient cells showed significantly less MBP-positive area coverage compared to control cells (Fig. 2E; n=4, p=0.0007). In contrast, function-blocking antibodies to the β1 subunit of α6β1 did not perturb the ability of IGF-1 to enhance MBP sheet area of oligodendrocytes grown on laminin substrates (Supplementary Fig. S3). Together these data indicated that the ability of IGF-1 to enhance the differentiation of oligodendrocytes grown on laminin was dependent on the presence of dystroglycan.
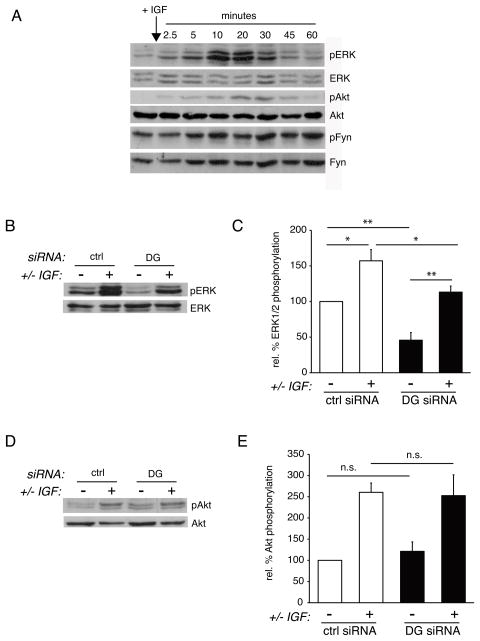
Dystroglycan influences the ability of IGF-1 to induce a maximal stimulation of MAPK, but not Akt, phosphorylation
MAPK and PI3K signaling cascades are key intrinsic regulators of oligodendrocyte development (Baron et al. 2005). IGF-1 has previously been shown to promote both MAPK and PI3K signaling in oligodendrocytes (Bibollet-Bahena and Almazan 2009). To confirm and further characterize the signaling events activated by IGF-1 during oligodendrocyte differentiation on laminin substrates, we serum-starved oligodendrocytes for 2 hours, followed by treatment with 50 ng/ml IGF-1 for indicated time points (Fig. 3A). Western blots indicated that during the time course of IGF-1 stimulation, both ERK1/2 and Akt phosphorylation increased, peaking at approximately 20 minutes post-stimulation, and then decreased to near baseline by 1 hour. In contrast, Fyn, a Src Family Kinase implicated in oligodendrocyte development as Fyn null mice are hypomyelinated, did not show substantial changes in phosphorylation during the IGF-1 time course (Fig. 3A). To determine whether dystroglycan was necessary for IGF-1 mediated activation of ERK1/2, control and dystroglycan-deficient cells were differentiated on laminin, serum-starved as above, and then treated with 50 ng/ml IGF-1 for 20 minutes (Fig. 3B). Control cells showed a significant increase in ERK1/2 phosphorylation (relative to total ERK1/2 protein) following IGF-1 treatment (Fig. 3C; 157.2±15.8% of untreated cells, n=4, p=0.0214). In contrast, dystroglycan-deficient cells showed significantly less relative ERK1/2 phosphorylation in the absence of IGF-1 (Fig. 3C; 45.7±10.7% that of control, untreated cells, n=4, p=0.0038).
Fig 3. Dystroglycan influences IGF-1 activation of MAPK/ERK, but not PI3K/Akt.
(A) Oligodendrocytes were treated for the indicated time points with 100 ng/ml IGF-1, lysed, and evaluated by Western Blot to visualize the phosphorylation of ERK1/2, Akt, or Fyn. (B) Oligodendrocytes were transfected with control (ctrl) or dystroglycan (DG) siRNA and differentiated on laminin substrate for 1 day. Oligodendrocytes were then serum-starved for 2 hours, incubated in the presence or absence IGF-1 for 20 minutes, then evaluated by Western blot to visualize ERK1/2 phosphorylation (pERK1/2) relative to total ERK1/2 protein. A representative set of blots from 4 independent experiments is shown. (C) Densitometry to determine levels of ERK1/2 phosphorylation (pERK) induced IGF-1, relative to total ERK protein. Bars represent the mean (± sem) relative change in ERK1/2 phosphorylation from 4 independent experiments (*p<0.05, **p<0.01). (D) Oligodendrocytes were transfected with control (ctrl) or dystroglycan (DG) siRNA and differentiated on laminin substrate for 1 day. Oligodendrocytes were then serum-starved for 2 hours, incubated in the presence or absence IGF-1 for 20 minutes, then evaluated by Western blot to visualize Akt phosphorylation (pAkt) relative to total Akt protein. A representative set of blots from 4 independent experiments is shown. (E) Densitometry to determine levels of Akt phosphorylation (pAkt) induced IGF-1, relative to total Akt protein. Bars represent the mean (± sem) relative change in Akt phosphorylation from 4 independent experiments (*p<0.05).
Dystroglycan-deficient cells did however show increased ERK1/2 phosphorylation when treated with IGF-1, although the maximal amount of ERK1/2 phosphorylation was significantly lower than that in control IGF-1-treated cells (Fig. 3C; 113.2±8.5%, n=4, p=0.0319). Yet, because of the lower baseline of ERK1/2 phosphorylation, the effect of IGF-1 treatment on dystroglycan-deficient cells remained a significant change (Fig. 3C; n=4, p=0.0041). In summary, while dystroglycan-deficient oligodendrocytes in contact with laminin are able to respond to IGF-1 and induce ERK1/2 phosphorylation, dystroglycan was required for the maximal level of ERK1/2 phosphorylation under these conditions.
In contrast to ERK1/2 activation, the ability of oligodendrocytes on laminin to elicit a normal IGF-1-induced AKT phosphorylation response was not significantly affected by the loss of dystroglycan (Fig. 3D). Thus, Akt phosphorylation in response to IGF-1 remained unchanged in the presence or absence of dystroglycan. Untreated control cells were therefore not significantly different from dystroglycan-deficient cells (Fig. 3E; 121.2±22.4%, n=3, p=0.6834), and IGF-1 stimulated control cells were not significantly different form IGF-1 stimulated dystroglycan-deficient cells (Fig. 3E; 260.5±21.9% in control cells versus 252.6±49.1% in dystroglycan-deficient cells, n=3, p=0.5149). Together, these data suggest that, in response to IGF-1, dystroglycan-laminin interactions were required to obtain a maximal level of ERK1/2 phosphorylation, but not Akt phosphorylation. This finding is consistent with our previous studies in which we reported that the enhancement of PDGF-mediated oligodendrocyte survival by laminin did not require dystroglycan but instead involved the activation of PI3K/Akt signaling through α6β1 integrin (Colognato et al. 2007).
We noted that ERK1/2 phosphorylation was lower in dystroglycan-deficient cells in the absence of exogenous IGF-1 and wondered whether IGF-1 that was synthesized by oligodendrocytes was a significant contributor (Fig. 3C). Indeed, IGF-1 synthesized by oligodendrocytes has previously been reported to promote autocrine signaling (Pfeiffer et al. 1993). Here we used the IGF-1 receptor inhibitor Tyrphostin 1-OMe-AG (Tyrphostin) to test whether blockade of IGF-1 autocrine effects could further dampen oligodendrocyte differentiation and/or MAPK signaling (Supplementary Fig. S4). Treatment with 10uM Tyrphostin caused a reduction in ERK1/2 phosphorylation and in MBP protein levels, when compared to cells treated with vehicle control (Supplementary Fig. S4). These data confirm that, in primary oligodendrocyte cultures, autocrine IGF-1 signaling activates MAPK/ERK signaling and promotes differentiation.
IGF-1-mediated differentiation on laminin substrates requires MAPK signaling
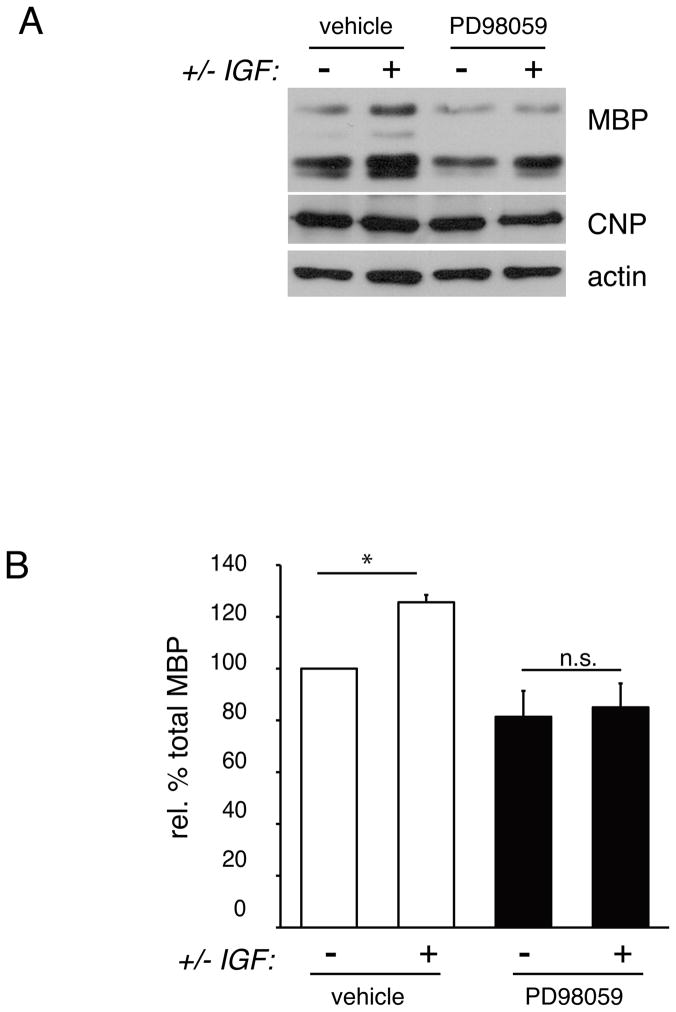
The ability of IGF-1 to enhance oligodendrocyte differentiation is known to require MAPK signaling (Baron et al. 2000; Bibollet-Bahena and Almazan 2009; Palacios et al. 2005). To ascertain whether IGF-1-mediated increases in oligodendrocyte differentiation were also MAPK-pathway-dependent when cells were grown on laminin, we compared cells treated for 3 days with MEK1/2 inhibitor (PD98059) or vehicle control (Fig. 4). As expected, oligodendrocytes grown on laminin and treated with IGF-1 for 3 days showed significantly increased levels of MBP protein relative to cells on laminin without added IGF-1 (Fig. 4A,B; 125.6±2.8 of untreated cells, n=4, p=0.296). In contrast, oligodendrocytes that were differentiated on laminin, in the presence of PD98059, showed no change in MBP levels following treatment with IGF-1 (81.4±10.0% without IGF-1 and 85.0±9.2% with IGF-1, n=4, p=0.9372). As in Fig. 2, relative MBP levels were calculated as a percentage of that found in control cells without added IGF-1, and normalized to actin. These data confirm and extend previous reports that IGF-1-mediated effects on oligodendrocyte differentiation rely on an intact MAPK signaling cascade; here we additionally demonstrated that combined IGF-1/laminin effects on differentiation are also reliant on MAPK pathway transduction.
Fig 4. MAPK/ERK signaling is required for IGF-1-enhancement of oligodendrocyte differentiation on laminin.
(A) Oligodendrocytes were differentiated on laminin for 3 days in the presence or absence of MEK inhibitor (PD98059) or vehicle control, as well as in the presence or absence of 50 ng/ml IGF-1. Following treatment, oligodendrocyte lysates were evaluated by Western blot to determine MBP and CNP protein relative to actin loading control. A representative western blot from 4 independent experiments is shown. (B) Densitometry to determine levels of MBP protein following IGF-1 treatment in vehicle or PD98059-treated oligodendrocytes. Bars represent the mean (± sem) change in MBP protein relative to actin from 4 independent experiments (*p<0.05). PD98059 abolishes IGF-1-induced enhancement of MBP expression.
Oligodendrocyte dystroglycan associates with Grb2 and IRS1
The adaptor protein Grb2, important in many contexts for appropriate MAPK signal transduction, has been reported to associate with dystroglycan in fibroblasts through interaction of its SH3 domains with the C-terminal region of beta dystroglycan (Spence et al. 2004). In addition, the IGF-1 signaling protein, IRS1, has been shown to interact with Grb2 through interaction of its SH2 domain (Holgado-Madruga et al. 1996). Together these reports suggested that Grb2 participates in linking the dystrophin-glycoprotein complex with the IGF-1 signaling pathway. To test this hypothesis we used immunocytochemistry to determine whether dystroglycan, Grb-2 and IRS1 were expressed in primary oligodendrocytes cultures, and whether expression coincided with that of dystroglycan (Fig. 5A,B). Co-immunocytochemistry using antibodies specific for IRS1 (Fig. 5A) in conjunction with dystroglycan and MBP antibodies confirmed that IRS1 was expressed in differentiated oligodendrocytes, with partial overlap with the expression of dystroglycan (Fig. 5A). Co-immunocytochemistry using antibodies specific for Grb2 (Fig. 5B) in conjunction with dystroglycan and MBP antibodies showed that Grb2 was expressed in differentiated oligodendrocytes, with partial overlap with the expression of dystroglycan (Fig. 5A). Next, we asked whether, using co-immunoprecipitation, we could identify Grb2, dystroglycan, and/or IRS-1 together in a protein complex (Fig. 5B). Indeed, immunoprecipitations using the Grb2 antibody revealed both dystroglycan and IRS-1 in the isolated complexes, while the IRS1 antibody immunoprecipitated dystroglycan and Grb2 (Fig. 5B). These associations however, were not dependent on laminin substrate, that is, they were observed in cells grown on PDL substrate (not shown). These data suggested that dystroglycan may be able to influence IGF-1-mediated signal transduction through its association with Grb2 and thus with IRS-1, an important component of the IGF-1 receptor signaling apparatus.
Discussion
Oligodendrocyte maturation is characterized by the expression of myelin proteins such as myelin basic protein. Myelin, the insulating sheath that surrounds axons, is necessary for salutatory conduction in the vertebrate nervous system. Thus, increasing our understanding of factors that influence the myelination process will be pivotal to the development of treatments for demyelinating pathologies such as Multiple Sclerosis. In the current study we investigated the downstream mechanism(s) by which the extracellular matrix (ECM) receptor, dystroglycan, is able to promote oligodendrocyte maturation. We identified a functional link between the IGF-1 receptor and dystroglycan during oligodendrocyte differentiation. Previous studies have shown that ECM-binding integrins potentiate oligodendroglial responses to a variety of growth factors, including PDGF and neuregulin (Baron et al. 2003; Colognato et al. 2002; Frost et al. 1999), however, the current study provides the first evidence that laminin-dystroglycan interactions are also capable of potentiating growth factor effects on oligodendrocyte differentiation.
Dystroglycan has previously been found by our group to promote the maturation of oligodendrocytes (Colognato et al. 2007), yet the underlying signaling mechanism(s) necessary for this role have not been elucidated. Dystroglycan protein complexes, of both signaling and structural varieties, have been identified in a variety of cell types, including other glial cells types. In Schwann cells, the myelinating cells of the peripheral nervous system (PNS), dystroglycan is required for proper clustering of sodium channels, formation of microvilli, and folding of myelin (Occhi et al. 2005). Several dystroglycan interacting proteins have been identified in Schwann cells including sarcoglycans, sarcospan, α-dystrobrevin 1, Dystrophin-related protein 2, DP116 (an alternatively spiced form of dystrophin) and α1-syntrophin (Saito et al. 2003). In astrocytes, dystroglycan has been shown to complex with, and mediate clustering of, the sodium channel Kir 4.1 and the water channel aquaporin 4 (AQP4) (Guadagno and Moukhles 2004). Dystroglycan’s role as a component of signal transduction cascades, however, has been best explored thus far in studies of fibroblasts, where dystroglycan has been shown to bind extracellular regulated kinase (ERK) and its upstream activator, mitogen activated protein kinase kinase 2 (MEK2) (Spence et al. 2004).
Here, we set out to elucidate the potential mechanisms involved in dystroglycan-mediated oligodendrocyte differentiation. We found that the dystroglycan ligand, laminin, was able to potentiate the effects of IGF-1 in promoting oligodendrocyte development. And, we found subsequently that laminin’s effect on IGF-1 was dystroglycan-dependent, and was thus sensitive to the depletion of dystroglycan using small interfering RNAs. α6β1 integrin, the other oligodendrocyte laminin receptor, was incapacitated using function-blocking antibodies to the β1 integrin subunit, however, this treatment did not effect the ability of IGF-1 to promote oligodendrocyte maturation on laminin substrates (Supplementary Fig. S3). Nonetheless, we cannot rule out the possibility that α6β1 integrin modulates other effects mediated by IGF-1 during oligodendrogenesis, such as oligodendrocyte progenitor proliferation. Insulin-like growth factor-1 (IGF-1) is a potent regulator of growth and development in nearly all tissue types. Functioning mainly as an endocrine hormone, systemic IGF-1 is produced primarily in the liver under the regulation of growth hormone (Juul 2003). However, in the CNS, it is synthesized independently of growth hormone regulation as a neurotrophic factor whose effects are the result of autocrine and paracrine signaling (Jones and Clemmons 1995). Indeed, neurons, astrocytes, and oligodendrocytes all synthesize IGF-1 and express its receptor, IGF1R. In the current study we confirmed the presence of autocrine IGF-1 signaling in primary oligodendrocyte cultures by pharmacological inhibition of the IGF1R (Supplementary Fig. S4).
As a key regulator of myelinogenesis, IGF-1 has been shown to promote the generation, proliferation, survival, and differentiation of oligodendrocytes (Barres et al. 1992; Hsieh et al. 2004; Jiang et al. 2001; McMorris et al. 1986). Studies in transgenic mice overexpressing IGF-1 have furthermore revealed that excess IGF-1 results in increased myelin thickness as well as an increase in the percentage of myelinated axons (Ye et al. 2002a; Ye et al. 2002b). IGF-1-null mice, as well as in mice that overexpress IGFBP-1, an IGF-1 binding protein that interferes with endogenous IGF-1 signaling, both show a decreased percentage of myelinated axons (Ye et al. 2002a; Ye et al. 2002b). IGF-1 signaling has also been implicated in remyelination, as genetic disruption of the IGF-1 receptor (IGF1R) leads to impaired myelin repair following recovery from cuprizone-mediated demyelination (Mason et al. 2003). Here we confirmed that IGF-1 promotes oligodendroglial differentiation, yet additionally provide evidence to suggest that, during oligodendrocyte maturation, the IGF-1 signaling pathway has the ability to intersect with signaling mediated by the extracellular matrix protein laminin through at least one of its receptors, dystroglycan.
IGF-1 signaling is initiated by the binding of IGF-1 to its receptor tyrosine kinase, IGF1R. Activation of the receptor is marked by conformational changes and the phosphorylation of tyrosines along the cytoplasmic beta subunit (Gronborg et al. 1993). Some of these phosphorylated sites serve as docking sites for insulin receptor substrates (IRS1-IRS4), a group of molecules that mediate IGF-1 signaling downstream of IGF1R. Activated IRSs can activate both MAPK and PI3K signaling through the recruitment of, and association with, certain adaptor and signaling proteins. Activated IRS-1 is known to recruit and interact with the adaptor protein, growth factor receptor-bound protein-2 (Grb2) (Holgado-Madruga et al. 1996). Indeed, overexpression of Grb2 in a myoblast cell line enhanced insulin-induced MAPK signaling through Ras and resulted in increased association among GRB2, Sos and IRS1 (Skolnik et al. 1993a; Skolnik et al. 1993b). Importantly, previous studies reported that oligodendrocytes express IRS1, IRS2, IRS4 (Freude et al. 2008) as well as Grb2 (Weinger et al. 2008), suggesting that interplay between these various adaptor molecules may occur in the oligodendrocyte lineage. Here we report that, in differentiating oligodendrocytes, Grb2 and IRS1 are found associated in a protein complex that includes dystroglycan (Fig. 5), suggesting that dystroglycan may interact with the IGF1R, at least in part, via these adaptor proteins. We did not observe any ligand-dependency to this interaction, however, as immunoprecipitated complexes were similar from cells grown in the presence or absence of IGF-1 (not shown).
Besides IRS1, however, another IRS protein, IRS2, has been recently implicated in the correct timing of myelination, as mice deficient for IRS2 show a small but pronounced delay in myelination onset in the developing cerebral cortex (Freude et al. 2008). In future studies it will be important to determine therefore whether IRS2 (or IRS4) also associates with dystroglycan, or, alternatively, with additional ECM receptors such as integrins. Interestingly, IRS1 and IRS2 have been shown to be at least partly compensatory to each other during oligodendrocyte development, as gene deletion of one leads to upregulation of the other (Freude et al. 2008; Ye et al. 2002a). Yet, loss of IRS1 leads to defects in the ability of oligodendrocytes to respond to exogenous IGF-1, suggesting that even if some compensation by IRS2 occurs, IRS1 remains as an important downstream effector of IGF1R (Ye et al. 2002a).
In addition to its well documented role in cell division of oligodendrocyte progenitors (Jiang et al. 2001), evidence suggests that MAPK/ERK activation drives differentiation and process extension in maturing oligodendrocytes (Althaus et al. 1997; Baron et al. 2000; Stariha et al. 1997; Stariha and Kim 2001). And, a recent study in which Raf-2 (a key upstream activator of MAPK/ERK signaling) was specifically ablated in CNS neural precursor cells revealed that MAPK signaling is necessary for normal CNS myelination (Galabova-Kovacs et al. 2008). In the current study we found that the ability of IGF-1 to stimulate MAPK signaling, and indeed to promote oligodendrocyte differentiation, could be modulated by dystroglycan interactions with the ECM protein laminin. Given that laminins have been found associated with future white matter tracts prior to the onset of myelination (Colognato et al. 2002), a potential mechanism is that contact with laminins drives oligodendrocytes to exhibit a more robust IGF-1 response that, in turn, further stimulates oligodendrocyte maturation.
In summary, we report that laminin enhanced the ability of IGF-1 to promote oligodendrocyte differentiation, and, that the laminin receptor dystroglycan is a critical component of this effect. These data suggest that laminin’s ability to modulate growth factor actions are not limited to laminin interactions with integrin receptors, and instead may depend on the precise repertoire of laminin receptors that are available and/or engaged. Indeed, preliminary studies indicate that myelination may be delayed in mice that lack oligodendroglial dystroglycan (Eyermann, McClenahan, and Colognato, unpublished observations). Further investigations will be required to determine whether additional mechanisms exist whereby dystroglycan is able to promote oligodendrocyte differentiation, and whether IGF-1 and dystroglycan similarly cooperate either during developmental myelination or during myelin repair following injury.
Supplementary Material
(A) Oligodendrocytes differentiated for 3 days in the presence or absence of 50 ng/ml IGF-1 were evaluated by Western Blot to evaluate relative levels of myelin basic protein (MBP); a representative blot of 3 independent experiments is shown. Actin blots were performed to control for equal protein loading. (B) Oligodendrocytes differentiated for 3 days in the presence or absence of 50 ng/ml IGF-1 were evaluated by MBP immunocytochemistry. Representative micrographs of MBP immunocytochemistry (green) and DAPI nuclear stain (blue) are shown. Scale bar, 50 μM.
Oligodendrocyte progenitor cells were transfected with control (ctrl) or dystroglycan (DG) siRNA and evaluated by Western Blot for dystroglycan protein levels (βDG) at 24 hours differentiation. Actin blots were performed to as loading controls.
Differentiated oligodendrocytes in the presence of either β1 integrin or control antibodies were evaluated using MBP Immunocytochemistry to determine MBP-positive sheet areas. (A) Mean MBP-positive sheet areas in the presence or absence of IGF-1 or anti-β1 integrin blocking antibodies (±SEM) are shown. (B) MBP immunocytochemistry (red), in conjunction with DAPI nuclear stain (blue). Representative micrographs are shown. Scale bar, 50 μM.
(A) Oligodendrocytes were treated with 10μM of the IGF1R inhibitor, tyrphostin1-OMe-AG (Tyrphostin) or vehicle (75% ethanol, 25% DMSO) for 30 minutes (following a 2 hour incubation in serum-free medium). Cell lysates were evaluated by Western blot to visualize ERK1/2 phosphorylation (pERK) relative to total ERK1/2 protein (ERK). (B) Oligodendrocytes were treated with 10μM of the IGF1R inhibitor, tyrphostin1-OMe-AG (Tyrphostin) or vehicle (75% ethanol, 25% DMSO) for 3 days in differentiation medium. Cell lysates were evaluated by Western blot to visualize myelin basic protein (MBP) relative to actin (loading control).
Oligodendrocytes were differentiated for 3 days on laminin in the presence or absence of 50 ng/ml IGF-1 and monitored for dystroglycan protein levels either using immunocytochemistry or using Western Blot. (A) Differentiated oligodendrocytes were evaluated using dystroglycan (DG) immunocytochemistry. Representative micrographs of DG (green) immunoreactivity, in conjunction with DAPI nuclear stain (blue), are shown. Scale bar, 50 μM. (B) Bars represent the mean (± sem) dystroglycan(+) oligodendrocyte cell area after differentiation in the presence or absence of IGF-1 (n=4). (C) Oligodendrocyte lysates were evaluated by Western blot to determine β-dystroglycan (β1DG) protein relative to actin loading control. A representative western blot from 3 independent experiments is shown.
Fig 6. Putative dystroglycan and IGF-1 interactions that promote oligodendrocyte differentiation.
Grb2 binds to both β-dystroglycan and IRS1; this complex then fosters the ability of the IGF-1 receptor (IGF1R) to promote MAPK signal transduction. Following IGF-1 ligand binding, MAPK signaling and subsequent differentiation may be more efficiently activated through Ras/Raf via association of Grb2 with SOS (son of sevenless).
Acknowledgments
This study was supported by a National Multiple Sclerosis Society Career Transition Fellowship (H.C.), the National Institute of Neurological Disorders and Stroke (5R01NS054042), and the NY State Department of Health (C020929). The authors wish to thank the members of the Colognato lab for helpful advice and discussion.
References
- Althaus HH, Hempel R, Kloppner S, Engel J, Schmidt-Schultz T, Kruska L, Heumann R. Nerve growth factor signal transduction in mature pig oligodendrocytes. J Neurosci Res. 1997;50(5):729–742. doi: 10.1002/(SICI)1097-4547(19971201)50:5<729::AID-JNR10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Baron W, Colognato H, ffrench-Constant C. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia. 2005;49(4):467–479. doi: 10.1002/glia.20132. [DOI] [PubMed] [Google Scholar]
- Baron W, Decker L, Colognato H, ffrench-Constant C. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr Biol. 2003;13(2):151–155. doi: 10.1016/s0960-9822(02)01437-9. [DOI] [PubMed] [Google Scholar]
- Baron W, Metz B, Bansal R, Hoekstra D, de Vries H. PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci. 2000;15(3):314–329. doi: 10.1006/mcne.1999.0827. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death in the oligodendrocyte lineage. J Neurobiol. 1992;23(9):1221–1230. doi: 10.1002/neu.480230912. [DOI] [PubMed] [Google Scholar]
- Barros CS, Nguyen T, Spencer KS, Nishiyama A, Colognato H, Muller U. {beta}1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development. 2009;136(16):2717–2724. doi: 10.1242/dev.038679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger Y, Colognato H, Thurnherr T, Franklin RJ, Leone DP, Atanasoski S, Nave KA, Ffrench-Constant C, Suter U, Relvas JB. Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J Neurosci. 2006;26(29):7665–7673. doi: 10.1523/JNEUROSCI.0444-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibollet-Bahena O, Almazan G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem. 2009;109(5):1440–1451. doi: 10.1111/j.1471-4159.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- Blum G, Gazit A, Levitzki A. Substrate competitive inhibitors of IGF-1 receptor kinase. Biochemistry. 2000;39(51):15705–12. doi: 10.1021/bi001516y. [DOI] [PubMed] [Google Scholar]
- Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14(3):199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- Camara J, Wang Z, Nunes-Fonseca C, Friedman HC, Grove M, Sherman DL, Komiyama NH, Grant SG, Brophy PJ, Peterson A, ffrench-Constant C. Integrin-mediated axoglial interactions initiate myelination in the central nervous system. J Cell Biol. 2009;185(4):699–712. doi: 10.1083/jcb.200807010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capello E, Voskuhl RR, McFarland HF, Raine CS. Multiple sclerosis: re-expression of a developmental gene in chronic lesions correlates with remyelination. Ann Neurol. 1997;41(6):797–805. doi: 10.1002/ana.410410616. [DOI] [PubMed] [Google Scholar]
- Chun SJ, Rasband MN, Sidman RL, Habib AA, Vartanian T. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol. 2003;163(2):397–408. doi: 10.1083/jcb.200304154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E, ffrench-Constant C. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4(11):833–841. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- Colognato H, Galvin J, Wang Z, Relucio J, Nguyen T, Harrison D, Yurchenco PD, Ffrench-Constant C. Identification of dystroglycan as a second laminin receptor in oligodendrocytes, with a role in myelination. Development. 2007;134(9):1723–1736. doi: 10.1242/dev.02819. [DOI] [PubMed] [Google Scholar]
- Colognato H, Ramachandrappa S, Olsen IM, ffrench-Constant C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 2004;167(2):365–375. doi: 10.1083/jcb.200404076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freude S, Leeser U, Muller M, Hettich MM, Udelhoven M, Schilbach K, Tobe K, Kadowaki T, Kohler C, Schroder H, Krone W, Bruning JC, Schubert M. IRS-2 branch of IGF-1 receptor signaling is essential for appropriate timing of myelination. J Neurochem. 2008;107(4):907–917. doi: 10.1111/j.1471-4159.2008.05631.x. [DOI] [PubMed] [Google Scholar]
- Frost EE, Buttery PC, Milner R, ffrench-Constant C. Integrins mediate a neuronal survival signal for oligodendrocytes. Curr Biol. 1999;9(21):1251–1254. doi: 10.1016/s0960-9822(99)80506-5. [DOI] [PubMed] [Google Scholar]
- Galabova-Kovacs G, Catalanotti F, Matzen D, Reyes GX, Zezula J, Herbst R, Silva A, Walter I, Baccarini M. Essential role of B-Raf in oligodendrocyte maturation and myelination during postnatal central nervous system development. J Cell Biol. 2008;180(5):947–955. doi: 10.1083/jcb.200709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronborg M, Wulff BS, Rasmussen JS, Kjeldsen T, Gammeltoft S. Structure-function relationship of the insulin-like growth factor-I receptor tyrosine kinase. J Biol Chem. 1993;268(31):23435–23440. [PubMed] [Google Scholar]
- Guadagno E, Moukhles H. Laminin-induced aggregation of the inwardly rectifying potassium channel, Kir4.1, and the water-permeable channel, AQP4, via a dystroglycan-containing complex in astrocytes. Glia. 2004;47(2):138–149. doi: 10.1002/glia.20039. [DOI] [PubMed] [Google Scholar]
- Holgado-Madruga M, Emlet DR, Moscatello DK, Godwin AK, Wong AJ. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature. 1996;379(6565):560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004;164(1):111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Frederick TJ, Wood TL. IGF-I synergizes with FGF-2 to stimulate oligodendrocyte progenitor entry into the cell cycle. Dev Biol. 2001;232(2):414–423. doi: 10.1006/dbio.2001.0208. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Jones KJ, Morgan G, Johnston H, Tobias V, Ouvrier RA, Wilkinson I, North KN. The expanding phenotype of laminin alpha2 chain (merosin) abnormalities: case series and review. J Med Genet. 2001;38(10):649–657. doi: 10.1136/jmg.38.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul A. Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm IGF Res. 2003;13(4):113–170. doi: 10.1016/s1096-6374(03)00038-8. [DOI] [PubMed] [Google Scholar]
- Lee KK, de Repentigny Y, Saulnier R, Rippstein P, Macklin WB, Kothary R. Dominant-negative beta1 integrin mice have region-specific myelin defects accompanied by alterations in MAPK activity. Glia. 2006;53(8):836–844. doi: 10.1002/glia.20343. [DOI] [PubMed] [Google Scholar]
- Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci. 2003;23(20):7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris FA, Smith TM, DeSalvo S, Furlanetto RW. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc Natl Acad Sci U S A. 1986;83(3):822–826. doi: 10.1073/pnas.83.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451(2):170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Occhi S, Zambroni D, Del Carro U, Amadio S, Sirkowski EE, Scherer SS, Campbell KP, Moore SA, Chen ZL, Strickland S, Di Muzio A, Uncini A, Wrabetz L, Feltri ML. Both laminin and Schwann cell dystroglycan are necessary for proper clustering of sodium channels at nodes of Ranvier. J Neurosci. 2005;25(41):9418–9427. doi: 10.1523/JNEUROSCI.2068-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios N, Sanchez-Franco F, Fernandez M, Sanchez I, Cacicedo L. Intracellular events mediating insulin-like growth factor I-induced oligodendrocyte development: modulation by cyclic AMP. J Neurochem. 2005;95(4):1091–1107. doi: 10.1111/j.1471-4159.2005.03419.x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3(6):191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Relucio J, Tzvetanova ID, Ao W, Lindquist S, Colognato H. Laminin alters fyn regulatory mechanisms and promotes oligodendrocyte development. J Neurosci. 2009;29(38):11794–11806. doi: 10.1523/JNEUROSCI.0888-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo K, Di Stasio E, Macchia G, Rosa G, Brancaccio A, Petrucci TC. Characterization of the beta-dystroglycan-growth factor receptor 2 (Grb2) interaction. Biochem Biophys Res Commun. 2000;274(1):93–98. doi: 10.1006/bbrc.2000.3103. [DOI] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, Brophy PJ, Schmelzer JD, Low PA, Wrabetz L, Feltri ML, Campbell KP. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38(5):747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Skolnik EY, Batzer A, Li N, Lee CH, Lowenstein E, Mohammadi M, Margolis B, Schlessinger J. The function of GRB2 in linking the insulin receptor to Ras signaling pathways. Science. 1993a;260(5116):1953–1955. doi: 10.1126/science.8316835. [DOI] [PubMed] [Google Scholar]
- Skolnik EY, Lee CH, Batzer A, Vicentini LM, Zhou M, Daly R, Myers MJ, Jr, Backer JM, Ullrich A, White MF, et al. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993b;12(5):1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence HJ, Dhillon AS, James M, Winder SJ. Dystroglycan, a scaffold for the ERK-MAP kinase cascade. EMBO Rep. 2004;5(5):484–489. doi: 10.1038/sj.embor.7400140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stariha RL, Kikuchi S, Siow YL, Pelech SL, Kim M, Kim SU. Role of extracellular signal-regulated protein kinases 1 and 2 in oligodendroglial process extension. J Neurochem. 1997;68(3):945–953. doi: 10.1046/j.1471-4159.1997.68030945.x. [DOI] [PubMed] [Google Scholar]
- Stariha RL, Kim SU. Protein kinase C and mitogen-activated protein kinase signalling in oligodendrocytes. Microsc Res Tech. 2001;52(6):680–688. doi: 10.1002/jemt.1052. [DOI] [PubMed] [Google Scholar]
- Sunada Y, Campbell KP. Dystrophin-glycoprotein complex: molecular organization and critical roles in skeletal muscle. Curr Opin Neurol. 1995;8(5):379–384. [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger JG, Gohari P, Yan Y, Backer JM, Varnum B, Shafit-Zagardo B. In brain, Axl recruits Grb2 and the p85 regulatory subunit of PI3 kinase; in vitro mutagenesis defines the requisite binding sites for downstream Akt activation. J Neurochem. 2008;106(1):134–146. doi: 10.1111/j.1471-4159.2008.05343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Jung D, Motto D, Meyer J, Koretzky G, Campbell KP. SH3 domain-mediated interaction of dystroglycan and Grb2. J Biol Chem. 1995;270(20):11711–11714. doi: 10.1074/jbc.270.20.11711. [DOI] [PubMed] [Google Scholar]
- Ye P, Carson J, D'Ercole AJ. In vivo actions of insulin-like growth factor-I (IGF-I) on brain myelination: studies of IGF-I and IGF binding protein-1 (IGFBP-1) transgenic mice. J Neurosci. 1995;15(11):7344–7356. doi: 10.1523/JNEUROSCI.15-11-07344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Li L, Lund PK, D'Ercole AJ. Deficient expression of insulin receptor substrate-1 (IRS-1) fails to block insulin-like growth factor-I (IGF-I) stimulation of brain growth and myelination. Brain Res Dev Brain Res. 2002a;136(2):111–121. doi: 10.1016/s0165-3806(02)00355-3. [DOI] [PubMed] [Google Scholar]
- Ye P, Li L, Richards RG, DiAugustine RP, D'Ercole AJ. Myelination is altered in insulin-like growth factor-I null mutant mice. J Neurosci. 2002b;22(14):6041–6051. doi: 10.1523/JNEUROSCI.22-14-06041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger M, Popken G, Zhang J, Xuan S, Lu QR, Schwab MH, Nave KA, Rowitch D, D'Ercole AJ, Ye P. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55(4):400–411. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Oligodendrocytes differentiated for 3 days in the presence or absence of 50 ng/ml IGF-1 were evaluated by Western Blot to evaluate relative levels of myelin basic protein (MBP); a representative blot of 3 independent experiments is shown. Actin blots were performed to control for equal protein loading. (B) Oligodendrocytes differentiated for 3 days in the presence or absence of 50 ng/ml IGF-1 were evaluated by MBP immunocytochemistry. Representative micrographs of MBP immunocytochemistry (green) and DAPI nuclear stain (blue) are shown. Scale bar, 50 μM.
Oligodendrocyte progenitor cells were transfected with control (ctrl) or dystroglycan (DG) siRNA and evaluated by Western Blot for dystroglycan protein levels (βDG) at 24 hours differentiation. Actin blots were performed to as loading controls.
Differentiated oligodendrocytes in the presence of either β1 integrin or control antibodies were evaluated using MBP Immunocytochemistry to determine MBP-positive sheet areas. (A) Mean MBP-positive sheet areas in the presence or absence of IGF-1 or anti-β1 integrin blocking antibodies (±SEM) are shown. (B) MBP immunocytochemistry (red), in conjunction with DAPI nuclear stain (blue). Representative micrographs are shown. Scale bar, 50 μM.
(A) Oligodendrocytes were treated with 10μM of the IGF1R inhibitor, tyrphostin1-OMe-AG (Tyrphostin) or vehicle (75% ethanol, 25% DMSO) for 30 minutes (following a 2 hour incubation in serum-free medium). Cell lysates were evaluated by Western blot to visualize ERK1/2 phosphorylation (pERK) relative to total ERK1/2 protein (ERK). (B) Oligodendrocytes were treated with 10μM of the IGF1R inhibitor, tyrphostin1-OMe-AG (Tyrphostin) or vehicle (75% ethanol, 25% DMSO) for 3 days in differentiation medium. Cell lysates were evaluated by Western blot to visualize myelin basic protein (MBP) relative to actin (loading control).
Oligodendrocytes were differentiated for 3 days on laminin in the presence or absence of 50 ng/ml IGF-1 and monitored for dystroglycan protein levels either using immunocytochemistry or using Western Blot. (A) Differentiated oligodendrocytes were evaluated using dystroglycan (DG) immunocytochemistry. Representative micrographs of DG (green) immunoreactivity, in conjunction with DAPI nuclear stain (blue), are shown. Scale bar, 50 μM. (B) Bars represent the mean (± sem) dystroglycan(+) oligodendrocyte cell area after differentiation in the presence or absence of IGF-1 (n=4). (C) Oligodendrocyte lysates were evaluated by Western blot to determine β-dystroglycan (β1DG) protein relative to actin loading control. A representative western blot from 3 independent experiments is shown.