Abstract
Background
Compositional changes within the normal intestinal microbiota have been associated with the development of various intestinal inflammatory disorders such as pouchitis and inflammatory bowel diseases (IBD). Therefore, it has been speculated that manipulation of a dysbiotic intestinal microbiota has the potential to restore microbial homeostasis and attenuate inflammation.
Methods
We performed community composition analyses by terminal restriction fragment length polymorphism (T-RFLP) of the bacterial 16S ribosomal RNA gene to investigate the impact of the probiotic VSL#3 on colonic microbial community composition and development of TNBS-induced colitis in rats.
Results
TNBS-induced chronic colitis was significantly reduced in VSL#3 fed rats compared to controls (p < 0.05). T-RFLP analysis revealed distinct microbial communities at luminal versus mucosal sites. Within the luminal microbiota, chronic colitis was associated with an overall decrease in bacterial richness and diversity (Margalef's richness, p < 0.01; Shannon diversity, p <0.01). This decrease in luminal microbial diversity was enhanced in TNBS-treated rats fed VSL#3 (Margalef's richness, p < 0.001; Shannon diversity, p < 0.001) and significantly correlated with reduced clinical colitis scores (Pearson correlation p < 0.05).
Conclusions
Our data demonstrate that the probiotic VSL#3 alters the composition of the intestinal microbiota, and these changes correlate with VSL#3-induced disease protection.
Keywords: Microbiota, VSL#3, inflammatory bowel disease, probiotic, T-RFLP
Introduction
The human intestinal tract may host as many as 36,000 bacterial species amounting to approximately 100 trillion microorganisms, collectively referred to as the intestinal microbiota (1-3). This complex microbial community has co-evolved with its eukaryotic host and has maintained its privileged niche by contributing a wide array of essential functions. In fact, the microbiota and its collective prokaryotic-genome contribute to key physiological processes such as development of the mucosal immune system, metabolism of polysaccharides, intestinal epithelial cell proliferation/differentiation, intestinal development and barrier function (4-7).
While it is clear that our symbiotic relationship with bacteria is integral to the normal function of the human gut, this relationship becomes unbalanced under certain circumstances resulting in disease. For example, Crohn's disease and ulcerative colitis, collectively termed inflammatory bowel diseases (IBD), appear to result from a combined loss of intestinal barrier function and overzealous immune response to the intestinal microbiota (8, 9). Current treatment regimens for IBD include administration of immunosuppressants such as prednisone, 6-mercaptopurine (6-MP) and azathioprine (Imuran) (Prometheus Therapeutics and Diagnostics; San Diego, CA) or biologics such as infliximab (Remicade) (Centocor Ortho Biotech; Horsham, PA) or adalimumab (Humira) (Abbott Laboratories; Abbott Park, IL) that block activity of the inflammatory cytokine TNFα. While these therapies have shown efficacy in treating the symptoms of IBD, they often confer serious side effects and further do not address the underlying basis of the disease, namely microbial dysbiosis.
The commercially available probiotic VSL#3 (VSL Pharmaceuticals, Fort Lauderdale FL) is a mixture of 8 strains of lactic acid-producing bacteria (Lactobacillus plantarum, Lactobacillus delbrueckii subsp. Bulgaricus, Lactobacillus casei, Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis and Streptococcus salivarius subsp. thermophilus). VSL#3 has been shown not only to prevent, but also to ameliorate established colitis in the Il10-/- murine model (10, 11) and to prevent development of ileitis in the SAMP1/YitFc mice (12). While the mechanism underlying the therapeutic effect of VSL#3 is currently unknown, it has been speculated that the beneficial activities of probiotics are propagated through a wide range of effects including inhibition of bacterial translocation, release of bacteriocidin and lactic acid, production of butyric acid, anti-oxidative effects, enhancement of epithelial barrier function and the modulation of immune cell responses (2, 10, 12-22). These disparate effects may be achieved via the action of by-products generated by these living organisms or indirectly by altering the population dynamics of the gut microbiota, thereby resulting in changes to the microbial metabolic spectrum. The impact of VSL#3 on microbial composition is currently unknown.
In this study we used community fingerprinting of the bacterial 16S rRNA gene to assess colonic microbial community composition in colitic rats. The presence of chronic colitis coincided with an overall decrease in bacterial species richness and diversity within the colonic lumen. In addition, we observed distinct microbial community signatures between the luminal and mucosally-adherent compartment. Interestingly, we observed that administration of the probiotic VSL#3 alters species richness and diversity of the luminal, but not the mucosally-adherent microbial community. Furthermore, decreased richness and biodiversity of the luminal community correlated with reduced colitis severity in VSL#3-treated rats. Together our data suggest that the beneficial effects of VSL#3 are mediated, at least in part, through restructuring of the colonic microbiota.
Materials and Methods
Rats and TNBS administration
Male Sprague-Dawley rats (∼ 6 weeks of age) were obtained from Southern Veterinary Service, PSM, Puerto Rico, USA). Animals were maintained in temperature-controlled rooms and a 12 hour light-dark cycle. Animals were housed one per cage and received standard laboratory chow ad libitum. Chronic colitis was induced by prolonged reactivation as previously described (Appleyard and Wallace, 1995). A volume of 0.5 ml Trinitrobenzene sulfonic acid (TNBS) at a concentration of 60 mg/ml in 50% ethanol was administered intracolonically by catheter inserted approximately 8 cm proximal to the anus under ether anesthesia. Six weeks after the initial TNBS instillation, reactivation was performed by intravenous tail vein injection of TNBS (5mg/kg body weight in 0.9% saline) once a day for 3 days. Animals continued to receive TNBS intravenously twice a week for 10 weeks as previously described (23). Animals were sacrificed by intraperitoneal administration of sodium phenobarbital (65 mg/ml). All procedures involving animals were approved by the Animal Care and Use Committee at Ponce School of Medicine.
VSL#3 administration
VSL#3 was administered to the VSL#3-fed group (n = 16) in the drinking water while the non-VSL#3 group (n = 17) received only water beginning one week prior to the initial induction of colitis until the time of sacrifice (total = 18 weeks). VSL#3 dose (CFU/ml water) was determined by measuring the number of colony forming units consumed daily by the animals and adjusted to ensure consumption of at least 5 billion CFU of bacteria per 100 g bodyweight. These calculations were based on a daily intake of 3,600 billion bacteria for an adult human with a mean weight of 70 kg.
Necropsy and colonic damage
At the time of sacrifice, the colon was dissected and fecal pellets were collected and snap frozen in liquid nitrogen. The colon was splayed longitudinally, cut in half in the same plane and scored for macroscopic damage based on a well-defined scoring system (23). The scoring system consisted of four criteria including: presence of adhesions (0, 1, or 2 for none, minor and major respectively), diarrhea (0, or 1 for absent or present respectively), colon thickness (mm), and ulceration (0 for no damage, with increasing scores depending on the extent of ulceration). Approximately 100 mg of proximal tissue was collected and snap frozen in liquid nitrogen for T-RFLP analysis.
DNA extraction, T-RFLP and community composition analysis
DNA was extracted from between 50-200 mg of fecal material or 100 mg proximal colon tissue. Samples were resuspended in lysis buffer containing 20 mg/ml lysozyme and incubated for 30 minutes at 37°C. Further lysis was performed by adding 10% SDS and proteinase K to 350 μg/ml. Samples were homogenized using a bead beater and 0.1mm zirconium beads (BioSpec Products) then processed using a DNA extraction kit (DNeasy; Qiagen). The bacterial 16S ribosomal RNA gene was amplified by PCR from each sample using fluorescently labeled primers (forward primer 8F FAM 5′AGAGTTTGATCCTGGCTCAG 3′ and reverse primer 1492R Hex 5′ GGTTACCTTGTTACGACTT 3′). Products were purified using a Qiagen PCR purification kit then digested with HhaI to generate terminal restriction fragments (T-RFs) of varying size. The T-RFs were processed by capillary electrophoresis on the ABI 3100 genetic analyzer. Genemapper software (Applied Biosystems) was used to determine the size, area, and height of each T-RF. The size of these T-RFs corresponds to different bacteria or bacterial groups with because of polymorphisms in the 16S RNA gene. Size and abundance data from Genemapper were compiled into a data matrix using Sequentix software (Sequentix, Germany). These data were normalized (individual T-RF peak height as a proportion of total T-RF peak heights within that sample), transformed by square root, and compiled into a Bray Curtis similarity matrix using PRIMER v6 (Primer-e Ltd). The T-RF data was subjected to hierarchical cluster followed by Analysis of Similarity (ANOSIM) to test for differences in global community composition. Biodiversity of each sample was measured by Margalef's test for richness and Shannon-Weiner diversity index; differences in richness or diversity between treatment groups were assessed by t test. The contribution of specific T-RFs to differences in bacterial composition between groups was assessed by similarity percentages (SIMPER). SIMPER results were used to generate pie charts of percent contribution of T-RFs within each group, after a 10% cutoff for low contributors.
Quantitative Real-Time PCR (qPCR) analysis
To assay the number of S. thermophilus in stool and mucosal tissue samples, qPCR was performed on total DNA extracted from fecal and intestinal tissue samples using the forward primer, 5′ TTATTTGAAAGGGGCAATTGCT 3′ and reverse primer, 5′ GTGAACTTTCCACTCTCACAC 3′ (24). Fold changes in the concentration of S. thermophilus in intestinal samples were calculated using the ΔΔCt method.
Statistical analysis
Values are presented as mean ± SEM where “n” represents one tissue from one animal used for a single replicate of an experiment. Pairwise comparisons were evaluated for statistically significant differences using two-tailed Student's t test, α = 0.05. Pearson correlation, α = 0.05, was used to assess linear relationships between clinical score and species richness, or clinical score and species diversity. Statistical analyses were performed using Graph Pad Instat V3.0 (Graph Pad Software, San Diego CA), and Graph Pad Prism V3.0 (Graph Pad Software, San Diego CA).
Results
TNBS-induced colitis is significantly reduced by consumption of VSL#3
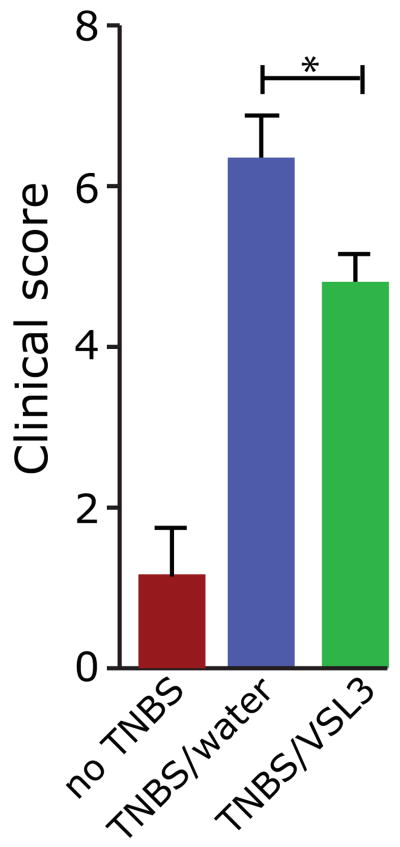
VSL#3 and other probiotics can elicit beneficial effects in various experimental models of intestinal inflammation and in pouchitis and IBD (25), although the mechanism by which these protective effects are achieved remains to be elucidated. To investigate the effect of the probiotic mixture VSL#3 on experimental colitis in rats, we orally administered VSL#3 to wild type rats beginning 1 week prior to and throughout 18 weeks of treatment with the colitogenic agent trinitrobenzene sulfonic acid (TNBS). We confirmed that VSL#3 was successfully administered using quantitative real-time PCR to measure levels of S. thermophilus, the concentration of which is highest among the components of VSL#3 and maintained at high levels in the intestine after consumption (12). We found a 64- and 36-fold increase in S. thermophilus in the feces and colonic mucosa of VSL#3 fed rats compared to water controls, indicating successful administration of VSL#3. We next evaluated colitis in these animals and as expected, TNBS induced robust colitis, indicated by significantly higher clinical scores than non-TNBS-treated rats (p < 0.001) (Figure 1). Importantly, administration of VSL#3 significantly reduced chronic colitis in TNBS-treated rats (p < 0.05), suggesting a beneficial effect of this probiotic on colonic inflammation.
Figure 1.
VSL#3 reduces the severity of chronic colitis in TNBS-treated rats. Proximal colon clinical scores for non-TNBS-treated rats and TNBS-treated rats that received either VSL#3 or water. Non-TNBS, n=3; TNBS +water, n = 17, TNBS +VSL#3, n = 16. *p = 0.05. Non-TNBS vs. TNBS +water, or vs. TNBS +VSL#3, p < 0.001.
VSL#3 consumption decreases the biodiversity of the luminal microbiota
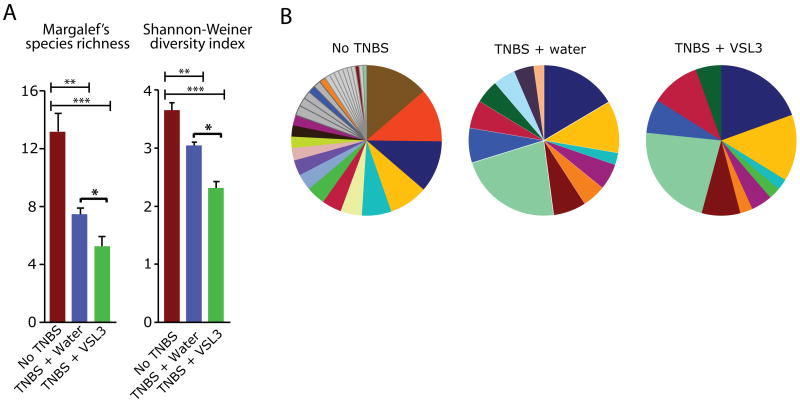
Although the consumption of VSL#3 has been associated with various health benefits such as amelioration of intestinal inflammation (12, 25) (Figure 1), its impact on the endogenous intestinal microbiota has not been explored. To address this important question, we assessed microbial community composition in the stool and colonic tissue of TNBS-treated rats using terminal restriction fragment length polymorphism (T-RFLP) analysis. We amplified the bacterial 16S rRNA gene from fecal DNA samples and digested with a restriction endonuclease to generate terminal restriction fragments (T-RFs). Based upon the abundance of individual T-RFs within each sample, we assessed species richness (Margalef's richness) and biodiversity (Shannon diversity) then compared mean values between groups – No TNBS, TNBS +water, TNBS +VSL#3 (Figure 2A). Interestingly, colitic rats displayed a significant decrease in luminal microbial diversity (p < 0.01) and richness (p < 0.01) compared to healthy control rats. Administration of VSL#3 to TNBS-treated rats further reduced microbial diversity and richness of the luminal microbiota (Margalef's richness and Shannon diversity: No TNBS vs. TNBS +VSL#3, p < 0.001; TNBS +water vs. TNBS +VSL#3, p < 0.05) (Figure 2A). We computed the percent contribution of T-RFs within each treatment group and found that the abundance of individual T-RFs differed between groups (Figure 2B and Table 1). These findings indicate that administration of VSL#3 alters the richness and diversity of the luminal microbial community during chronic colitis.
Figure 2.
VSL#3 administration decreases the biodiversity of the luminal microbiota. A. Measures of microbial biodiversity in the luminal compartment of each treatment group. Red = Non-TNBS (n=7); Blue = TNBS +water (n=6); Green = TNBS +VSL#3 (n=7). *p < 0.05; **p < 0.01; ***p < 0.001. B. Percent contribution of T-RFs within the luminal compartment of each group (from top 90%). Each color represents a different sized T-RF, indicating a different bacterial group. Dark grey comprises all species contributing between 1% and 2%, and light gray comprises species contributing below 1% within each group. Unless shared among groups, T-RFs contributing < 2% are colored in grey.
Table 1.
Luminal T-RF abundance
| No TNBS | TNBS + water | TNBS + VSL#3 | |
|---|---|---|---|
| T-RF | |||
| 399 | 14.48 ± 1.519 | 0.86 ± 0.831*** | 0.33 ± 0.331*** |
| 205 | 19.04 ± 1.446 | 0.17 ± 0.170*** | 1.08 ± 0.707*** |
| 355 | 2.13 ± 0.238 | NP*** | NP*** |
| 95 | 0.75 ± 0.124 | NP*** | NP*** |
| 191 | 0.27 ± 0.032 | NP*** | NP*** |
| 207 | 0.22 ± 0.123 | 23.06 ± 2.534*** | 22.38 ± 6.084* |
| 75 | 1.29 ± 0.238 | 0.34 ± 0.276* | 0.25 ± 0.116** |
| 346 | 0.64 ± 0.127 | NP** | NP** |
| 407 | 0.26 ± 0.062 | 0.17 ± 0.174 | NP** |
| 411 | 0.20 ± 0.042 | NP** | NP** |
| 213 | 0.20 ± 0.051 | NP** | NP** |
| 97 | 0.16 ± 0.045 | NP** | NP** |
| 199 | 0.15 ± 0.031 | NP** | NP** |
| 397 | 2.77 ± 0.968 | NP* | NP* |
| 390 | 1.78 ± 0.734 | NP* | NP* |
| 404 | 5.11 ± 1.561 | 0.50 ± 0.449* | 4.02 ± 2.941 |
| 405 | 0.60 ± 0.206 | 4.04 ± 1.169* | 3.89 ± 1.385 |
| 362 | 0.11 ± 0.067 | 0.64 ± 0.207* | 0.24 ± 0.157 |
| 46 | NP | 1.40 ± 0.461* | 1.56 ± 1.265 |
| 226 | 16.02 ± 2.564 | 15.11 ± 3.061 | 16.98 ± 4.689 |
| 401 | 8.65 ± 1.722 | 8.94 ± 2.412 | 11.09 ± 2.881 |
| 115 | 4.28 ± 1.520 | 3.55 ± 1.175 | 3.86 ± 1.267 |
| 81 | 4.07 ± 0.653 | 3.44 ± 1.632 | 2.84 ± 1.502 |
| 377 | 1.91 ± 0.967 | 3.23 ± 0.690 | 2.38 ± 0.666 |
| 57 | 1.73 ± 0.708 | NP | 0.11 ± 0.073 |
| 248 | 1.69 ± 0.699 | NP | NP |
| 58 | 1.21 ± 0.410 | 0.33 ± 0.204 | 0.32 ± 0.182 |
| 62 | 1.17 ± 0.318 | 5.22 ± 2.067 | 2.80 ± 1.592 |
| 48 | 1.00 ± 0.362 | NP | NP |
| 186 | 0.98 ± 0.350 | 4.10 ± 1.599 | 1.25 ± 0.540 |
| 402 | 0.35 ± 0.200 | 3.14 ± 1.277 | 3.43 ± 1.543 |
| 30 | 0.38 ± 0.145 | 0.34 ± 0.102 | 0.21 ± 0.172 |
| 369 | 0.29 ± 0.310 | NP | NP |
| 192 | NP | 5.21 ± 2.690 | 1.00 ± 0.946 |
Mean +/- SEM of normalized T-RF peak heights from top 90% of contributors within either group
“NP” denotes T-RFs not present within a that group
*Astericks denote significant differences between “No TNBS” and either “TNBS +water” or “TNBS +VSL#3”
No significant differences in individual T-RF abundance were found between “TNBS +water” and “TNBS +VSL#3”
p < 0.05,
p < 0.01,
p < 0.001 by t test
VSL#3 does not alter the biodiversity or species distribution of the mucosally-adherent microbiota
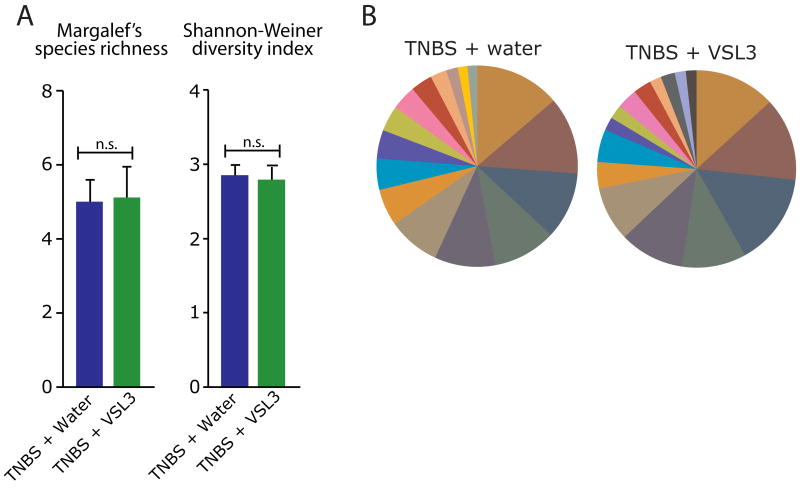
We next sought to determine if VSL#3 also induces a shift in microbial composition of the mucosally-adherent microbiota. Using proximal colon tissue DNA of TNBS-treated rats administered either VSL#3 or water, we utilized T-RFLP to analyze the mucosally-adherent microbial community. In contrast to its effects on the luminal microbiota, VSL#3 consumption did not affect species richness (p > 0.05) or biodiversity (p > 0.05) (Figure 3A), nor did it affect bacterial distribution in the mucosally-adherent microbiota (Figure 3B and Table 2). These findings suggest that VSL#3 affects only the composition of the luminal microbiota.
Figure 3.
VSL#3 does not affect the biodiversity or species distribution of the mucosally-adherent microbiota. A. Measures of microbial biodiversity within the mucosally-adherent compartment of each treatment group (TNBS +water n=17, TNBS +VSL#3 n=16). “ns” denotes no significant difference between groups. B. Percent contribution of T-RFs within the luminal compartment of each group (from top 90%). Each color represents a different sized T-RF indicating a different bacterial group.
Table 2.
Mucosal T-RF abundance
| TNBS + water | TNBS + VSL#3 | |
|---|---|---|
| T-RF | ||
| 30 | 10.62 ± 1.955 | 10.81 ± 2.425 |
| 92 | 8.70 ± 4.135 | 8.15 ± 4.345 |
| 29 | 7.34 ± 1.302 | 7.82 ± 1.561 |
| 23 | 7.06 ± 1.422 | 7.85 ± 1.514 |
| 250 | 6.27 ± 2.668 | 5.33 ± 2.080 |
| 46 | 5.78 ± 1.460 | 5.69 ± 1.696 |
| 33 | 5.36 ± 3.505 | 2.78 ± 1.810 |
| 226 | 4.89 ± 1.315 | 2.97 ± 1.029 |
| 24 | 4.68 ± 0.764 | 4.76 ± 1.030 |
| 189 | 4.57 ± 1.387 | 3.65 ± 1.959 |
| 27 | 4.53 ± 0.803 | 4.68 ± 0.942 |
| 26 | 3.52 ± 0.635 | 3.38 ± 0.695 |
| 20 | 2.87 ± 0.731 | 3.80 ± 1.375 |
| 31 | 2.23 ± 1.051 | 1.78 ± 0.615 |
| 404 | 2.13 ± 0.883 | 2.62 ± 0.948 |
| 207 | 1.88 ± 0.714 | 1.29 ± 0.652 |
| 198 | 1.79 ± 0.727 | 2.58 ± 0.969 |
| 377 | 1.66 ± 0.496 | 1.99 ± 0.684 |
| 62 | 1.56 ± 0.472 | 1.54 ± 0.561 |
Mean +/- SEM of normalized T-RF peak heights from top 90% of contributors within either group
No significant differences in individual T-RF abundance were found between the groups
The luminal and mucosally-adherent microbiota display distinct microbial community signatures
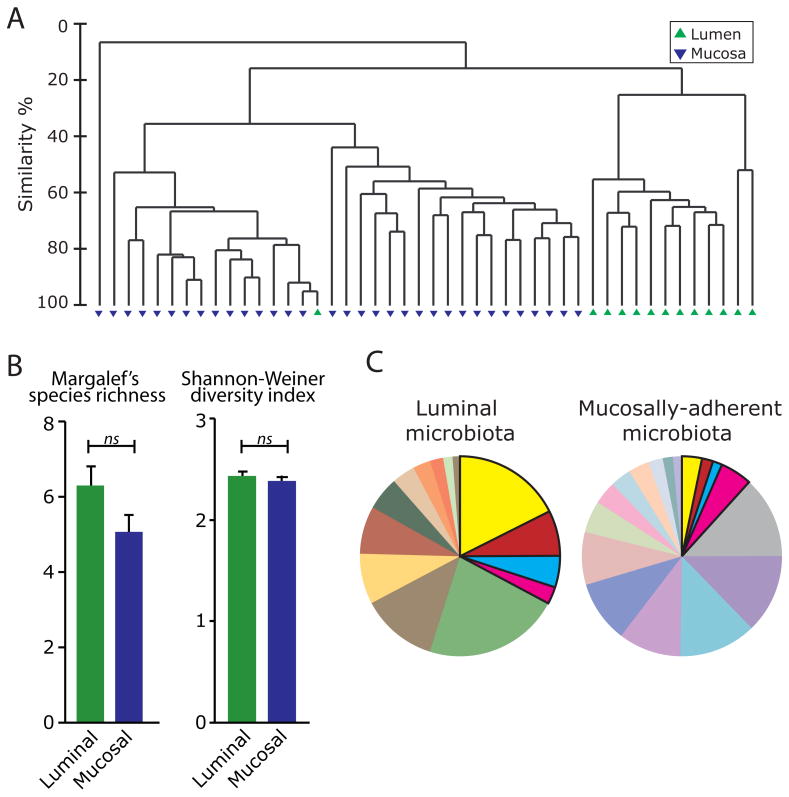
Microbial communities have been shown to differ in composition across different anatomical sites in humans (26, 27). Accordingly, we employed community fingerprinting by T-RFLP to evaluate the microbiota of TNBS-treated rats within two distinct sites of the colon: the luminal contents, and the surface mucosa of the proximal colon. Evaluation of microbial community composition revealed striking differences between sites. Hierarchical cluster analysis indicated that microbial communities in the lumen cluster independently from those inhabiting the surface of the colonic mucosa (Figure 4A). Analysis of Similarity (ANOSIM) testing confirmed that community composition differed significantly between luminal and mucosal sites (p < 0.001). Interestingly, the extent of microbial richness and biodiversity did not differ between luminal and mucosal compartments (Figure 4B). However, a comparison of T-RFs representing different populations within the bacterial community showed that only 4 out of the 30 T-RFs identified were shared among the top 90% contributing T-RFs in the luminal and mucosally-adherent microbiota (Table 3 and Figure 4C). These observations indicate that although microbial richness and diversity do not differ between luminal and mucosal sites of the colon, these sites harbor highly distinct microbial communities.
Figure 4.
Distinct microbial community communities reside in the colonic lumen and mucosal tissue. A. Hierarchical cluster analysis of T-RF data from TNBS-treated rats. Mucosally-adherent populations are represented by blue triangles and luminal populations by green triangles. B. Measures of microbial biodiversity in the colonic lumen and mucosal tissue. Blue = luminal (n=13); Green = mucosal (n=33). “ns” denotes no significant difference between groups. C. Percent contribution of T-RFs from each site (from top 90%). Each color represents a different sized T-RF indicating a different bacterial group. Regions in bold represent the 4 bacterial groups within the top 90% contributors in the microbiota of both the colonic lumen and mucosa.
Table 3.
Luminal and mucosal T-RF abundance
| Luminal | Mucosal | |
|---|---|---|
| T-RF | ||
| 207 | 22.69 ± 3.349 | 1.59 ± 0.466*** |
| 226 | 16.12 ± 2.792 | 3.96 ± 0.821*** ◊ |
| 401 | 10.10 ± 1.857 | 0.20 ± 0.197*** |
| 30 | 0.27 ± 0.101 | 10.71 ± 1.477*** |
| 20 | NP | 3.32 ± 0.734*** |
| 23 | NP | 7.44 ± 0.992*** |
| 26 | NP | 3.45 ± 0.448*** |
| 27 | NP | 4.60 ± 0.588*** |
| 29 | NP | 7.58 ± 0.966*** |
| 405 | 3.96 ± 0.883 | 0.47 ± 0.191** |
| 115 | 3.72 ± 0.837 | 0.18 ± 0.092** |
| 46 | 1.49 ± 0.688 | 5.74 ± 1.063** |
| 24 | 1.20 ± 0.960 | 4.72 ± 0.607** |
| 250 | 0.54 ± 0.267 | 5.81 ± 1.630** |
| 92 | 0.19 ± 0.189 | 8.43 ± 2.859** |
| 198 | 0.09 ± 0.095 | 2.17 ± 0.577** |
| 402 | 3.30 ± 0.978 | 0.64 ± 0.432* |
| 81 | 3.12 ± 1.061 | NP* |
| 189 | 1.05 ± 0.549 | 4.13 ± 1.137* |
| 362 | 0.43 ± 0.135 | 0.06 ± 0.044* |
| 33 | NP | 4.11 ± 1.932* |
| 62 | 3.92 ± 1.274 | 1.55 ± 0.348 ◊ |
| 192 | 2.94 ± 1.413 | 0.04 ± 0.037 |
| 377 | 2.77 ± 0.475 | 1.82 ± 0.400 ◊ |
| 186 | 2.57 ± 0.858 | 0.76 ± 0.269 |
| 404 | 2.40 ± 1.620 | 2.36 ± 0.618 ◊ |
| 118 | 1.67 ± 1.130 | 0.09 ± 0.052 |
Mean +/- SEM of normalized T-RF peak heights from top 90% of contributors within either group
Indicates T-RFs shared among the top 90% contributors of both groups
“NP” denotes T-RFs not present within that group
*Astericks denote significant differences between the groups
p < 0.05,
p < 0.01,
p < 0.001 by t test
Colitis severity correlates with luminal species richness and diversity in VSL#3-treated animals
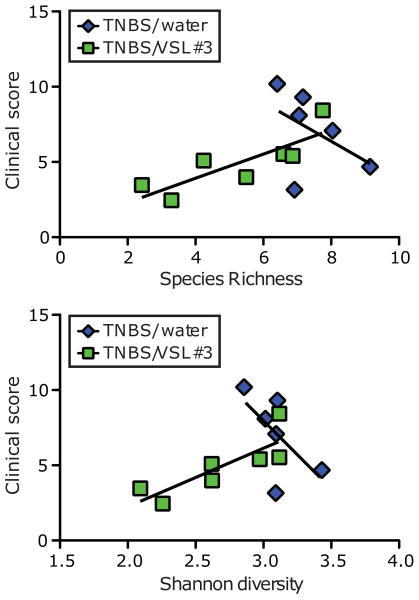
We had observed that VSL#3 decreased the severity of colitis in TNBS-treated rats (Figure 1), and reduced species richness and diversity of the luminal microbiota (Figure 2). We therefore predicted that colitis severity would correlate with VSL#3-induced changes in luminal microbial diversity. Accordingly, we assessed the correlation between clinical scores and luminal species richness or diversity by Pearson correlation analysis. We found no correlation between clinical score and species richness, or clinical score and species diversity in TNBS-treated rats that were not fed VSL#3. However, we found that the clinical scores of rats fed VSL#3 during TNBS treatment correlated with both species richness and species diversity (Figure 5). Together, these findings demonstrate that VSL#3-mediated anti-inflammatory effects correlate with changes in both richness and diversity of the luminal microbiota.
Figure 5.
Clinical score of VSL#3-fed rats correlates with luminal species richness and biodiversity. Luminal microbial Margalef's richness vs. clinical score (top panel); Pearson correlation for TNBS/water group, p=0.348; for TNBS/VSL#3, *p=0.021. Luminal microbial Shannon diversity vs. clinical score (bottom panel); Pearson correlation for TNBS/water group, p=0.183; for TNBS/VSL#3, p=0.021.
Discussion
There is a growing body of evidence suggesting that probiotic consumption promotes gastrointestinal health, representing a new avenue for the treatment of diseases such as pouchitis and IBD (28, 29). Understanding how these healthful effects are conferred will allow for more rational design of probiotic mixtures and aid in the identification and development of bacterial strains with greater efficacy. In this study we show that distinct microbial community signatures exist between the luminal and mucosally-adherent microbiota of colitic rats. Long-term VSL#3 consumption decreases the biodiversity of the luminal microbiota, whereas bacterial populations associated with the colonic mucosa remained relatively unchanged. These findings demonstrate that habitual consumption of a probiotic mixture is capable of reshaping specific compartments of the intestinal microbiota.
Although it is not clear how probiotics confer their beneficial effects on the intestine, it has been speculated that consumption of adequate quantities of certain bacteria may result in the competitive exclusion of disease promoting bacterial strains (30). Here, we demonstrate using T-RFLP and community composition analyses that TNBS-induced chronic colitis in rats results in a significant decrease in biodiversity within the colonic microbiota. This finding is consistent with recent work by Dore and coworkers who used a metagenomic approach to demonstrate that the diversity of the fecal microbiota in Crohn's patients is markedly reduced compared to that in healthy controls (31). The loss of key microbial constituents in the intestine may result in dysfunction of homeostatic processes including nutrient absorption, metabolism and epithelial barrier function (7). Interestingly, we found that VSL#3 consumption further enhanced the TNBS-induced decrease in luminal microbial biodiversity. While these findings may appear counterintuitive, it is plausible that VSL#3 promotes its effects through competitive exclusion of specific deleterious microorganisms. If this were the case, one would posit that the presence of inflammation would either suppress the growth of beneficial bacteria or promote the growth of those that are deleterious. A subsequent influx of VSL#3 may then cause a shift back from this dysbiotic community in favor of health-promoting species, thus attenuating inflammation but resulting in an overall decrease in biodiversity. While this decrease in biodiversity was apparent within the luminal microbiota, it was not observed in the mucosally adherent community of the proximal colon. Yet it is likely that microbial differences are present at other colonic locations such as cecum, mid-, and distal colon. Investigating microbial composition at multiple sites will help establish site specific effects of VSL#3.
At this stage of our investigation, it is unclear how VSL#3-induced changes in microbial composition affect the status of intestinal inflammation. It is possible that VSL#3 promotes the presence of protective microorganisms; likely constituents of the probiotic itself that directly influence host innate responses. It has been reported that VSL#3 modulates barrier function, intestinal permeability and innate host functions, which if altered, could have a profound impact on the state of colitis. VSL#3 has been shown to enhance barrier function through decreased epithelial permeability in Il10-/- mice, correlating with a reduction in intestinal inflammation (10). Another recent study demonstrated that VSL#3 prevents the development of ileitis in SAMP1/YitFc mice by enhancing epithelial barrier function and activating innate signaling leading to NF-kB activition (12). These effects appear to be mediated by VSL#3 secreted products (12). This is consistent with reports showing that probiotic-derived factors are capable of modulating host responses and experimental colitis (32-35). Additionally, structural components present in probiotic bacteria could profoundly impact immune response and favor the production of immunosuppressive molecules such as IL-10 (36).
Overall, our results clarify the impact of probiotic consumption on microbial composition in the colon. Although the community fingerprinting method used in this study did not allow us to identify specific bacteria suppressed by VSL#3 consumption, it provides a global perspective of the changes in architecture of the intestinal microbiota. Undoubtedly, future studies employing next-generation sequencing will offer the resolution needed to identify individual constituents in this vast microbial community and provide a clearer picture of the role of the microbiota in the context of healthy and diseased individuals. Through metagenomic analyses, it may be possible to pinpoint metabolic pathways altered in disease states and those modulated by probiotic consumption. When combined, these studies will yield essential information about the mechanism of action of probiotics in promoting healthful effects in the intestine.
Acknowledgments
The authors would like to thank A.N. McCoy and F. Araujo-Perez for technical assistance and data analysis and also Dr. Claudio De Simone for generously donating the VSL#3 probiotic used in this study
This work was supported by NIH RO1 grants DK047700 and DK073338 to C. Jobin and by NIH T32 Fellowship DK007737 to J. Arthur and J. Uronis.
List of abbreviations
- IBD
inflammatory bowel disease
- T-RFLP
terminal restriction fragment length polymorphism
- TNBS
Trinitrobenzene sulfonic acid
- T-RF
terminal restriction fragment
References
- 1.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper LV, Stappenbeck TS, Hong CV, et al. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 5.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Ley RE, Hamady M, et al. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Gordon JI. Inaugural Article: Honor thy symbionts. Proc Natl Acad Sci U S A. 2003;100:10452–10459. doi: 10.1073/pnas.1734063100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 9.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 11.Madsen KL, Doyle JS, Jewell LD, et al. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–1114. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 12.Pagnini C, Saeed R, Bamias G, et al. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci U S A. 2010;107:454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn C, Stiles ME. Antibacterial activity of lactic acid bacteria isolated from vacuum-packaged meats. J Appl Bacteriol. 1990;69:302–310. doi: 10.1111/j.1365-2672.1990.tb01520.x. [DOI] [PubMed] [Google Scholar]
- 14.Madsen K. Probiotics and the immune response. J Clin Gastroenterol. 2006;40:232–234. doi: 10.1097/00004836-200603000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Marteau PR, de Vrese M, Cellier CJ, et al. Protection from gastrointestinal diseases with the use of probiotics. Am J Clin Nutr. 2001;73:430S–436S. doi: 10.1093/ajcn/73.2.430s. [DOI] [PubMed] [Google Scholar]
- 16.Pessi T, Sutas Y, Marttinen A, et al. Probiotics reinforce mucosal degradation of antigens in rats: implications for therapeutic use of probiotics. J Nutr. 1998;128:2313–2318. doi: 10.1093/jn/128.12.2313. [DOI] [PubMed] [Google Scholar]
- 17.Pessi T, Sutas Y, Saxelin M, et al. Antiproliferative effects of homogenates derived from five strains of candidate probiotic bacteria. Appl Environ Microbiol. 1999;65:4725–4728. doi: 10.1128/aem.65.11.4725-4728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, O'Gorman MR, Bu HF, et al. Probiotic preparation VSL#3 alters the distribution and phenotypes of dendritic cells within the intestinal mucosa in C57BL/10J mice. J Nutr. 2009;139:1595–1602. doi: 10.3945/jn.109.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin MY, Chang FJ. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig Dis Sci. 2000;45:1617–1622. doi: 10.1023/a:1005577330695. [DOI] [PubMed] [Google Scholar]
- 20.Isolauri E, Majamaa H, Arvola T, et al. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology. 1993;105:1643–1650. doi: 10.1016/0016-5085(93)91059-q. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto T, Sasaki M, Tsujikawa T, et al. Preventive efficacy of butyrate enemas and oral administration of Clostridium butyricum M588 in dextran sodium sulfate-induced colitis in rats. J Gastroenterol. 2000;35:341–346. doi: 10.1007/s005350050358. [DOI] [PubMed] [Google Scholar]
- 22.Corr SC, Li Y, Riedel CU, et al. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci U S A. 2007;104:7617–7621. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santiago C, Pagan B, Isidro AA, et al. Prolonged chronic inflammation progresses to dysplasia in a novel rat model of colitis-associated colon cancer. Cancer Res. 2007;67:10766–10773. doi: 10.1158/0008-5472.CAN-07-1418. [DOI] [PubMed] [Google Scholar]
- 24.Furet JP, Quenee P, Tailliez P. Molecular quantification of lactic acid bacteria in fermented milk products using real-time quantitative PCR. Int J Food Microbiol. 2004;97:197–207. doi: 10.1016/j.ijfoodmicro.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Yan F, Polk DB. Probiotics: progress toward novel therapies for intestinal diseases. Curr Opin Gastroenterol. 2010;26:95–101. doi: 10.1097/MOG.0b013e328335239a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costello EK, Lauber CL, Hamady M, et al. Bacterial community variation in human body habitats across space and time. Science. 2009;326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gionchetti P, Rizzello F, Morselli C, et al. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum. 2007;50:2075–2082. doi: 10.1007/s10350-007-9068-4. discussion 2082-2074. [DOI] [PubMed] [Google Scholar]
- 29.Sood A, Midha V, Makharia GK, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–1209. 1209, e1201. doi: 10.1016/j.cgh.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Gorbach SL, Barza M, Giuliano M, et al. Colonization resistance of the human intestinal microflora: testing the hypothesis in normal volunteers. Eur J Clin Microbiol Infect Dis. 1988;7:98–102. doi: 10.1007/BF01962192. [DOI] [PubMed] [Google Scholar]
- 31.Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prisciandaro L, Geier M, Butler R, et al. Probiotics and their derivatives as treatments for inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1906–1914. doi: 10.1002/ibd.20938. [DOI] [PubMed] [Google Scholar]
- 33.Sokol H, Lay C, Seksik P, et al. Analysis of bacterial bowel communities of IBD patients: what has it revealed? Inflamm Bowel Dis. 2008;14:858–867. doi: 10.1002/ibd.20392. [DOI] [PubMed] [Google Scholar]
- 34.Heuvelin E, Lebreton C, Grangette C, et al. Mechanisms involved in alleviation of intestinal inflammation by bifidobacterium breve soluble factors. PLoS One. 2009;4:e5184. doi: 10.1371/journal.pone.0005184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seth A, Yan F, Polk DB, et al. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1060–1069. doi: 10.1152/ajpgi.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grangette C, Nutten S, Palumbo E, et al. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci U S A. 2005;102:10321–10326. doi: 10.1073/pnas.0504084102. [DOI] [PMC free article] [PubMed] [Google Scholar]