Abstract
Congestive heart failure (CHF) alters vascular reactivity and an up regulation in Urotensin II (UTII), a potent vasoactive peptide. The aim of this study was to investigate the interaction between CHF and UTII in altering vascular reactivity in a rat model of volume overload heart failure. Animals were divided into 4 groups: control, UTII infused (UTII), volume overload only (VO) or volume overload+UTII (VO+UTII). Volume overload was established by the formation of an aorto-caval fistula. Following fistula formation animals were administered UTII at a rate of 300 pmol/kg/hr for 4 weeks subcutaneously with mini-osmotic pumps. Thoracic aorta rings, with/without endothelium, were subjected to cumulative dose-responses to phenylephrine, sodium nitroprusside (SNP), acetylcholine (ACH), UTII, and the Rho-kinase inhibitor HA-1077. Aortas from VO animals exhibited increased sensitivity to phenylephrine and UTII with a decreased relaxation response to ACH and HA-1077. Aortas from animals subjected to chronic UTII with volume overload (VO + UTII) retained their sensitivity to phenylephrine and UTII while they improved their relaxation to HA-1077 but not ACH. The constrictive response to UTII was dose-dependent and augmented at concentrations < 0.01 μM in VO animals. The changes in vascular reactivity paralleled an elevation of both the UTII and α1a-adrenergic receptor while the Rho and Rho-kinase signalling proteins were diminished. We found that volume overload increased sensitivity to the vasoconstrictor agents that was inversely related to changes in the Rho-kinase expression. The addition of UTII with VO reversed the constrictive vascular response through alterations in the Rho-kinase signalling pathway.
Keywords: vascular smooth muscle; endothelial dependent relaxation; rho-kinase, thoracic aorta; rat
1. Introduction
Urotensin II is a recently discovered vasoactive peptide which has been linked to various cardiovascular pathologies [10,26,37,47]. The human form of urotensin II, (hUTII), is an 11 amino acid peptide cleaved from two distinct pre-propeptides, but resulting in identical bioactive peptides. The UTII biologically active region is conserved across species [9]. UTII binds to a G-protein coupled receptor, recently named the urotensin receptor (UTR), with high affinity. UTII binding causes prolonged activation of the ERK1/2 MAPK pathway in isolated cardiomyocytes [34], while also causing an increase in intracellular Ca2+ mobilization [1,30]. Generally, it has been shown that both circulating UTII and its receptor expression are widely distributed throughout the body at low levels[5]. However, both the receptor and ligand appear to be up-regulated in the presence of cardiovascular pathologies, including congestive heart failure [7,10]. In thoracic aortic rings UTII caused a vasoconstriction though a PKC/Rho Kinase-dependant signaling pathway [1,8,38,45]. However, the vascular effects of UTII may be dependent on the vascular bed being stimulated. Administration of UTII to small resistance vessels and renal artery has been reported to result in a NO-dependant vasodilation [44,47]. Furthermore, underlying cardiovascular pathologies alter the responsiveness of the UTII/UTR system. In patients with hypertension or congestive heart failure, administration of UTII to the forearm microvasculature resulted in vasoconstriction while administration to normal control subjects resulted in forearm vasodilation [28,43]. These reports suggested that the role of UTII in vasoreactivity is modified at least by underlying cardiac disease and perhaps other disease states as well.
Currently there is little information regarding the signaling mechanisms that contribute to alterations of vascular response in models of volume overload. It is widely accepted that the progression of heart failure is associated with increased vasoconstriction and impaired endothelium-dependent vasodilation [24,31]. Crespo and co-workers [6] used Syrian cardiomyopathic hamsters to measure the effects of progressive heart failure on vascular function. They found a shift in the vascular response curves to norepinephrine stimulation that increased maximal force generation with no difference in relaxation induced by SNP.
Because of the reported link of UTII to changes in peripheral vascular tone [16,19,28], we hypothesized that in a model of congestive heart failure induced by volume overload [14], UTII might contribute to altered vascular responses in order to maintain blood pressure in the presence of a failing myocardium. Therefore, the aim of this study was to evaluate the changes in aortic response in animals subjected to volume overload and volume overload+urotensin II.
2. Methods
2.1. Animals
Male Sprague-Dawley rats weighing 250-300 g were used in this study following procedures approved by the Brody School of Medicine Animal Care and Use Committee in accordance to the guidelines established by the National Institutes of Health. Animals were divided into 4 groups: control, UTII infused (UT II), volume overload only (VO) or volume overload+UTII (VO+UTII). Human Urotensin II (hUTII, American Peptide, Sunnyvale, CA) was administered using subcutaneous Alzet miniosmotic pumps for 4 weeks at a rate of 300 pmol/kg/hr. Infusion rates and concentration of UTII were based on those previously reported by Kompa and co-workers [23]. In animals in the VO+UTII group, UTII administration was begun immediately after aortocaval (A-V) shunt formation. At the end of 4 weeks animals were sacrificed and aortas were removed and cleaned of fat and surrounding connective tissue. Segments of aortas which were not used for force measurements were frozen in liquid nitrogen and stored at −80°C and used for protein and RNA biochemical evaluation.
2.2. Aortocaval Shunt Formation
The procedure for formation of the aortocaval (A-V) shunt was performed as described by Garcia and Diebold [12], and has been well-established in our hands (14). Briefly, a midline abdominal incision was made to visualize the abdominal segment of aorta/vena cava between the renal arteries and the iliac bifurcation. Aortic blood flow was occluded distal of the renal arteries and proximal of the iliac bifurcation. Using an 18-gauge needle, the aorta was punctured and the needle was passed into the adjacent vena cava. This connection was maintained for 60 seconds, after which the needle was removed and the aortic puncture site was sealed with 3M© vetbond. Aortic blood flow was restored by the release of the ligatures and fistula formation was confirmed by observing oxygenated, pulsatile blood flow in the vena cava. The laparotmy was repaired. Following the surgical procedure, the animals received a 1 mL bolus intra-peritoneal saline injection for rehydration/volume replacement, a single subcutaneous dose of antibiotic (Durapen 0.1 mg/100g) and analgesics (buprenophine 0.05 mg/kg).
2.3 Systemic Blood Pressure Measurements
All pressure measurements were made using a fluid-filled catheter connected to a pressure transducer and recorded using Grass Polyview software (Astro-Med Inc, West Warwick, RI). The right carotid artery was exposed and cannulated with PE40 tubing. Blood pressure was collected for 2 minutes and average values in mmHg are reported for 8 animals. The systolic and diastolic values were determined by averaging the maximal and minimal pressures recorded in 30 second windows. Mean arterial Pressure (MAP) was calculated from the systolic and diastolic pressures using the standard formula for MAP = [(2 × diastolic) + systolic] / 3. Reported heart rate was based on counting the number of systolic peaks in the 30 second window and multiplying by 2 and reported as average beats per minute (BPM).
2.4. In vitro measurements of vascular isometric force generation
The aorta (arch to diaphragm) was removed after sacrifice. Blood, connective tissue, and fat were carefully removed from each vessel to ensure smooth muscle and endothelial layer integrity. Cleaned aortas were placed in cold physiological saline solution (PSS, mM composition: NaCl, 140.0; KCl, 5.0; CaCl2, 1.6; MgSO4, 1.2; 3-N-morpholino-propane sulfonic acid (MOPS), 1.2; d-glucose, 5.6; EDTA 0.02; pH at 7.4 at 37 °C) and used immediately in assessing vascular responsiveness.
Aortic rings approximately 5 mm in length were cut from cleaned vessels. Rings were mounted in a DMT 610M myograph system (DMT-USA International, Atlanta, GA) and bathed in 37°C PSS bubbled with medical grade compressed air. Rings were incrementally stretched over a one hour period until a resting tension of 40 mN was maintained. A passive tension was established by rapidly resetting the ring length to 20 mN. This force corresponded to a tissue length to produce optimal force generation during K+-depolarization (data not shown). Rings were stimulated with 109 mM K+PSS solution (in which Na+ has been substituted by K+ in an equal molar fashion) for 10 minutes to insure tissue viability. Rings which did not achieve a sustained force of 1 mN/mm2 during this depolarization were not included for subsequent analysis. After depolarization, aortic rings were rinsed with PSS until passive force was restored. Cumulative dose response curves were constructed for the vasoconstrictors α1 adrenergic agonist phenylephrine (PE, 0.1 – 30 μM) and urotensin II (UTII, 0.001 μM – 0.1 μM). Endothelial-dependent relaxation was assessed using the muscarinic agonist acetylcholine (ACH, 0.001 – 0.3 μM) in rings prestimulated with 1 μM PE for 5 minutes. Endothelial-independent relaxations were probed using sodium nitroprusside (0.001 μM – 10.0 μM) and Rho-kinase dependant relaxation with HA-1077 (0.1 μM – 100.0 μM) in rings prestimulated with 0.03 μM UTII for 10 minutes. Individual concentration-response curves were fit using the Hill algorithm (Sigma Plot ver 8.01, Systat Software, Point Richmond, CA) and analyzed to identify minimum and maximum responses and estimated effective concentrations for 50% of a maximal response (EC50).
2.5. Western Blot
Protein was extracted from aortas (10-12 mg tissue weight) in 1mL of RIPA (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 1 mM EDTA, 1% NP - 40, 0.01% TritonX 100) and subjected to SDS-PAGE electrophoresis. Tissue homogenates were separated on 4-15% SDS-polyacrylamide gels (Bio-Rad, Hercules, CA) to measure changes in Rho (using non-specific Rho isoform antibody), RhoA, ROCKI, ROCKII and urotensin II and α1-adrenergic receptors protein expression. The proteins were transferred to PVDF membranes and blocked with 1% Tween in 20 mM TRIS buffered saline pH 7.6 containing 5% non-fat milk overnight at 4°C. Membranes were then incubated with the appropriate primary antisera for 4 hours. Rho antiserum (BD Transduction Laboratories, San Jose, CA) was used at 1:250 dilution, RhoA antiserum (Santa Cruz Biotechnology, Santa Cruz, CA), was used at 1:250 dilution, ROCK I antiserum (BD Transduction Laboratories, San Jose, CA), was used at 1:500 dilution, ROCK II antiserum (BD Transduction Laboratories, San Jose, CA) was used at 1:1000 dilution, Urotensin Receptor antiserum (Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1:200 dilution, and α1a-adrenergic receptor antiserum (Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1:250 dilution. Horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG were used as the secondary antisera at a dilution of 1:5000 dilution (Bio-Rad, Hercules, CA). Antibody binding was visualized using a chemiluminescence detection system (GE Healthcare) and quantified using ImageQuant TL (GE Healthcare, Piscataway, NJ).
2.6. Quantitative Real-Time PCR (qRT-PCR)
RNA was isolated from extracted aorta using the TRIzol method and subjected to PCR to measure expression changes in urotensin receptor. Extracted RNA was DNAse treated and reverse transcription was performed using the Bio-Rad iSCRIPT reverse transcription kit (Bio-Rad Laboratories, Hercules, CA). Following cDNA synthesis, qPCR was performed using the Bio-Rad iQ w/SYBR green PCR system (Bio-Rad Laboratories, Hercules, CA). Concentrations were used as per the manufacturer’s instructions. PCR was performed on the Bio-Rad iCYCLER (Bio-Rad Laboratories, Hercules, CA) using the following conditions: The first cycle was performed at 95°C for 5 minutes. The next cycle was repeated 45 times at 95°C for 15 seconds followed by 58°C for 45 seconds. The final step was performed once at 95°C for 1 minute. UTR primers used were adapted from those reported by Behm et al. [2].
2.7. Statistics
Values were reported as means ± SEM. Differences between groups were compared using ANOVA with Fishers test for least significant difference. In all cases a p value of ≤ 0.05 was used to indicate statistical significance between groups.
3. Results
3.1. Effect of volume overload and chronic urotensin II administration on arterial blood pressure and heart rate
Changes in arterial blood pressure between the experimental groups are shown in Table 1. Four week administration of UTII resulted in a significant decrease in peak systolic blood pressure (103.6 ± 6.7 mmHg) compared to control animals (117.6 ± 4.5 mmHg) and increased diastolic blood pressure, without a change in mean arterial pressure (MAP). Animals subjected to 4 weeks of volume overload demonstrated significantly decreased peak systolic blood pressure (83.5 ± 3.4 mmHg) and mean arterial blood pressure (65.3 ± 6.6 mmHg) as compared to controls (117.6 ± 4.5 mmHg, 86.1 ± 6.6 mmHg, respectively). Four week administration of urotensin II to animals subjected to volume overload partially reversed the changes in blood pressure, increasing both systolic (100.0 ± 3.1 mmHg) and mean arterial blood pressure, (74.7 ± 1.5 mmHg) measurements compared to volume overload alone. We observed no significant differences in heart rates between the experimental treatments (Table 1).
Table 1.
Arterial systolic, diastolic, and mean arterial pressures (MAP) and Heart Rate from control and experimental groups. UTII − 4 week administration of urotensin II; VO − 4 week volume overload; VO+UTII − 4 week VO + 4 week UTII administration (Data are reported as mean ± sem with n = 8 in all groups)
| Groups |
Systolic (mmHg) |
Diastolic (mmHg) |
MAP (mmHg) |
Heart Rate (Beats/minute) |
|---|---|---|---|---|
| Control | 117.6 ± 4.5 | 70.3 ± 8.3 | 86.1 ± 6.6 | 314 ± 25 |
| UTII | 103.6 ± 6.7* | 83.0 ± 6.7 | 89.9 ± 6.5$ | 335 ± 14 |
| VO | 83.5 ± 3.4*†# | 56.2 ± 8.3 | 65.3 ± 6.6*† | 326 ± 16 |
| VO+UTII | 100.0 ± 3.1* | 62.0 ± 1.3 | 74.7 ± 1.5 | 341 ± 21 |
p<0.05 vs. Control
p<0.05 vs. VO+UTII
p<0.05 vs. UTII
p<0.05 vs. VO
3.2. Effects of volume overload and urotensin II administration on urotensin II receptor mRNA and protein expression in aortas
As shown in Figure 1, neither 4 weeks of urotensin II nor volume overload alone provided significant increases in urotensin receptor gene expression, compared to the sham/control condition. However, the mean expression of the urotensin receptor message was increased ~12 fold when VO and UTII were combined. Receptor protein expressions paralleled gene expression trends in VO and UTII only groups, but in contrast to the gene expression, the VO+UTII combination did not significantly increase UTII receptor protein expression (Figure 2).
Figure 1.
Urotensin Receptor mRNA expression following 4 week administration of Urotensin II, Volume Overload (VO) or VO+UTII as measured by qRT-PCR. Bars represents mean ± sem (n = 4). All samples were performed in triplicate. * indicates statistical significance p < 0.05.
Figure 2.
Protein expression by Western Blot of A) urotensin receptor, B) α1A-receptor, and C) GAPDH as a loading control. Panels D and E are densitometric representations of urotensin II receptor and α1A-receptor protein expression. Reported are mean ± sem for n = 3. * indicates statistical significance p < 0.05.
3.3. Effects of volume overload and urotensin II administration on aortic rings vasoreactivity
Chronic volume overload has been reported to be associated with a progressive loss of left ventricular function that may be accompanied by altered vascular responses [11,46]. Therefore, contractile and relaxation responses of thoracic aortic rings were assessed using phenylephrine, urotensin II, acetylcholine, and sodium nitroprusside, to investigate mechanism responsible for any altered vascular reactivity in this animal model.
Maximal active stress following PE, UTII and KPSS stimulations are reported in Table 2. Aortic rings from animals in all experimental groups exhibited nearly a 50% reduction in force generation capacity when stimulated with 30 μM PE as compared to the response of aortic rings from control animals. The force generation in response to exogenously applied 0.01 μM UTII was significantly lower from controls values only in the UTII group. However, we only found a diminished maximal contractile response to K+PSS in the VO group.
Table 2.
Maximal active stress response (mN/mm2) following 30 μM phenylephrine, 0.01 μM urotensin II, and 109 mM K+PSS. Control – Vehicle animals (n=9), UTII − 4 week administration of urotensin II (n=6); VO − 4 week volume overload (n=5); VO+UTII − 4 week VO + 4 week UTII administration (n = 4). Data are reported as mean ± sem
| Groups | PE (30 μM) | UTII (0.01 μM) | 109 mM K+PSS |
|---|---|---|---|
| Control | 4.15 ± 0.42 | 1.88 ± 0.31 | 4.57 ± 0.37 |
| UTII | 2.25 ± 0.43* | 0.65 ± 0.13* | 4.27 ± 0.43 |
| VO | 2.86 ± 0.29* | 1.70 ± 0.13 | 1.99 ± 0.36* |
| VO+UTII | 2.23 ± 0.25* | 1.37 ± 0.15 | 3.73 ± 1.12 |
p<0.05 vs. Control
Acetylcholine induced sensitivity was not changed in the UTII, VO or VO+UTII groups when compared to control (data not shown). However, the magnitude of acetylcholine induced maximal relaxation by 0.1 μM acetylcholine (Ach) was reduced by more than 20% in aortas from both VO and VO+UTII animals (Table 3).
Table 3.
Calculated EC50s following constriction by PE, UTII in intact rings and relaxation to SNP and HA-1077in denuded rings. Maximal active stress relaxation (%) by 0.1 μM acetylcholine following pre-stimulation with 1.0 μM phenylephrine. Control – Vehicle animals (n=9); UTII − 4 week administration of urotensin II; VO − 4 week volume overload (n=5); VO+UTII − 4 week VO+4 week UTII administration (n = 4). Data are reported as mean ± sem
| Groups |
Intact PE (μM) |
Intact UTII (μM) |
Denuded HA-1077 (μM) |
Denuded SNP (μM) |
Intact ACH (%) |
|---|---|---|---|---|---|
| Control | 0.14 ± 0.10 | 0.010 ± 0.004 | 2.56 ± 0.04 | 0.014 ± 0.010 | 95.8 ± 1.0 |
| UTII | 0.34 ± 0.13† | 0.024 ± 0.004*†$ | 3.07 ± 0.93 | 0.011 ± 0.004 | 94.7 ± 2.2†$ |
| VO | 0.03 ± 0.02*# | 0.002 ± 0.001*# | 8.18 ± 0.55*† | 0.040 ± 0.001*# | 70.1 ± 5.1*# |
| VO+UTII | 0.06 ± 0.02*# | 0.003 ± 0.001 | 2.02 ± 0.21 | 0.027 ± 0.003 | 68.3 ± 7.7* |
p<0.05 vs. Control
p<0.05 vs. VO+UTII
p<0.05 vs. UTII
p<0.05 vs. VO
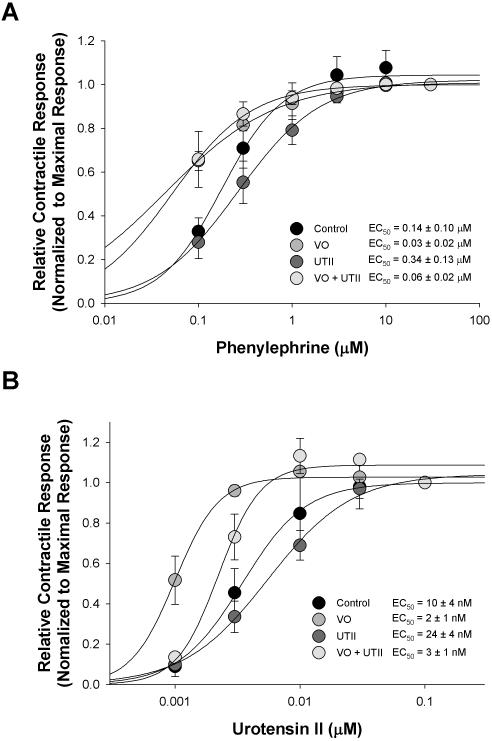
Chronic administration of UTII decreased sensitivity to phenylephrine (EC50 = 0.34 ± 0.13 μM) compared to control aorta sensitivity (EC50 = 0.14 ± 0.1 μM). Meanwhile, the phenylephrine sensitivity was increased in both the volume overload (VO) animals (EC50 = 0.03 ± 0.02 μM) and VO +UTII (EC50 = 0.06 ± 0.02 μM) experimental groups (Table 3, Figure 3).
Figure 3.
Contractile dose-responses following administration of phenylephrine and exogenous Urotensin II in aortic rings from Control, Volume Overload (VO), Urotensin II, and VO+UTII animals. Data points and EC50 values are means ± sem for n = 5 - 9. Curved lines represent Hill fit to mean data.
Because of the reported different vasoactive responses to UTII in heart failure [28-30] we examined the response of the intact aortic rings. We found the sensitivity to urotensin II was increased in both the VO (EC50 = 0.002 ± 0.001 μM) and VO+UTII (EC50 = 0.003 ± 0.001 μM) groups as compared to control (EC50 = 0.010 ± 0.004 μM)(see Table 3 and Figure 3), while the maximal stress generating ability were not different between these groups (Table 2). However, chronic UTII infusion reduced maximal force generation and depressed the sensitivity to exogenous administration of UTII in isolated aortic rings (Table 3 and Figure 3). Such a response is likely to reflect a desensitization response of the receptor in the continued presence of high levels of the ligand.
There were no significant changes in sensitivity to the NO donor sodium nitroprusside in aortic rings from the VO or VO+UTII groups when compared to control responses (Table 3) although there was a trend towards a reduction in sensitivity as EC50 values rose (Figure 4). In addition, the magnitude of relaxation at the lowest concentrations (< 0.1 μM) of sodium nitroprusside in the VO and VO+UTII groups were smaller than that of the control group (Figure 5a).
Figure 4.
Denuded aortic relaxation responses following administration A) sodium nitroprusside and B) HA-1077 in 0.1 mm UTII prestimulated rings from Control, Volume Overload (VO), Urotensin II and VO+UTII experimental groups. Data points and EC50 values are means ± sem for n = 5 - 9. Curved lines represent Hill fit to mean data.
Figure 5.
Aortic protein expression by western blot of A) Rho, B) RhoA, C) ROCKI, and D) ROCKII expression from Control, Urotensin II, Volume Overload (VO), or VO+UTII. Data are reported as means ± sem for n = 5 - 9. * indicates statistical significance p < 0.05.
3.4. Effects of volume overload and urotensin II administration on Rho-kinase mediated force maintenance
Rho-kinase signaling has been linked to vasoconstriction in the vasculature and to changes in the performance of the failing rat heart [22,33]. HA-1077, a Rho-kinase inhibitor, was used to probe the effect of VO and VO+UTII on UTII force maintenance by Rho-kinase. As shown in Table 3, VO resulted in a decreased sensitivity to HA-1077 (EC50 = 8.18 ± 0.93 μM) while this effect was reversed with the addition of UTII (EC50 = 2.02 ± 0.21 μM) compared to control (EC50 = 2.56 ±0.04 μM). The magnitude of relaxation of submaxially stimulated aortic rings was smaller in the VO group at concentrations > 1.0 μM HA-1077 (Figure 4B).
3.5. Effects of volume overload and urotensin II on Rho, RhoA, ROCKI, ROCKII protein expression
The Rho-Rho-Kinase system has been recently implicated in the progression of heart failure in both human and animal models [33]. Due to recent evidence suggesting UTII may exert its actions through Rho-Rho-kinase [38,41], we measured the effect of VO and VO+UTII on the protein expression of Rho (using a isoform non-specific antibody), RhoA, Rho-Kinase I (ROCKI), and Rho-Kinase II (ROCKII) in aortic tissue from control, VO, and VO+UTII animals. In general, the expression of Rho and RhoA were lower in all experimental groups compared to control. There were significant reductions seen in the VO groups for both total Rho protein and RhoA with a similar reduction in RhoA in the UTII+ VO group (Figure 5A and B). Downstream targets of RhoA are the rho-associated kinases ROCK I and II reveled differential expression patterns in theses experimental groups. ROCK I protein expression was elevated in the UTII group and reduced in the UTII+VO group; however, the expression of ROCKII was reduced in all groups when compared to control (Figure 5C and D).
In order to demonstrate a potential relationship between the expression patterns of the Rho-kinases to the experimental condition of VO, we calculated the ratio of ROCK I to ROCK II detected by Western blot. This analysis revealed a relative increase in the ratio of ROCK I to ROCK II expression in the VO condition which was reversed with UTII administration (VO + UTII), and actually fell below the relative expression ratio seen in the control group (Figure 6).
4. Discussion
The findings of the present study report novel information with regard to UTII vasoactive effects in a model of heart failure, where the changes in aortic constrictor behavior were associated with alterations in Rho/Rho-kinase signaling. Urotensin II has been shown to be increased in the circulation of patients diagnosed with congestive heart failure and the increase was in proportion to the severity of disease [26,32,36]. While several studies have investigated the role of urotensin II in regards to vascular function, most of these studies have examined the effect of UTII following acute administration on vessels from normal animals [13,25,27,38,40]. The first study of the effects UTII found it to be a potent vasoconstrictor [1]. Results of subsequent studies have been variable, reporting UTII as having vasoconstrictive or dilatory effects, depending on the vascular bed examined [35].
Consistent with models of shunts and heart failure, our data revealed that 4 weeks of volume overload reduced arterial systolic pressure as well as mean arterial pressure. The reduction was partially reversed following concurrent administration of urotensin II, supporting a constrictor role for UTII in this setting. Peripheral vascular constriction is a characteristic of failing myocardium, leading to both impaired peripheral perfusion, [11], and increased cardiac afterload [20,46]. The precise mechanisms remain unclear, in that peripheral constriction and exercise intolerance can be identified in individuals with diastolic impairment only, and blood pressure and cardiac output within normal limits. Empirically, alpha blockers and angiotensin II inhibitors have shown benefit, suggesting involvement for both of these signaling pathways in the end-response. We suggest that the endogenous UTII system in the animals subjected to VO also may be activated to support blood pressure maintenance by promoting vasoconstriction in the vasculature, in part by raising UTII receptor levels. Additionally, because force generation in response to phenylephrine or exogenously applied UT II was decreased in both the VO and VO+UTII animals, the increased vascular sensitivity of vasoconstrictor agents may be related a calcium-sensitization mechanism associated with receptor-coupled signaling pathways in arterial smooth muscle [4,15,17]. The increased sensitivity may contribute to the small increase in MAP and systolic blood pressure in the VO+UTII animals may reflect the action of circulating UTII and not one of an increased sympathetic outflow. Although Urotensin II has been reported to have chronotrophic or inotrophic actions on the myocardium [39] that could influence blood pressure in a manner consistent with our findings, heart rate data in these experiments do not support a direct cardiac effect as the basis for the observed blood pressure response, and instead suggested a peripheral vascular mechanism. A conclusion that can be drawn from these data is that the reduction in systolic blood pressure in the VO and VO+UTII groups may result from decreased vascular tone associated with uncoupled UTII receptor activation.
Previous work has shown upregulation of UTR in the myocardium of heart failure patients [10], though no studies have previously measured UTR expression in the aortic smooth muscle in response to progressive heart failure. Therefore, we measured both mRNA expression and protein expression in these tissues. While we found no significant differences in the mRNA levels, there was a trend towards increased expression in the three experimental groups UTII, VO and VO+UTII. However, there was significant elevation in the UTII receptor protein in the UTII treated group, but no change in the UTII+VO group. Such an increased expression of UTR in the UTII group might suggest an uncoupled mechanism of ligand signaling for maintaining force production in these tissues. A mechanism to compensate for altered force generation could be through the increased expression of the α-adrenergic receptor. There is a well documented recognition, in heart failure models as well as in the human disease, of an increased neural-hormonal effect on the sympathetic out flow which contributes to increased vascular tone [11,20,46]. We found elevated α-adrenergic receptor protein levels in the UTII and the VO group. However, the change in α-adrenergic receptor protein expression levels did not parallel the contractile responses, and furthermore, when the conditions were combined (UTII+VO), there was no difference in the levels of α-adrenergic receptor expression. Therefore, we suggest that UTII modulation of adrenergic receptor-mediated vascular tone is regulated at levels in additional to receptor expression.
Vascular tone may be modulated by direct effects on vascular smooth muscle, or indirectly, by endothelial modulation of vascular smooth muscle responses. Previous reports have shown that acute UTII exposure promoted vasodilation via NO in some vascular beds [18,25,27,47], suggesting that UTII enhanced endothelial modulation of vasodilation. While we did not see a direct vasorelaxant effect of UTII alone in aortic rings, chronic administration of UTII in vivo did generate an improved endothelium independent sensitivity to sodium nitroprusside (SNP) in heart failure animals (VO vs VO+UTII). Combined with the previous results reported Syrian cardiomyopathic hamsters model [6], these data might indicate that UTII might alter vascular tone via endothelium, NO-dependent pathways, but also by modulating vascular smooth muscle signaling directly. Although the impaired response to SNP in volume overload was improved by UTII, UTII did not alter the magnitude of relaxation to ACH. One possible explanation for the apparent uncoupling may lie with the NO generating capacity of the tissue. Numerous studies have documented the potential role of reactive oxygen species (ROS) generation for their ability to obscure the normal relaxation effects of NO. The possible role of ROS and/or reduced NO bioavailability are directions that remain to be investigated, but were beyond the scope of the current investigation. Regardless, our results support the supposition that multiple signaling pathways are involved in vascular responses in early compensated heart failure independent of UTII, and these may underlie the variation of reported effects of UTII on vascular responses [48].
The Rho-Rho-kinase (Rho-ROCK) pathway is an important regulator of smooth muscle contraction and is thought to play a vasoconstrictive role in various pathologies [3]. In particular, one study reported an improvement in vascular resistance by ROCK inhibition in the face of impaired vasodilation in patients with congestive heart failure [21]. In our VO animals, the aortic relaxation to HA-1077 was reduced and associated with higher EC50 values than control, suggesting that vascular associated with chronic volume overload involves an alteration in RhoA-Rho-kinase signaling mechanism that impacts the Ca2+-sensitization of force maintenance [42]. The poor relaxation seen with Rho-kinase inhibition in the VO group was reversed in the VO+ UTII group. Other reports have suggested the vasoconstrictive activity of UTII is mediated by the Rho-Rho-kinase pathway. Sauzeau et. al. demonstrated hUTII contraction of rat aorta could be abolished when RhoA activity was inhibited using a membrane-permeant inhibitor [41]. The role of ROCK was confirmed by Rossowski and co-workers in a study that used a series of pharmacological inhibitors and showed the addition of HA-1077 reduced UTII induced vasoconstriction [38].
To elucidate a role of Rho-ROCK in the vascular reactivity of VO and VO+UTII groups, we examined the expression patterns of Rho, RhoA, ROCKI, and ROCKII. There are four known isoforms of Rho; RhoA, RhoB, RhoC, and RhoE. Both RhoA and RhoE have been associated with modulation of smooth muscle contractility while the other isoforms are responsible for a host of other cellular functions [37,49]. Using an antibody capable of recognizing all Rho isoforms, we saw Rho protein expression reduced in the VO group and partially restored with the addition of UTII. However, when we used an antibody specific for RhoA, we found a persistent reduction in RhoA protein expression, which could contribute to the decreased vascular contractility through a impaired activation of a Ca+-sensitization that contribute to smooth muscle force maintenance [42]. A downstream target of RhoA is Rho-kinase of which there are currently two isoforms designated ROCKI (also known as ROKβ) and ROCKII (also known as ROKα) [50]. Most of the recent literature has focused on using pharmacological ROCK inhibitors to demonstrate Ca2+-sensitization in smooth muscle. The loss of RhoA and ROCKII in the different experimental models would favor the depressed constrictor responses we observed with receptor-coupled agonists. It appeared that volume overload and treatment with UTII could alter the expression of Rho and ROCK isoforms and change the sensitivity to relaxation by Rho-kinase inhibition as suggested by others [29,33,37].
The role of Urotensin II in the progression of cardiovascular disease is poorly understood as are the vascular changes associated with heart failure. We have demonstrated alterations in vascular function in the presence of compensated heart failure with partial recovery of relaxation by Rho-kinase inhibition following administration of UTII. The role of the ROCK isoforms in regards to specific vascular functions in disease are generally undefined, but we suggest the ROCK expression plays a role in regulating aortic reactivity in the presence of heart failure. Our results suggest that inhibition of the Rho-Kinase signaling might improve peripheral blood flow in the face of abnormal vascular reactivity and aid in the heart failure patient.
Research Highlights.
Volume overload alters vascular reactivity and up regulates Urotensin II.
Changes in thoracic aorta reactivity were sensitive to Rho-kinase inhibition.
Sensitivity changes were opposite of UTII receptor or Rho-kinase protein expression.
Acknowledgements
The authors would like to thank April Bofferding and Jonathan DeAntonio for their help in aorta preparation and western blotting. This work was supported in part by National Institutes of Health grants, HL-60047 awarded to LCK, DK-59467 and ES5016246 to CJW and funds from the Shared Resources program of ECU Brody School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, Louden CS, Foley JJ, Sauermelch CF, Coatney RW, Ao Z, Disa J, Holmes SD, Stadel JM, Martin JD, Liu WS, Glover GI, Wilson S, McNulty DE, Ellis CE, Elshourbagy NA, Shabon U, Trill JJ, Hay DW, Ohlstein EH, Bergsma DJ, Douglas SA. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999 Sep;401(6750):282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- 2.Behm DJ, Harrison SM, Ao Z, Maniscalco K, Pickering SJ, Grau EV, Woods TN, Coatney RW, Doe CP, Willette RN, Johns DG, Douglas SA. Deletion of the UT receptor gene results in the selective loss of urotensin-II contractile activity in aortae isolated from UT receptor knockout mice. Br J Pharmacol. 2003 May;139(2):464–472. doi: 10.1038/sj.bjp.0705254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budzyn K, Marley PD, Sobey CG. Targeting Rho and Rho-kinase in the treatment of cardiovascular disease. Trends Pharmacol Sci. 2006 Feb;27(2):97–104. doi: 10.1016/j.tips.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Christ G, Wingard C. Calcium sensitization as a pharmacological target in vascular smooth-muscle regulation. Curr Opin Investig Drugs. 2005 Sep;6(9):920–933. [PubMed] [Google Scholar]
- 5.Conlon JM. Liberation of urotensin II from the teleost urophysis: an historical overview. Peptides. 2008 May;29(5):651–657. doi: 10.1016/j.peptides.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 6.Crespo MJ. Vascular alterations during the development and progression of experimental heart failure. J Card Fail. 1999 Mar;5(1):55–63. doi: 10.1016/s1071-9164(99)90025-8. [DOI] [PubMed] [Google Scholar]
- 7.Doggrell SA. Urotensin-II and the cardiovascular system--the importance of developing modulators. Expert Opin Investig Drugs. 2004 May;13(5):479–487. doi: 10.1517/13543784.13.5.479. [DOI] [PubMed] [Google Scholar]
- 8.Douglas SA. Human urotensin-II as a novel cardiovascular target: ‘heart’ of the matter or simply a fishy ‘tail’? Curr Opin Pharmacol. 2003 Apr;3(2):159–167. doi: 10.1016/s1471-4892(03)00012-2. [DOI] [PubMed] [Google Scholar]
- 9.Douglas SA, Ohlstein EH. Human urotensin-II, the most potent mammalian vasoconstrictor identified to date, as a therapeutic target for the management of cardiovascular disease. Trends Cardiovasc Med. 2000 Aug;10(6):229–237. doi: 10.1016/s1050-1738(00)00069-4. [DOI] [PubMed] [Google Scholar]
- 10.Douglas SA, Tayara L, Ohlstein EH, Halawa N, Giaid A. Congestive heart failure and expression of myocardial urotensin II. Lancet. 2002 Jun;359(9322):1990–1997. doi: 10.1016/S0140-6736(02)08831-1. [DOI] [PubMed] [Google Scholar]
- 11.Flaim SF. Peripheral vascular effects of nitroglycerin in a conscious rat model of heart failure. Am J Physiol. 1982 Dec;243(6):H974–H981. doi: 10.1152/ajpheart.1982.243.6.H974. [DOI] [PubMed] [Google Scholar]
- 12.Garcia R, Diebold S. Simple, rapid, and effective method of producing aortocaval shunts in the rat. Cardiovasc Res. 1990 May;24(5):430–432. doi: 10.1093/cvr/24.5.430. [DOI] [PubMed] [Google Scholar]
- 13.Giebing G, Tolle M, Jurgensen J, Eichhorst J, Furkert J, Beyermann M, Neuschafer-Rube F, Rosenthal W, Zidek W, van der GM, Oksche A. Arrestin-independent internalization and recycling of the urotensin receptor contribute to long-lasting urotensin II-mediated vasoconstriction. Circ Res. 2005 Sep;97(7):707–715. doi: 10.1161/01.RES.0000184670.58688.9F. [DOI] [PubMed] [Google Scholar]
- 14.Harris GS, Lust RM, Katwa LC. Hemodynamic effects of chronic urotensin II administration in animals with and without aorto-caval fistula. Peptides. 2007 Aug;28(8):1483–1489. doi: 10.1016/j.peptides.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilgers RH, Webb RC. Molecular aspects of arterial smooth muscle contraction: focus on Rho. Exp Biol Med (Maywood ) 2005 Dec;230(11):829–835. doi: 10.1177/153537020523001107. [DOI] [PubMed] [Google Scholar]
- 16.Hillier C, Berry C, Petrie MC, O’Dwyer PJ, Hamilton C, Brown A, McMurray J. Effects of urotensin II in human arteries and veins of varying caliber. Circulation. 2001 Mar;103(10):1378–1381. doi: 10.1161/01.cir.103.10.1378. [DOI] [PubMed] [Google Scholar]
- 17.Hirano K. Current topics in the regulatory mechanism underlying the Ca2+ sensitization of the contractile apparatus in vascular smooth muscle. J Pharmacol Sci. 2007 Jun;104(2):109–115. doi: 10.1254/jphs.cp0070027. [DOI] [PubMed] [Google Scholar]
- 18.Ishihata A, Ogaki T, Aita T, Katano Y. Role of prostaglandins in urotensin II-induced vasodilatation in the coronary arteries of aged rats. Eur J Pharmacol. 2005 Oct;523(1-3):119–126. doi: 10.1016/j.ejphar.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Ishihata A, Sakai M, Katano Y. Vascular contractile effect of urotensin II in young and aged rats: influence of aging and contribution of endothelial nitric oxide. Peptides. 2006 Jan;27(1):80–86. doi: 10.1016/j.peptides.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Katz AM. Proliferative signaling and disease progression in heart failure. Circ J. 2002 Mar;66(3):225–231. doi: 10.1253/circj.66.225. [DOI] [PubMed] [Google Scholar]
- 21.Kishi T, Hirooka Y, Masumoto A, Ito K, Kimura Y, Inokuchi K, Tagawa T, Shimokawa H, Takeshita A, Sunagawa K. Rho-kinase inhibitor improves increased vascular resistance and impaired vasodilation of the forearm in patients with heart failure. Circulation. 2005 May;111(21):2741–2747. doi: 10.1161/CIRCULATIONAHA.104.510248. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi N, Horinaka S, Mita S, Nakano S, Honda T, Yoshida K, Kobayashi T, Matsuoka H. Critical role of Rho-kinase pathway for cardiac performance and remodeling in failing rat hearts. Cardiovasc Res. 2002 Sep;55(4):757–767. doi: 10.1016/s0008-6363(02)00457-1. [DOI] [PubMed] [Google Scholar]
- 23.Kompa AR, Thomas WG, See F, Tzanidis A, Hannan RD, Krum H. Cardiovascular role of urotensin II: effect of chronic infusion in the rat. Peptides. 2004 Oct;25(10):1783–1788. doi: 10.1016/j.peptides.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991 Oct;84(4):1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 25.Lacza Z, Busija W. Urotensin-II is a nitric oxide-dependent vasodilator in the pial arteries of the newborn pig. Life Sci. 2006 May;78(23):2763–2766. doi: 10.1016/j.lfs.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Lapp H, Boerrigter G, Costello-Boerrigter LC, Jaekel K, Scheffold T, Krakau I, Schramm M, Guelker H, Stasch JP. Elevated plasma human urotensin-II-like immunoreactivity in ischemic cardiomyopathy. Int J Cardiol. 2004 Mar;94(1):93–97. doi: 10.1016/j.ijcard.2003.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Yuan WJ, Su DF. Effects of rat urotensin II on coronary flow and myocardial eNOS protein expression in isolated rat heart. Acta Pharmacol Sin. 2004 Nov;25(11):1444–1449. [PubMed] [Google Scholar]
- 28.Lim M, Honisett S, Sparkes CD, Komesaroff P, Kompa A, Krum H. Differential effect of urotensin II on vascular tone in normal subjects and patients with chronic heart failure. Circulation. 2004 Mar;109(10):1212–1214. doi: 10.1161/01.CIR.0000121326.69153.98. [DOI] [PubMed] [Google Scholar]
- 29.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006 Feb;98(3):322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 30.Maguire JJ, Davenport AP. Is urotensin-II the new endothelin? Br J Pharmacol. 2002 Nov;137(5):579–588. doi: 10.1038/sj.bjp.0704924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miche E, Herrmann G, Nowak M, Wirtz U, Tietz M, Hurst M, Zoller B, Radzewitz A. Effect of an exercise training program on endothelial dysfunction in diabetic and non-diabetic patients with severe chronic heart failure. Clin Res Cardiol. 2006 Jan;95(Suppl 1):i117–i124. doi: 10.1007/s00392-006-1106-z. [DOI] [PubMed] [Google Scholar]
- 32.Ng LL, Loke I, O’Brien RJ, Squire IB, Davies JE. Plasma urotensin in human systolic heart failure. Circulation. 2002 Dec;106(23):2877–2880. doi: 10.1161/01.cir.0000044388.19119.02. [DOI] [PubMed] [Google Scholar]
- 33.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006 Mar;290(3):C661–C668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onan D, Pipolo L, Yang E, Hannan RD, Thomas WG. Urotensin II promotes hypertrophy of cardiac myocytes via mitogen-activated protein kinases. Mol Endocrinol. 2004 Sep;18(9):2344–2354. doi: 10.1210/me.2003-0309. [DOI] [PubMed] [Google Scholar]
- 35.Ovcharenko E, Abassi Z, Rubinstein I, Kaballa A, Hoffman A, Winaver J. Renal effects of human urotensin-II in rats with experimental congestive heart failure. Nephrol Dial Transplant. 2006 May;21(5):1205–1211. doi: 10.1093/ndt/gfk049. [DOI] [PubMed] [Google Scholar]
- 36.Richards AM, Nicholls MG, Lainchbury JG, Fisher S, Yandle TG. Plasma urotensin II in heart failure. Lancet. 2002 Aug;360(9332):545–546. doi: 10.1016/s0140-6736(02)09709-x. [DOI] [PubMed] [Google Scholar]
- 37.Riento K, Villalonga P, Garg R, Ridley A. Function and regulation of RhoE. Biochem Soc Trans. 2005 Aug;33(Pt 4):649–651. doi: 10.1042/BST0330649. [DOI] [PubMed] [Google Scholar]
- 38.Rossowski WJ, Cheng BL, Taylor JE, Datta R, Coy DH. Human urotensin II-induced aorta ring contractions are mediated by protein kinase C, tyrosine kinases and Rho-kinase: inhibition by somatostatin receptor antagonists. Eur J Pharmacol. 2002 Mar;438(3):159–170. doi: 10.1016/s0014-2999(02)01341-9. [DOI] [PubMed] [Google Scholar]
- 39.Russell FD, Molenaar P. Investigation of signaling pathways that mediate the inotropic effect of urotensin-II in human heart. Cardiovasc Res. 2004 Sep;63(4):673–681. doi: 10.1016/j.cardiores.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 40.Saetrum OO, Nothacker H, Ehlert FJ, Krause DN. Human urotensin II mediates vasoconstriction via an increase in inositol phosphates. Eur J Pharmacol. 2000 Oct;406(2):265–271. doi: 10.1016/s0014-2999(00)00672-5. [DOI] [PubMed] [Google Scholar]
- 41.Sauzeau V, Le ME, Bertoglio J, Scalbert E, Pacaud P, Loirand G. Human urotensin II-induced contraction and arterial smooth muscle cell proliferation are mediated by RhoA and Rho-kinase. Circ Res. 2001 Jun;88(11):1102–1104. doi: 10.1161/hh1101.092034. [DOI] [PubMed] [Google Scholar]
- 42.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000 Jan;522(Pt 2):177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sondermeijer B, Kompa A, Komesaroff P, Krum H. Effect of exogenous urotensin-II on vascular tone in skin microcirculation of patients with essential hypertension. Am J Hypertens. 2005 Sep;18(9 Pt 1):1195–1199. doi: 10.1016/j.amjhyper.2005.03.748. [DOI] [PubMed] [Google Scholar]
- 44.Stirrat A, Gallagher M, Douglas SA, Ohlstein EH, Berry C, Kirk A, Richardson M, MacLean MR. Potent vasodilator responses to human urotensin-II in human pulmonary and abdominal resistance arteries. Am J Physiol Heart Circ Physiol. 2001 Feb;280(2):H925–H928. doi: 10.1152/ajpheart.2001.280.2.H925. [DOI] [PubMed] [Google Scholar]
- 45.Tasaki K, Hori M, Ozaki H, Karaki H, Wakabayashi I. Mechanism of human urotensin II-induced contraction in rat aorta. J Pharmacol Sci. 2004 Apr;94(4):376–383. doi: 10.1254/jphs.94.376. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Ren B, Liu S, Sentex E, Tappia PS, Dhalla NS. Characterization of cardiac hypertrophy and heart failure due to volume overload in the rat. J Appl Physiol. 2003 Feb;94(2):752–763. doi: 10.1152/japplphysiol.00248.2002. [DOI] [PubMed] [Google Scholar]
- 47.Wang ZJ, Shi LB, Xiong ZW, Zhang LF, Meng L, Bu DF, Tang CS, Ding WH. Alteration of vascular urotensin II receptor in mice with apolipoprotein E gene knockout. Peptides. 2006 Apr;27(4):858–863. doi: 10.1016/j.peptides.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe T, Kanome T, Miyazaki A, Katagiri T. Human urotensin II as a link between hypertension and coronary artery disease. Hypertens Res. 2006 Jun;29(6):375–387. doi: 10.1291/hypres.29.375. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004 Nov;301(1):43–49. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol. 2005 Aug;170(3):443–453. doi: 10.1083/jcb.200412043. [DOI] [PMC free article] [PubMed] [Google Scholar]