Abstract
INTRODUCTION
Both nitric oxide (NO) and adenosine A1 receptor activation mediate microvascular vasodilation during intestinal glucose absorption. Our overall hypothesis is that ATP utilization during glucose absorption would increase adenosine metabolite release, which acts on adenosine A1 receptors to alter endothelial production of NO and/or activate ATP-dependent potassium channels (K+ATP) to dilate intestinal microvessels.
METHODS
Intravital videomicroscopy of the rat jejunum was used to record the vascular responses of inflow (termed 1A) arterioles and proximal (p3A) and distal (d3A) premucosal arterioles during exposure to isotonic glucose or mannitol solutions alone or in the presence of the selective nitric oxide synthase (NOS) inhibitor (L-NMMA), an adenosine A1 receptor antagonist (8-cyclopentyl-1,3-dipropylxanthine (DPCPX)), or a K+ATP channel inhibitor (glibenclamide).
RESULTS
As expected, glucose exposure caused rapid dilation of both p3A and d3A arterioles, while mannitol exposure had no effect on microvascular diameters. Adenosine A1 receptor blockade completely prevented glucose-induced dilation of the premucosal arterioles. NOS inhibition significantly blunted the glucose-induced vasodilation of the premucosal arterioles, but had little effect in the mannitol group. Simultaneous application of both the NOS inhibitor and the adenosine A1 receptor antagonist gave the same reduction in glucose-induced dilation of the premucosal arterioles as the adenosine A1 receptor antagonist alone. Blockade of K+ATP channels with glibenclamide did not attenuate glucose-induced vasodilation of the premucosal arterioles.
CONCLUSIONS
These data suggest that glucose-induced vasodilation of premucosal jejunal arterioles is mediated through adenosine A1 receptors, and NO at least partially mediates the adenosine A1 receptor-induced vasodilation. In addition, K+ATP channels are not involved in premucosal arteriolar vasodilation during intestinal glucose exposure.
Keywords: glucose-induced intestinal hyperemia, adenosine A1 receptor, nitric oxide, ATP-dependent potassium channel, intravital videomicroscopy
INTRODUCTION
Postprandial intestinal hyperemia, or increased blood flow during absorption of ingested nutrients in the gastrointestinal tract, depends on vasodilation of the gastrointestinal microvasculature and microvessel recruitment to open previously unperfused premucosal arterioles and capillaries. Glucose and many L-amino acids are absorbed by sodium-linked, secondary active transport from the intestinal lumen into the mucosal epithelial cells, which requires ATP utilization by the epithelial cells. As an ATP degradation product, adenosine participates in many regulatory processes including vascular reactivity via adenosine receptor activation (8), and studies have shown that adenosine-related mechanisms are involved in absorptive hyperemia (15, 31, 34). Adenosine, an endogenous purine nucleoside, mediates many physiological actions through four known purinergic (P1) receptors, designated A1, A2a, A2b and A3 (3). The actions of adenosine are well described and include decreased heart rate (A1), coronary artery vasodilation (A2a) and bronchospasm (A2b). Adenosine receptors are thought to be ubiquitous, but the receptor subtypes most commonly implicated in vascular physiology are the A1 and A2a receptors and to a lesser extent A2b receptors (3,7,11). Adenosine A1 receptors mediate vasoconstriction in many vascular beds, while A2a receptors mediate vasodilation. The activity of each adenosine receptor subtype involves second messenger systems, either stimulatory (A2) or inhibitory (A1, A3) G-proteins and subsequent increase or decrease in cyclic AMP. A recent intravital videomicroscopy study in rat intestine suggested that glucose-induced vasodilation of the intestinal microvessels is primarily mediated by the A1 and A2b adenosine receptors (21).
The mechanisms of adenosine-induced vasodilation have been widely explored in ex vivo studies (17, 29, 32) and vary with tissue. Some have proposed that adenosine A2x receptors are the major subtypes involved in adenosine-related vasodilation (16, 17, 29), while activation of the adenosine A1 receptor (ADO A1R) produces vasoconstriction. However, adenosine A1 receptor-mediated vasodilation has been reported in some in vivo studies (3, 7, 11). Perhaps due to the diversity of effectors coupled with adenosine receptors, the adenosine A1 receptor appears to produce either constriction or dilation in a tissue-dependent and adenosine concentration-dependent manner. Coupling of nitric oxide (NO), ATP-dependent potassium channels (K+ATP) and/or prostaglandins to the activation of adenosine A1 receptors has been reported in some tissues, which also might explain the diversity of possible effects during adenosine A1 receptor activity (11). Also, an unusual low-affinity type of the adenosine A1 receptor has been proposed that might explain adenosine A1 receptor-induced vasodilation (3), which fits with the observation that high concentration of an adenosine A1 receptor agonist caused vasodilation in some vascular beds while low concentration of the agonist did not (32). The mechanisms of adenosine A1 receptor-induced vasodilation have not been reported in the intestinal microvasculature.
Nitric oxide (NO), a potent local vasodilator, has been shown to mediate intestinal hyperemia during nutrient absorption (5, 6, 24), and synergy between NO and adenosine has been suggested in different tissue and vascular beds (1, 2, 20, 30). We have previously shown that adenosine A1 receptors are involved in glucose-induced intestinal vasodilation (21), but the hypothesized predominant role of the adenosine A1 receptor subtype in mediating adenosine-related vasodilation during glucose absorption in the intestine has not been demonstrated. The focus of the present study was to examine the mechanisms of adenosine A1 receptor-mediated vasodilation during intestinal glucose absorption. We hypothesized that adenosine A1 receptor-mediated vasodilation of intestinal microvessels during glucose absorption involve NO release or K+ATP channel activation. To test this hypothesis, we performed intravital videomicroscopy in the rat jejunum during intestinal glucose exposure alone or in the presence of an adenosine A1 receptor antagonist, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), the selective NO synthase inhibitor, L-NMMA, or the K+ATP channel inhibitor, glibenclamide.
MATERIALS AND METHODS
Chemicals and solutions
All chemicals were purchased from Sigma Chemical Company (St. Louis, Missouri, USA). The baseline physiological salt solution (PSS) for the exposed intestinal segment was a modified glucose-free Krebs solution, which consisted (in mM) of 25.5 NaHCO3, 112.9 NaCl, 4.7 KCl, and 2.55 CaCl2·2H2O. This solution was used to bathe the intestinal tissue during both surgical preparation and the baseline period of the microcirculation protocol. To initiate glucose-mediated vasodilation, a modified salt solution with Tris pH buffering was used that contained (in mM) 36.29 Tris-HCl, 13.71 Tris-base, 11.1 D-glucose, 88.98 NaCl, 5.87 KCl, and 2.55 CaCl2·2H2O (25). An isotonic mannitol solution was used as an osmotic control for the glucose solution and had the same components with the exception that mannitol was substituted for glucose (11.1 mM). Selective NOS inhibitor (L-NMMA) and ATP-dependent potassium channel inhibitor (glibenclamide) were applied topically to the jejunum segment in the tissue bath at the final concentrations of 100 μM and 10 μM, respectively. We have previously utilized these inhibitors in other studies of the intestinal microcirculation and the doses used in the present study were based on EC50 levels determined from dose-response curves generated in previous animals. The doses used in this study are 100 fold greater than the previously observed EC50 doses for each inhibitor.
Animal and tissue preparation
Animals were maintained in a facility approved by the American Association for the Accreditation of Laboratory Animal Care in temperature and humidity controlled rooms with a 12-hour light/dark cycle. The research protocol was approved by the Institutional Animal Care and Use Committee, the Biohazard Safety Committee and the Research & Development Committee at the Louisville VA Medical Center. Fifty-six (56) male Sprague-Dawley rats (190-210 g body weight) were acclimated for 2 weeks and received standard rat chow (20 g/day) and water ad libitum prior to experimental use. Food, but not water, was withheld 16 hours prior to experimental use to minimize the presence of digestive products in the intestinal lumen. Rats were anesthetized via intra-peritoneal administration of pentobarbital (60 mg/kg). A surgical plane of anesthesia was maintained by regular administration of supplemental pentobarbital (2.5 mg/kg) as needed. Core body temperature was maintained at 37.0 ± 0.5 °C using a servo-controlled feedback controller, rectal thermister and heating pad. Tracheotomy was performed to maintain airway patency. The right carotid artery was cannulated to monitor mean arterial blood pressure and heart rate, and the left femoral vein was cannulated to infuse saline (1 mL/h) to maintain body fluid homeostasis.
A right paramedian abdominal laparotomy was performed and a segment of the upper jejunum was selected 5-6 cm from the attachment of the jejunum and transverse mesocolon. A jejunal segment (1.5-2 cm) supplied by a single mesenteric artery with intact neurovascular supply was exteriorized and both ends were ligated to exclude collateral circulation. The segment was opened along the anti-mesenteric border by electrocautery and the tissue was positioned over the optical port of a Plexiglas tissue bath. The segment was bathed with no-glucose Krebs solution and bubbled with CO2 and N2 to regulate bath pH at 7.40 ± 0.05 and to maintain physiologic O2 concentration. The temperature of the exteriorized segment was maintained at 37.0 ± 0.5 °C via feedback controller, bath thermister and bath heating element. The rat and board were positioned on the stage of a trinocular Zeiss microscope and 45 minutes were allowed for hemodynamic and microvascular equilibration before data collection began. Animals were considered equilibrated when hemodynamic parameters remained stable (within 10%) over two 10-minute baseline periods. Intestinal microvascular images were recorded via a closed-circuit CCD camera on PC and videotape.
Experimental measurements
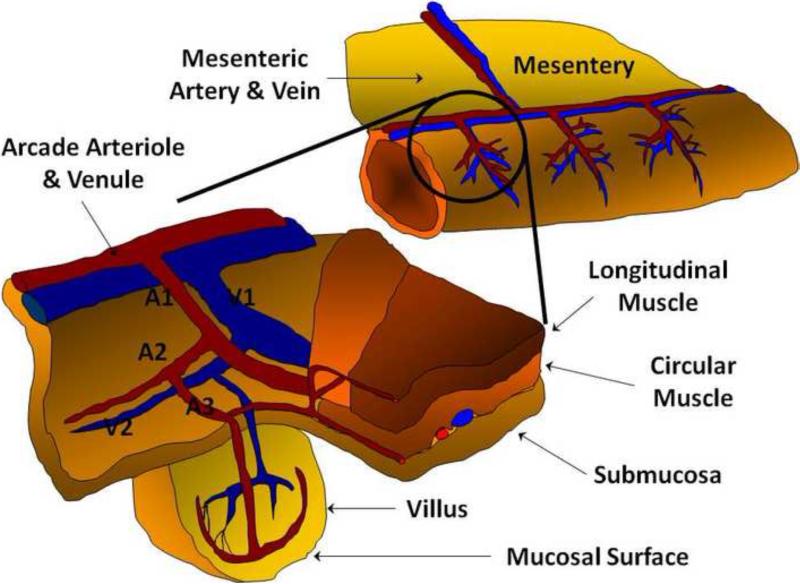
Mean arterial blood pressure and heart rate were recorded at each time point. As shown in Figure 1, the microvascular anatomy was identified according to the description of Bohlen and Gore (4), and arteriolar diameters were measured at three microvascular levels: large inflow distributing arterioles (first-order arteriole, A1), proximal premucosal arterioles (third-order arteriole, pA3), and distal premucosal arterioles (dA3). Centerline red blood cell velocity in A1 arterioles was measured by optical Doppler velocimetry (Optical Doppler Velocimeter, Microcirculation Research Institute, Texas A&M University, College Station, TX, USA). Microvascular flow in A1 arterioles was calculated from the equation A1 Flow (nL/sec) =(V/1.6)(πr2)(0.001), in which V is the centerline red blood cell velocity in mm/sec and r is the vessel radius in μm and 0.001 is a conversion factor to obtain nL/sec.
Figure 1.
a. Arteriolar branching structure of the rat jejunum. b. Inflow arteriole (A1) of the rat jejunum image captured from the intravital videomicroscopy set up. The muscular wall of the arteriole is clearly seen on the left edge of the vessel. To the right of the arteriole is the outflow venule. c. Premucosal (A3) arteriole of the rat jejunum prior to the addition of glucose to the topical bath solution showing the tapering of the arteriole from the proximal to the distal portions. The arteriole originates out of the frame from the upper left corner (proximal A3) and terminates in a villus to the right of the photograph. d. Premucosal (A3) arteriole after addition of glucose to the topical solution demonstrating significant dilation of the proximal and distal ends of the blood vessel.
Experimental Protocol
Rats were randomly assigned to one of seven experimental groups (n=8 per group): 1) glucose alone; 2) mannitol alone; 3) glucose with L-NMMA; 4) mannitol with L-NMMA; 5) glucose with glibenclamide; 6) glucose with L-NMMA and adenosine A1 receptor antagonist; and 7) glucose with glibenclamide and adenosine A1 receptor antagonist.
Within ten minutes of the equilibration period, baseline diameters in no-glucose Krebs at three vascular levels (A1, pA3 & dA3 arterioles) were measured every 10 seconds for 3 minutes and averaged for data analysis. In any group using blockers (L-NMMA, adenosine A1 receptor antagonist and/or glibenclamide), a second baseline period was performed ten minutes after drug application in the bath. Following Krebs wash-out, the blocker(s) was re-applied with glucose or mannitol solution. Hemodynamic and microvascular responses were recorded at 5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80 and 90 minutes. Sodium nitroprusside (10-4) was applied immediately after each experiment to obtain the maximum possible dilation of the vessels. Preliminary data showed that the average dilator capacity, which was calculated from the equation Dilator Capacity = (Vessel DiameterNitroprusside – Vessel DiameterBaseline) / Vessel DiameterBaseline, was 15% for A1 arterioles, 45% for pA3 arterioles and 60% for dA3 arterioles. If an animal exhibited dilator capacity <10% in the A1 arteriole or <30% in pA3 or dA3 arterioles, the animal was excluded from the study (none were excluded for this reason).
Statistical analysis
Vascular responses are presented as percent change from initial baseline diameter for glucose alone or mannitol alone groups or from the second baseline diameter in all other groups. All data are presented as mean ± standard error of the mean. Differences between groups were determined by 3-way and 2-way Analysis of Variance (ANOVA) tests, and then by Student Newman-Kuel multiple comparisons when the ANOVA tests indicated a significant difference (p < 0.05) between groups or time points.
RESULTS
Table 1 shows that the baseline levels of all measured variables (mean arterial blood pressure, heart rate, arteriolar diameters and inflow arteriolar (1A) flow) were consistent between groups. Further, the first and second baseline values were averaged since there were no differences between the initial baseline and the second baseline in blood pressure, heart rate, arteriolar diameters or inflow arteriolar (1A) flow in any group. Mean arterial blood pressure and heart rate remained stable in all groups throughout all experimental time points in all groups. Inflow arteriolar (1A) flow did vary between groups.
Table 1.
Baseline parameters of each experimental group
| Parameter | Glucose Alone | Mannitol Alone | Glucose + L-NMMA | Mannitol + L-NMMA | Glucose + Glibenclamide | Glucose + ADO A1 RA | Glucose + L-NMMA + ADO A1 RA | Glucose + Glibenclamide + ADO A1 RA |
|---|---|---|---|---|---|---|---|---|
| MAP (mmHg) | 118 ± 2 | 120 ± 4 | 114 ± 3 | 119 ± 2 | 120 ± 3 | 119 ± 4 | 116 ± 4 | 118 ± 3 |
| HR (beat/min) | 360 ± 15 | 375 ± 12 | 354 ± 20 | 377 ± 14 | 356 ± 18 | 365 ± 14 | 368 ± 21 | 355 ± 18 |
| A1 flow (nL/sec/100g) | 168 ± 15 | 187 ± 26 | 175 ± 16 | 137 ± 12 | 150 ± 15 | 160 ± 16 | 156 ± 16 | 140 ± 12 |
| A1 (μm) | 93.3 ± 2.6 | 88.4 ± 3.7 | 91.3 ± 3.5 | 87.4 ± 3.1 | 84.4 ± 3.7 | 83.9 ± 2.1 | 83.5 ± 3.2 | 85.0 ± 3.7 |
| pA3 (μm) | 13.7 ± 0.9 | 13.1 ± 0.9 | 13.2 ± 0.5 | 12.2 ± 0.6 | 12.3 ± 0.6 | 13.1 ± 0.5 | 12.7 ± 0.6 | 13.5 ± 0.5 |
| dA3 (μm) | 8.9 ± 0.4 | 8.7 ± 0.3 | 9.2 ± 0.5 | 9.2 ± 0.6 | 8.7 ± 0.5 | 9.1 ± 0.4 | 8.1 ± 0.5 | 9.7 ± 0.4 |
MAP = mean arterial blood pressure; HR = heart rate; A1 = distributing arteriole; pA3 = proximal premucosal arteriole; dA3 = distal premucosal arteriole; L-NMMA, nitric oxide synthase inhibitor; adenosine A1 RA, adenosine A1 receptor antagonist DPCPX; Glibenclamide, ATP-dependent potassium channel blocker. There are no significant differences of each baseline parameter among groups.
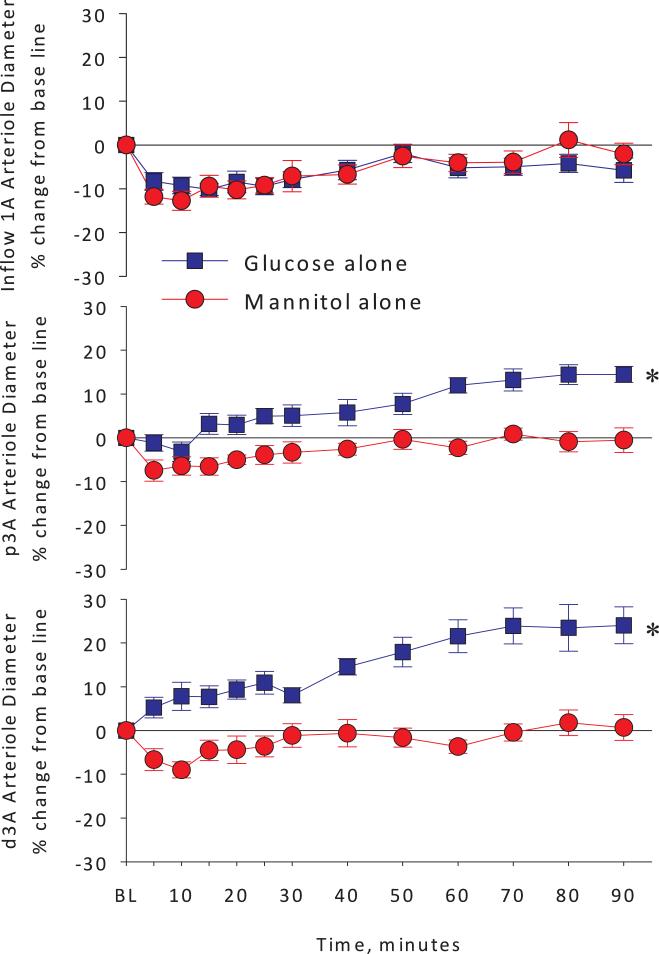
As expected, topical application of isotonic glucose solution caused a rapid and sustained dilation in the proximal and distal premucosal arterioles (p3A and d3A) but no such effect occurred in the mannitol alone group (Figure 2, middle and bottom panels). The inflow arteriole diameters did not change with topical glucose exposure or mannitol control (top panel). The addition of the NO synthase blocker L-NMMA did not alter the arteriolar diameter responses (inflow or premucosal) compared to the mannitol alone group (Figure 3). These observations suggest that the slight mannitol-induced constriction, observed in the mannitol alone group at all arteriolar levels was not due to decreased NO production during mannitol exposure, since pretreatment with L-NMMA did not alter the mannitol response. In the glucose + L-NMMA group, NO synthase blockade decreased inflow (1A) arteriolar diameters at 5, 10 and 15 minutes compared to the glucose alone group (Figure 4, top panel).
Figure 2.
Arteriolar diameter changes expressed as percent change from baseline in inflow 1A arterioles, and premucosal proximal p3A and distal d3A arterioles in glucose alone versus mannitol alone groups. Topical exposure of pre-warmed, pH-buffered, iso-osmotic glucose solution produced a rapid and sustained vasodilation in premucosal p3A and d3A arterioles, but not inflow 1A arterioles. * <0.05 vs. Mannitol alone by 2-way analysis of variance.
Figure 3.
Arteriolar diameter changes expressed as percent change from baseline in inflow 1A arterioles, and premucosal proximal p3A and distal d3A arterioles in mannitol alone versus mannitol plus nitric oxide synthase blockade groups. Topical exposure of pre-warmed, pH-buffered, iso-osmotic mannitol solution produced an early trend toward vasoconstriction that was not statistically significant in any arteriole measured. Pretreatment with the NO synthase inhibitor L-NMMA had no effect on the mannitol alone responses suggesting that altered NO is not a significant mediator in this response. * <0.05 vs. Mannitol alone by 2-way analysis of variance.
Figure 4.
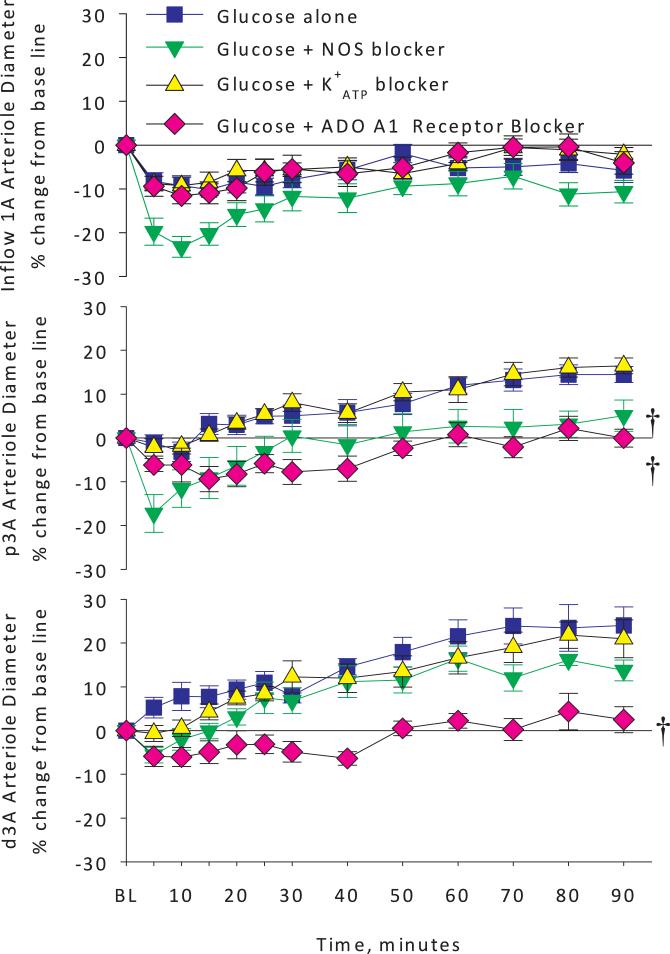
Arteriolar diameter changes expressed as percent change from baseline in inflow 1A arterioles, and premucosal proximal p3A and distal d3A arterioles in glucose alone versus glucose plus NO synthase blocker, glucose plus ATP-dependent potassium channel blocker or glucose plus adenosine A1 receptor blocker groups. Pretreatment with the NO synthase blocker worsened the A1 arteriolar vasoconstriction suggesting that NO synthase acts to blunt the inflow 1A arteriolar response. Also, NO blockade prevented glucose-induced p3A arteriolar vasodilation and partially prevented the d3A response. Pretreatment with topical glibenclamide to block ATP-dependent potassium channels had no effect and pretreatment with the adenosine A1 receptor antagonist DCPCX completely blocked the glucose-induced vasodilation in both p3A and d3A arterioles. † <0.05 vs. Glucose alone by 2-way analysis of variance.
Figure 4 shows the arteriolar diameters for the glucose alone group compared to glucose + L-NMMA, glucose + glibenclamide and glucose + adenosine A1 receptor antagonist groups. Adenosine A1 receptor antagonism with the DPCPX completely prevented the premucosal vasodilation during glucose exposure (Figure 4, middle and bottom panels), while NOS blockade with L-NMMA only partially blocked the glucose-induced proximal and distal premucosal arteriole (p3A and d3A) vasodilation. In the inflow (1A) arterioles, NOS blockade resulted in further vasoconstriction during glucose exposure, suggesting that NOS moderates the vasoconstriction observed during topical glucose exposure in this jejunal segment (top panel). Adenosine A1 receptor antagonism reduced glucose-induced distal premucosal arteriole (d3A) dilation as did NOS inhibition over the first 25 minutes of exposure to glucose. However, adenosine A1 receptor blockade decreased premucosal arteriole diameters more than NO synthase blockade did from 30 to 90 minutes during the observation period. Interestingly, the responses in the proximal vs distal premucosal arterioles differed from each other with adenosine and NO synthase blockade, suggesting that differences might exist with regard to adenosine receptor subtype distribution or regional NO synthase expression of either ecNOS or iNOS. ATP-dependent potassium channel inhibition with glibenclamide had no effect on any arteriolar diameters compared to glucose alone at any time point, suggesting that K+ATP channels are not required for glucose-induced premucosal arteriolar dilation.
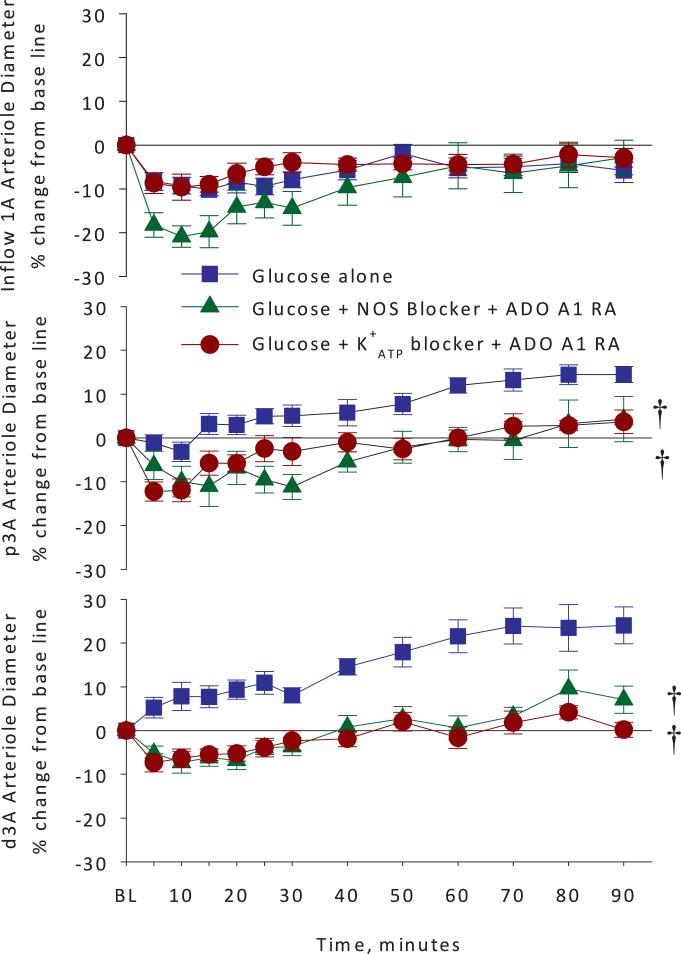
Figure 5 depicts the arteriolar diameters for the glucose alone group compared to glucose + L-NMMA + adenosine A1 receptor antagonist and glucose + glibenclamide + adenosine A1 receptor antagonist groups. Co-application of adenosine A1 receptor antagonist and NOS inhibitor revealed no significant difference compared to the NOS inhibitor alone in the inflow (1A) arterioles, again suggesting a protective role for NO in the glucose-induced inflow (1A) arteriolar vasoconstriction observed. Simultaneous exposure to both the NOS inhibitor and the adenosine A1 receptor antagonist gave the same reduction in glucose-induced dilation of the dA3 as did the adenosine A1 receptor antagonist alone. The effects of the NOS inhibitor and the adenosine A1 receptor antagonist on glucose-induced vasodilation were similar for the proximal premucosal (p3A) and distal premucosal (d3A) arterioles. Simultaneous inhibition of adenosine A1 receptor and K+ATP channels attenuated vasodilation of the premucosal arterioles to the same degree as adenosine A1 receptor antagonism alone.
Figure 5.
Arteriolar diameter changes expressed as percent change from baseline in inflow 1A arterioles, and premucosal proximal p3A and distal d3A arterioles in glucose alone versus glucose plus NO synthase blocker and adenosine A1 receptor antagonist or glucose plus ATP-dependent potassium channel blocker and adenosine A1 receptor blocker groups. Pretreatment with the NO synthase blocker and adenosine A1 receptor antagonist worsened the inflow 1A arteriolar vasoconstriction suggesting that NO synthase acts to blunt the A1 arteriolar response. Also, NO blockade prevented glucose-induced p3A arteriolar vasodilation and partially prevented the d3A response. Pretreatment with topical glibenclamide to block ATP-dependent potassium channels had no effect and pretreatment with the adenosine A1 receptor antagonist DCPCX completely blocked the glucose-induced vasodilation in both p3A and d3A arterioles. † <0.05 vs. Glucose alone by 2-way analysis of variance.
DISCUSSION
The current study is the first to show that glucose-induced intestinal hyperemia is mediated by both adenosine and nitric oxide, but not ATP-dependent potassium channels. Our over-arching hypothesis has been that ATP metabolism is increased in the active small intestine during glucose absorption, which occurs via sodium-linked, secondary active transport; and that ATP metabolism during glucose absorption results in increased adenosine metabolite release into the mucosal and premucosal microvascular and microlymphatic spaces. The data presented in the current study support the hypothesis that adenosine-mediated vasodilation in the small intestinal microcirculation involves nitric oxide. Clinically glucose-induced intestinal hyperemia is of interest for many reasons. This phenomenon might explain some of the benefits of early enteral feeding in critically ill trauma patients that have sometimes been attributed to the powers of immune-enhancing nutrients such as glutamine, arginine, fish oil and trace minerals. Enteral nutrition stimulates blood flow to the intestines in a nutrient- and time-dependent manner. Notably D-glucose is the single most potent nutrient for stimulating intestinal blood flow during postprandial hyperemia. Additionally, glucose present in peritoneal dialysis fluid also partially explains the intense vasodilation and hyperemia observed during peritoneal dwell of dialysis solution, in spite of the fact that the glucose is present in the peritoneal space rather than the intestinal lumin.
Nitric oxide (NO) is a ubiquitous paracrine vasodilator produced primarily by vascular endothelium and neurons and its involvement in regulating vascular basal tone in coronary and retinal vascular beds has been reported (12, 13). In the present study, the topical placement of a specific NOS inhibitor in the tissue bath prior to intestinal exposure to glucose or mannitol had no effect on the resting diameter of inflow (A1) or premucosal (pA3 or dA3) arterioles. These findings suggest that NO does not contribute significantly to the regulation of basal (resting) tone in the rat jejunum microvasculature examined herein. It has been hypothesized that NO produced by constitutive NO synthase in the gut instead functions to maintain sodium reabsorption and villous hyperosmolarity during the resting (unfed) state of the small intestine (5). However, the involvement of NO in regulating vascular reactivity and intestinal hyperemia during nutrient absorption (fed state) has been reported in the small intestinal microcirculation (6, 25, 26). Topical application of pre-warmed, pH-buffered, iso-osmotic glucose solution caused rapid premucosal arteriolar dilation and capillary recruitment in the rat ileum that was prevented by NOS inhibition with L-NAME (25). Also, NO metabolite levels (nitrate and nitrite) were increased in the portal vein during luminal glucose exposure (and presumably, glucose absorption) in the rat small intestine, which was inhibited by adenosine receptor antagonists (26). Further, Bohlen et al used NO-sensitive microelectrodes to detect increased NO in intestinal submucosal blood vessel walls in proportion to luminal glucose concentration (6).
In the current study, mucosal glucose exposure produced vasodilation of the premucosal A3 arterioles that was partially attenuated by NOS inhibition. On the other hand, NOS inhibition had little effect on premucosal A3 arteriolar diameter during intestinal mannitol exposure, which is metabolically inert. These findings support the hypothesis that NO, generated during sodium-linked secondary active transport of glucose, is a key regulator of glucose-induced premucosal arteriolar vasodilation. As noted above, NOS inhibition in the terminal ileum during topical glucose exposure completely abolished premucosal arteriolar vasodilation (25). The difference in the degree of loss of glucose-induced vasodilation during NOS inhibition between the proximal jejunum and the terminal ileum might be related to the functional difference between the two intestinal segments. For example, both constitutive and inducible nitric oxide synthase isoforms have been identified in the terminal ileum while only constitutive NOS has been identified in the jejunum (37). Furthermore, the vascular response to adenosine and the distribution of adenosine receptors also varies along the length of small intestine (21).
Topical glucose exposure in a discrete section of the small intestine was insufficient stimulus to induce vasodilation or increased blood flow in the inflow (1A) arterioles, in this study, as in our prior intravital videomicroscopy studies of glucose-mediated intestinal vasodilation. This observation appears to contradict prior studies that have identified glucose as the single most potent inducer of absorptive hyperemia during intestinal nutrient exposure (9, 14). The possible interpretations of this phenomenon have been previously discussed and include insufficient area of glucose exposure and/or insufficient involvement of bile recirculation, which might regulate glucose-induced intestinal hyperemia via inflow (1A) arteriolar vasodilation (25). In the present study, the inflow (1A) arterioles responded similarly to glucose and mannitol, suggesting that slight constriction of inflow (1A) vessels was not related to nutrient (glucose) absorption processes. During NO synthase inhibition, inflow (1A) vessels responded similarly to either sugar (actively absorbed glucose versus passively diffused mannitol) and constricted even more in the absence of NOS inhibition during 90 minutes exposure, which indicates that constitutive NO synthase activity counteracts the A1 arteriolar vasoconstriction induced by intestinal exposure to either glucose or mannitol. The constrictor process involved in this phenomenon is not known.
The role of adenosine in absorptive intestinal hyperemia is well established (15, 31, 32, 34). However, the relative contributions of adenosine receptor subtypes in glucose-induced premucosal arteriolar vasodilation have not been determined. Proctor identified the adenosine A2b receptor as the subtype most likely to mediate vasodilation in small intestinal microvessels (32). Our prior work suggested that the adenosine A1 receptor subtype mediated vasodilation in the resting (unfed) intestinal microcirculation in the rat (21). The current study suggests that the adenosine A1 receptor plays a predominant role in regulating dilation of the premucosal vessels during glucose absorption. Adenosine A1 receptor-mediated vasodilation has been demonstrated in several other vascular beds; however, vasoconstriction via activation of the adenosine A1 receptor has been more commonly reported in the literature. Adenosine A1 receptors have been identified on both vascular endothelium and smooth muscle cells (8) suggesting that adenosine A1 receptor-mediated vasodilation could be either endothelium dependent or independent.
The mechanisms underlying adenosine A1 receptor moderated vasodilation have been investigated in different tissues (3, 11, 22). Both A1 and A2x adenosine receptors have been reported to co-regulate NO production in both in vivo and ex vivo studies. Endothelium-derived NO release has been shown during activation of adenosine A2x receptors in both in vitro (20) and in vivo studies (2, 30). Adenosine A1 receptor-mediated vasodilation involving increased NO synthesis has been reported in rat hindlimb during systemic hypoxia (7). In addition, adenosine A1 receptor-induced negative inotropic effect has been associated with increased NO production (33). Signaling transduction between adenosine receptor activation and NO production has also been suggested in biochemical studies (35). Activation of adenosine A1 receptors results in inositol trisphosphate accumulation, which triggers an intracellular cascade including increased intracellular calcium and subsequent activation of constitutive NO synthase. The current study indicates that NO is involved in glucose-induced vasodilation of the premucosal arterioles and that NO release is likely mediated through adenosine A1 receptor activation. However, adenosine A1 receptor-mediated vasodilation cannot be solely explained by increased NO release.
ATP-dependent K+ channels are associated with adenosine A1 receptors via G proteins (18) or protein kinase C (PKC) pathways (27). Activation of adenosine A1 receptors is thought to open K+ATP channels on vascular smooth muscle cells, which causes hyperpolarization of the cell membrane and a subsequent decrease in intracellular Ca2+ concentration and vasodilation. Additionally, opening of K+ATP channels could also be triggered by increased intracellular cGMP, a well-known effector of NO (19). K+ATP channels could thereby be indirectly associated with NO release via activation of adenosine A1 receptors. Adenosine A1 receptor-induced opening of K+ATP channels has also been reported in coronary, diaphragm, and retinal microvasculature beds (11, 22, 38). The association of adenosine A1 receptors and K+ATP channels has also been reported in ex vivo rat mesenteric artery (36) and in cat vascular (hindquarter) studies (3). However, Bivalacqua et al did not define the roles of NO, prostaglandins, or K+ATP channels in mediating adenosine A1 receptor-mediated vasodilation in cat hindquarter circulation (3).
Generally, adenosine A1 receptor-mediated vasodilation is controversial. The possible existence of an unusual low-affinity receptor type would support the notion that adenosine A1 receptor activity might be dependent on adenosine agonist concentration. This might explain studies in which application of high doses of adenosine A1 receptor antagonist reduced blood flow in the large submucosal arterioles in the intestine, while low concentrations had no effect (32). A single study has shown that activation of adenosine A1 receptors increased intracellular cAMP levels (which would lead to vessel relaxation) as opposed to numerous studies of adenosine A1 receptor signaling pathways (23). Our present study found that inhibition of K+ATP channels had no effect on glucose-induced premucosal arteriolar vasodilation and that co-application of K+ATP blocker with adenosine A1 receptor antagonist had no effect on premucosal arteriolar diameters. These findings support the notion that adenosine A1 receptor-mediated vasodilation in the intestinal terminal arterioles does not involve activation of K+ATP channels.
In summary, we have previously demonstrated that glucose-induced intestinal hyperemia is mediated through both adenosine A1 and A2a receptors (21). In the present study, we examined the role of nitric oxide synthase and ATP-dependent potassium channels in adenosine A1 receptor-mediated vasodilation. Our data suggests that nitric oxide plays a significant role in glucose-induced intestinal hyperemia, which supports our prior findings in both intestinal intravital videomicroscopy studies of glucose-induced intestinal hyperemia (25) and in studies examining nitric oxide metabolite levels in the portal circulation following absorption of glucose (26). That prior study showed that glucose absorption increased wash out of nitric oxide metabolites into the portal circulation (suggestive of increased NO production) and, further, that adenosine blockade prevented the glucose-induced increase in NO metabolite wash out. These findings suggest that the involvement of adenosine receptors in glucose-induced intestinal hyperemia is “up-stream” of the nitric oxide synthase involvement and necessary for NO production. Other factors that could be associated with adenosine A1 receptor-mediated vasodilation in the intestinal microvasculature are adenosine A2b receptors and/or prostaglandins. Adenosine A2b receptors are distributed on the intestinal microvasculature and might play a role in glucose-induced vasodilation of the terminal A4 arterioles (experimental observation not published). Additionally, interactions between adenosine A1 receptor and adenosine A2b receptor-activated signaling pathways have been indicated in some studies (28). Prostaglandins have been implicated in studies of adenosine A1 receptor-induced cardiac effects in isolated guinea pig hearts (33). Prostaglandins are also involved in absorptive hyperemia in the intestine (10) and might be released during activation of adenosine receptors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abebe W, Hussain T, Olanrewaju H, Mustafa SJ. Role of nitric oxide in adenosine receptor-mediated relaxation of porcine coronary artery. Am JPhysiol Heart Circ Physiol. 1995;269:H1672–H1678. doi: 10.1152/ajpheart.1995.269.5.H1672. [DOI] [PubMed] [Google Scholar]
- 2.Ali S, Metzger WJ, Olanrewaju HA, Mustafa SJ. Adenosine receptor-mediated relaxation of rabbit airway smooth muscle: a role for nitric oxide. Am J Physiol. 1997;273(3 Pt 1):L581–L587. doi: 10.1152/ajplung.1997.273.3.L581. [DOI] [PubMed] [Google Scholar]
- 3.Bivalacqua TJ, Champion HC, Lambert DG, Kadowitz PJ. Vasodilator responses to adenosine and hyperemia are mediated by A1 and A2 receptors in the cat vascular bed. Am J Physiol Regulatory Integrative Comp Physiol. 2002;282:R1696–R1709. doi: 10.1152/ajpregu.00394.2001. [DOI] [PubMed] [Google Scholar]
- 4.Bohlen HG, Gore RW. Preparation of rat intestinal muscle and mucosa for quantitative microcirculatory studies. Microvasc Res. 1976;11:103–110. doi: 10.1016/0026-2862(76)90081-9. [DOI] [PubMed] [Google Scholar]
- 5.Bohlen HG, Lash JM. Intestinal absorption of sodium and nitric oxide-dependent vasodilation interact to dominate resting vascular resistance. Circ Res. 1996;78(2):231–237. doi: 10.1161/01.res.78.2.231. [DOI] [PubMed] [Google Scholar]
- 6.Bohlen HG. Mechanism of increased vessel wall nitric oxide concentrations during intestinal absorption. Am J Physiol. 1998;275(2 Pt 2):H542–H550. doi: 10.1152/ajpheart.1998.275.2.H542. [DOI] [PubMed] [Google Scholar]
- 7.Bryan PT, Marshall JM. Adenosine receptor subtypes and vasodilatation in rat skeletal muscle during systemic hypoxia: a role for A1 receptors. J Physiol. 1999;514(1):151–162. doi: 10.1111/j.1469-7793.1999.151af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnstock G. Current state of purinoceptor research. Pharm Acta Helv. 1995;69(4):231–242. doi: 10.1016/0031-6865(94)00043-u. Review. [DOI] [PubMed] [Google Scholar]
- 9.Chou CC, Hsieh CP, Yu YM, Kvietys P, Yu LC, Pittman R, Dabney JM. Localization of mesenteric hyperemia during digestion in dogs. Am J Physiol. 1976;230(3):583–589. doi: 10.1152/ajplegacy.1976.230.3.583. [DOI] [PubMed] [Google Scholar]
- 10.Chou CC, Alemayehu A, Mangino MJ. Prostanoids in regulation of postprandial jejunal hyperemia and oxygen uptake. Am J Physiol. 1989;257(5 Pt 1):G798–G808. doi: 10.1152/ajpgi.1989.257.5.G798. [DOI] [PubMed] [Google Scholar]
- 11.Danialou G, Vicaut E, Sambe A, Aubier M, Boczkowski J. Predominant role of A1 adenosine receptors in mediating adenosine induced vasodilatation of rat diaphragmatic arterioles: involvement of nitric oxide and the ATP-dependent K+ channels. Br J Pharmacol. 1997;121:1355–1363. doi: 10.1038/sj.bjp.0701247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorner GT, Garhofer G, Kiss B, Polska E, Polak K, Riva CE, Schmetterer L. Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol. 2003;285(2):H631–H636. doi: 10.1152/ajpheart.00111.2003. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez N, Sanchez MA, Martinez MA, Garcia-Villalon AL, Monge L, Gomez B, Dieguez G. Role of nitric oxide in vascular tone and in reactivity to isoproterenol and adenosine in the goat coronary circulation. Eur J Pharmacol. 2000;387(1):93–99. doi: 10.1016/s0014-2999(99)00766-9. [DOI] [PubMed] [Google Scholar]
- 14.Gallavan RH, Jr, Chou CC, Kvietys PR, Sit SP. Regional blood flow during digestion in the conscious dog. Am J Physiol. 1980;238(2):H220–H225. doi: 10.1152/ajpheart.1980.238.2.H220. [DOI] [PubMed] [Google Scholar]
- 15.Granger HJ, Norris CP. Role of adenosine in local control of intestinal circulation in the dog. Circ Res. 1980;46(6):764–770. doi: 10.1161/01.res.46.6.764. [DOI] [PubMed] [Google Scholar]
- 16.Hein TW, Belardinelli L, Kuo L. Adenosine A(2A) receptors mediate coronary microvascular dilation to adenosine: role of nitric oxide and ATP-sensitive potassium channels. J Pharmacol Exp Ther. 1999;291(2):655–64. [PubMed] [Google Scholar]
- 17.Hinschen AK, Rose'Meyer RB, Headrick JP. Adenosine receptor subtypes mediating coronary vasodilation in rat hearts. J Cardiovasc Pharmacol. 2003;41(1):73–80. doi: 10.1097/00005344-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Kirsch GE, Codina J, Birnbaumer L, Brown AM. Coupling of ATP-sensitive K+ channels to A1 receptors by G proteins in rat ventricular myocytes. Am J Physiol. 1990;259(3 Pt 2):H820–H826. doi: 10.1152/ajpheart.1990.259.3.H820. [DOI] [PubMed] [Google Scholar]
- 19.Kubo M, Nakaya Y, Matsuoka S, Saito K, Kuroda Y. Atrial natriuretic factor and isosorbide dinitrate modulate the gating of ATP-sensitive K+ channels in cultured vascular smooth muscle cells. Circ Res. 1994;74:471–476. doi: 10.1161/01.res.74.3.471. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Fenton RA, Wheeler HB, Powell CC, Peyton BD, Cutler BS, Dobson JG., Jr Adenosine A2a receptors increase arterial endothelial cell nitric oxide. J Surg Res. 1998;80:357–364. doi: 10.1006/jsre.1998.5439. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Harris PD, Zakaria el R, Matheson PJ, Garrison RN. Microvascular responses to adenosine help explain functional and pathologic differences between intestinal segments. Am J Surg. 2004;188(5):526–531. doi: 10.1016/j.amjsurg.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Puro DG. Adenosine activates ATP-sensitive K(+) currents in pericytes of rat retinal microvessels: role of A1 and A2a receptors. Brain Res. 2001;907(1-2):93–99. doi: 10.1016/s0006-8993(01)02607-5. [DOI] [PubMed] [Google Scholar]
- 23.Linden J. Structure and function of A1 adenosine receptors. FASEB J. 1991;5:2668–2676. doi: 10.1096/fasebj.5.12.1916091. Review. [DOI] [PubMed] [Google Scholar]
- 24.Maher MM, Gontarek JD, Jimenez RE, Cahill PA, Yeo CJ. Endogenous nitric oxide promotes ileal absorption. J Surg Res. 1995;58(6):687–692. doi: 10.1006/jsre.1995.1108. [DOI] [PubMed] [Google Scholar]
- 25.Matheson PJ, Wilson MA, Spain DA, Harris PD, Anderson GL, Garrison RN. Glucose-induced intestinal hyperemia is mediated by nitric oxide. J Surg Res. 1997;72(2):146–154. doi: 10.1006/jsre.1997.5176. [DOI] [PubMed] [Google Scholar]
- 26.Matheson PJ, Spain DA, Harris PD, Garrison RN, Wilson MA. Glucose and glutamine gavage increase portal vein nitric oxide metabolite levels via adenosine A2b activation. J Surg Res. 1999;84(1):57–63. doi: 10.1006/jsre.1999.5604. [DOI] [PubMed] [Google Scholar]
- 27.Mubagwa K, Flameng W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc Res. 2001;52(1):25–39. doi: 10.1016/s0008-6363(01)00358-3. [DOI] [PubMed] [Google Scholar]
- 28.Murthy KS, McHenry L, Grider JR, Makhlouf GM. Adenosine A1 and A2b receptors coupled to distinct interactive signaling pathways in intestinal muscle cells. J Pharmacol Exp Ther. 1995;274(1):300–306. [PubMed] [Google Scholar]
- 29.Ngai AC, Coyne EF, Meno JR, West GA, Winn HR. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2329–H2335. doi: 10.1152/ajpheart.2001.280.5.H2329. [DOI] [PubMed] [Google Scholar]
- 30.Nieri P, Martinotti E, Calderone V, Breschi MC. Adenosine-mediated hypotension in in vivo guinea-pig: receptors involved and role of NO. Br JPharmacol. 2001;134(4):745–52. doi: 10.1038/sj.bjp.0704301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proctor KG. Possible role for adenosine in local regulation of absorptive hyperemia in rat intestine. Circ Res. 1986;59(4):474–481. doi: 10.1161/01.res.59.4.474. [DOI] [PubMed] [Google Scholar]
- 32.Proctor KG. Intestinal arteriolar responses to mucosal and serosal applications of adenosine analogues. Circ Res. 1987;61(2):187–193. doi: 10.1161/01.res.61.2.187. [DOI] [PubMed] [Google Scholar]
- 33.Rubio R, Ceballos G. Sole activation of three luminal adenosine receptor subtypes in different parts of coronary vasculature. Am J Physiol Heart Circ Physiol. 2003;284:H204–H214. doi: 10.1152/ajpheart.00068.2002. [DOI] [PubMed] [Google Scholar]
- 34.Sawmiller DR, Chou CC. Role of adenosine in postprandial and reactive hyperemia in canine jejunum. Am J Physiol. 1992;263(4 Pt 1):G487–G493. doi: 10.1152/ajpgi.1992.263.4.G487. [DOI] [PubMed] [Google Scholar]
- 35.Sterin-Borda L, Gomez RM, Borda E. Role of nitric oxide/cyclic GMP in myocardial adenosine A1 receptor-inotropic response. Br JPharmacol. 2002;135(2):444–450. doi: 10.1038/sj.bjp.0704487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tabrizchi R, Lupichuk SM. Vasodilatation produced by adenosine in isolated rat perfused mesenteric artery: a role for endothelium. Naunyn Schmiedebergs Arch Pharmacol. 1995;352(4):412–418. doi: 10.1007/BF00172778. [DOI] [PubMed] [Google Scholar]
- 37.Weisbrodt NW, Pressley TA, Li YF, Zembowicz MJ, Higham SC, Zembowicz A, Lodato RF, Moody FG. Decreased ileal muscle contractility and increased NOS II expression induced by lipopolysaccharide. Am J Physiol. 1996;271(3 Pt 1):G454–G460. doi: 10.1152/ajpgi.1996.271.3.G454. [DOI] [PubMed] [Google Scholar]
- 38.Yao Z, Gross GJ. Glibenclamide antagonizes adenosine A1 receptor-mediated cardioprotection in stunned canine myocardium. Circulation. 1993;88(1):235–244. doi: 10.1161/01.cir.88.1.235. [DOI] [PubMed] [Google Scholar]