Abstract
Proposals that adaptation with left-shifting prisms induces neglect-like symptoms in normal individuals rely on a dissociation between the post-adaptation performance of individuals trained with left- versus right-shifting prisms (e.g., Colent, et al. 2000). A potential problem with this evidence is that normal young adults have an a priori leftward bias (e.g., Jewell & McCourt, 2000). In Experiment 1, we compared the line bisection performance of young adults to that of aged adults, who as a group may lack a leftward bias in line bisection. Participants trained with both left- and right-shifting prisms. Consistent with our hypothesis, while young adults demonstrated aftereffects for left, but not right prisms, aged adults demonstrated reliable aftereffects for both prisms. In Experiment 2, we recruited a larger sample of young adults, some of whom were right-biased at baseline. We observed an interaction between baseline bias and prism-shift, consistent with the results of Experiment 1: Left-biased individuals showed a reduced aftereffect when training with right-shifting prisms and right-biased individuals showed a reduced aftereffect when training with left-shifting prisms. These results suggest that previous failures to find generalizable aftereffects with right-shifting prisms may be driven by participants’ baseline biases rather than specific effects of the prism itself.
Keywords: hemispatial neglect, prism adaptation, line bisection, attention
Introduction
Research on the treatment of left spatial neglect following right-hemisphere stroke has elucidated a promising therapy with the potential for long-lasting benefits: prism adaptation (see Lauté et al., 2006, for a review). Because prism adaptation may rehabilitate neglect, investigators have been challenged to explain its effectiveness (e.g., Streimer, Sablatnig, & Danckert, 2006). Emerging from this research is the claim that prism adaptation may induce a model of spatial neglect in healthy individuals (e.g., Michel, 2006), marked by an asymmetrical adaptation to left- and right-shifting prisms. Here, we provide evidence that asymmetrical prism adaptation may depend on pre-existing biases in healthy individuals, and therefore may not be an appropriate model for neglect.
Spatial neglect is characterized by a functionally debilitating failure (Barrett & Burkholder, 2006) to recognize or attend to stimuli on the side of space contralateral to a brain injury (Heilman, Watson, & Valenstein, 2003). It is more likely to occur after right- than after left-brain stroke (c.f. Ringman, Saver, Woolson, Clarke, & Adams, 2004), suggesting a special role for the right hemisphere in spatial attention (e.g., Heilman, et al.; Mesulam, 2000). In prism adaptation treatment for left spatial neglect, patients don prism goggles that displace vision rightward and make repeated pointing movements to targets. During training, the initial part of their hand trajectory is blocked from view (Redding, Rossetti, & Wallace, 2005). While wearing the prisms, patients initially err rightward, but quickly return to accurate pointing performance: evidence of adaptation to the visual displacement. A rehabilitative effect is observed when patients remove the prisms: patients demonstrate an aftereffect – rightward pointing bias is reduced and patients may demonstrate leftward pointing bias. For patients, measured improvement transfers broadly to paper-and-pencil bedside tasks (e.g., line bisection and copy a scene tasks; Rossetti et al, 1998) as well as to functional tasks (e.g., Keane, Turner, Sherrington, & Beard, 2006). Although neglect patients demonstrate adaptation and post-adaptation effects when training with right-shifting prisms, they do not demonstrate these effects when training with left-shifting prisms (Rossetti, et al., 1998).
Conversely, when healthy individuals adapt to left-shifting prisms, they acquire post-adaptation rightward bias. However, right-shifting prsms fail to produce a generalizeable post-adaptation leftward bias in the healthy young (Colent et al. 2000; Girardi, McIntosh, Michel, Vallar, & Rossetti, 2004; Michel, Rossetti, Rodes, & Tilikete, 2006; Michel Pisella, Halligan, Luauté, Rode, & Boisson, 2003; c.f. Michel, Vernet, Courtine, Ballay, & Pozzo, 2008, Streimer et al., 2006). One interpretation of this asymmetry in post-adaptation errors is that left-shifting prisms induce a neglect-like spatial bias because they preferentially engage the right hemisphere of the brain, which plays a special role in spatial attention (Colent et al., 2000; Michel, 2006).
A major confound in examining prism adaptation in young people may be the pre-existing capacity for change: Young individuals have a priori spatial biases that can create ceiling or floor effects in their performance (Streimer, et al., 2006). On visuospatial tasks, including the line bisection task, young individuals exhibit a distinct leftward bias (e.g., Charles, Sahraie, & McGeorge, 2007; Jewell & McCourt, 2000; Nicholls, Bradshaw, & Mattingley, 1999). Therefore, young individuals may fail to acquire a leftward bias after training with right-shifting prisms because they are as left-biased as possible at baseline.
In two experiments, we investigated whether the asymmetric effect of left versus right prism training may be consistent with baseline asymmetric spatial biases. In Experiment 1 we recruited healthy young and aged adults for training with both left- and right-shifting prisms. Although younger adults demonstrate a leftward bias, older adults demonstrate no bias or a rightward bias (Barrett & Craver-Lemley, 2008; Failla, Sheppard, & Bradshaw, 2003: Fujii, Fukatsu, Yamadori, & Kimura, 1995). Reducing or reversing the effect of the leftward baseline bias should clarify whether left-shifting prisms exert a special effect. If an a priori leftward bias of young individuals produces a ceiling effect limiting the post-adaptation leftward shift, then in an aged group lacking leftward bias, training with left- and right-shifting prisms should induce either symmetrical aftereffects, or the reversed asymmetry.
EXPERIMENT 1
Method
Participants
Twenty-four right-handed, neurologically unimpaired individuals received $30 for participating. Twelve individuals (6 female) aged 21 to 33 (M = 25.3) comprised the young group and twelve individuals (6 female) aged 61 to 85 (M = 72.8) comprised the aged group. All participants had normal or corrected-to-normal vision. All received informed consent and were treated in accordance with the Declaration of Helsinki, and as approved by the IRB of Kessler Foundation Research Center.
Apparatus and Stimuli
During prism adaptation, participants wore Bernell™ Deluxe Prism Training Glasses fitted with an optical wedge prism shifting participants’ vision 12.4° laterally. During baseline and post-test, participants wore placebo goggles: Bernell™ frames fitted with plain glass lenses.
At pre-test, training, and post-test participants bisected lines (23.5 cm × 0.3 cm) oriented horizontally on a sheet of paper (21.6 cm × 27.9 cm). Participants sat and performed these bisections from the open end of a box (61 cm high × 91.4 cm wide × 61 cm long) similar to that of Redding and Wallace (2001). A shelf (91.4 cm wide × 15.2 cm long) blocked participants’ view of their initial hand movement, while permitting subjects a distal, terminal view of the hand movement. To-be-bisected lines were placed 59.1 cm from the torso of the each participant directly aligned with the participant’s body midline, or 29.8 cm to the right or left of body midline.
Procedure
All participants completed the procedure described below for both the left- and right-shifting prisms in separate sessions, counterbalanced across participants. Participants returned for their second session no sooner than 48 hrs after the first. On average, 110 hrs passed between experimental sessions (range, 48 to 288 hrs).
During line bisection trials, participants were seated while wearing either placebo or prism goggles. Participants kept their hands at the center of their chest at heart-level, and bisected lines using a single, ballistic, out-and-back movement. Within each block of trials, participants bisected lines presented in left, central, and right space, the order of which was randomly determined.
During the pre-test and post-test, participants donned placebo goggles and performed two blocks of three line bisection trials in left, central, and right space. During adaptation training, participants donned prisms and performed ten blocks of six bisection trials (two each in left, central, and right space).
Results and Discussion
Participants’ error on each line-bisection trial was recorded as deviation from the true center of the line in cm, with leftward deviations coded as negative and rightward deviations, positive. Preliminary analyses of the counterbalancing factor – order of right/left prism administration – failed to yield significant effects. Thus, it was excluded from further analyses. All repeated-measures analyses were performed using the MANOVA Pillai’s Trace procedure.
Baseline line bisection performance
Participants exhibited the expected group biases in their baseline performance. Comparing the average bisection error to zero revealed that younger adults exhibited a leftward bias [M = −0.28, SD = .38, t(11) = −2.6, p = .026] while older adults did not exhibit a bias [M = 0.06, SD = .44, t(11) = .48, p = .638]. A MANOVA on the bisection error revealed a main effect of age group, F(1, 22) = 4.3, p = .050,, and a line position by age group interaction, F(2, 21) = 8.3, p = .002, . As can be seen in Table 1, performance of the aged participants did not differ across the three line positions; however, the leftward bias of the young participants emerged in both left and center spaces, but not in right space.
Table 1.
Mean bisection error at baseline in Experiment 1 as a function of age group and line position. Standard deviations appear in parentheses.
| Left | Center | Right | |
|---|---|---|---|
| Young | −0.50 (0.50) | −0.40 (0.45) | −0.05 (0.43) |
| Aged | 0.16 (0.40) | 0.09 (0.50) | −0.07 (0.59) |
Adaptation
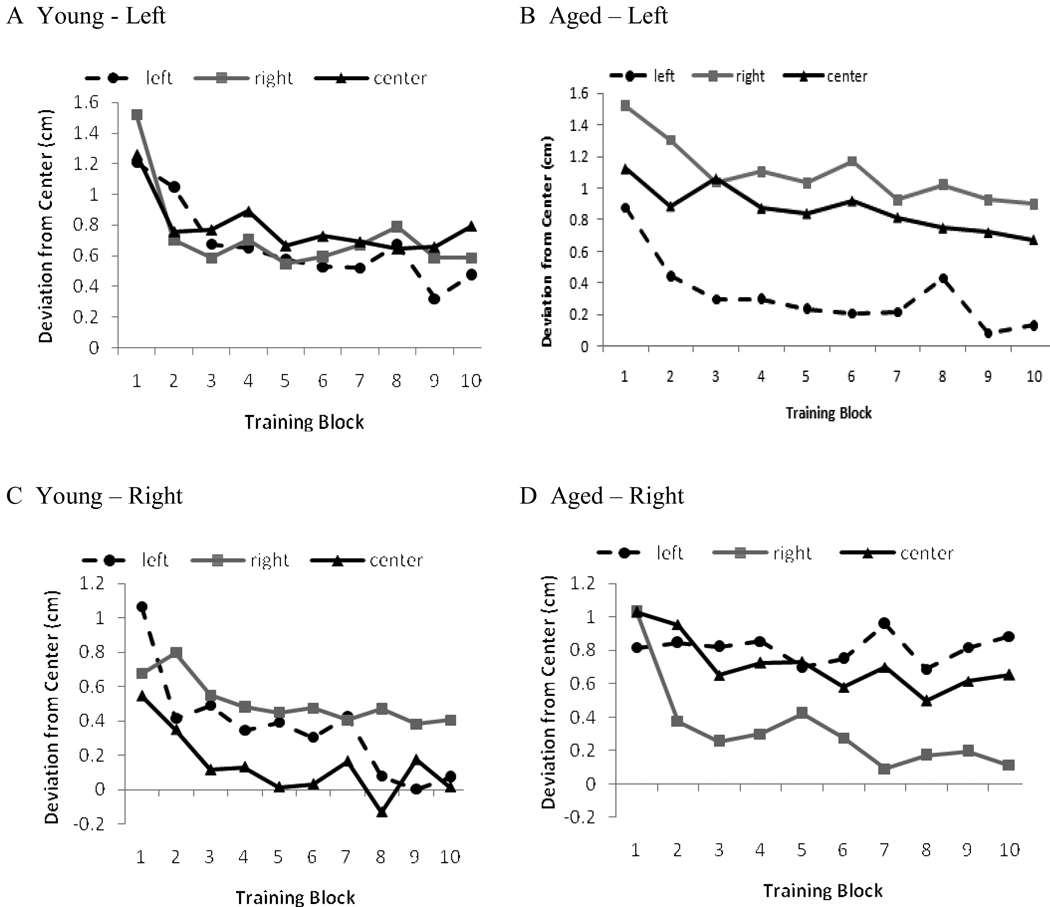
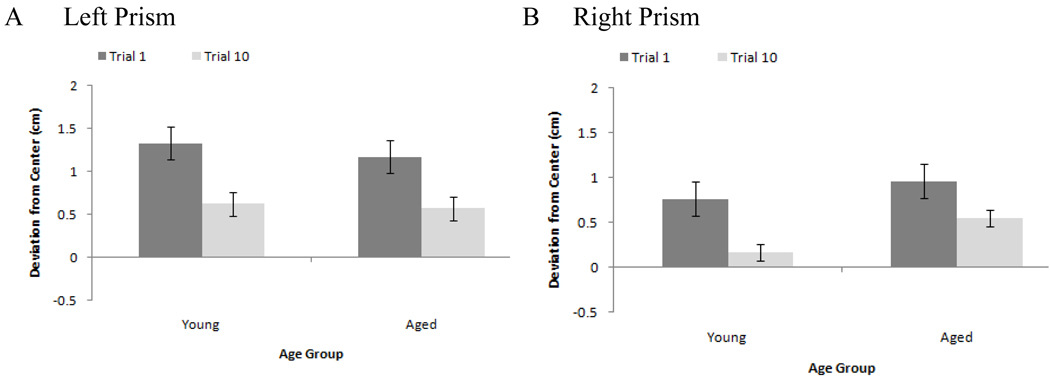
So that adaptation to left and right prisms could be statistically compared, the error for all bisections made while wearing left-shifting prisms was multiplied by negative one. Thus, positive values indicate error in the direction of the prism displacement and negative values indicate error in the opposite direction. Figure 1 depicts bisection error across blocks. To assess adaptation, we compared participants’ performance in the first adaptation block to that in their last (Figure 2). Both young and aged participants exhibited a decrease in their error from blocks one to ten for both prisms (all ts > 2.8 and ps < .019).
Figure 1.
Bisection error across training trials in Experiment 1 as a function of prism, line position and age group. Positive values indicate error in the direction of the prism shift and negative values error opposite the prism shift.
Figure 2.
Comparison of average deviation from true center on the first and last blocks of adaptation training for (A) left and (B) right prisms as a function of age group. Positive values indicate error in the direction of the prism shift, negative values error in the direction opposite the prism shift. Error bars = 1 SE.
The MANOVA yielded main effects of trial, F(1, 22) = 40.0, p < .001,, and prism, F(1, 22) = 6.1, p = .002, , and a four way interaction, F(2, 21) = 5.3, p = .014,. Overall, participants’ error decreased from trials one (M = 1.06, SE = 0.11) to ten (M = 0.48, SE = 0.06), and overall, participants erred more in the direction of the prism displacement when wearing left (M = 0.92, SE = 0.11) as opposed to right prisms (M = 0.61, SE = 0.09), an effect that may in part be driven by lingering leftward baseline biases. The interaction can be seen in Table 3. For the left prism, both groups exhibited significant decreases in their bisection error between trials one and ten for all lines (ts > 2.4, ps < .025). For the right prism, however, young participants improved for lines in left and center space (ts > 2.4, ps < .034), but not for those in right space. Aged participants improved for lines in right and center space (ts > 2.9, ps < .016), but not for those in left space.
Table 3.
Mean bisection error during adaptation trials one and ten in Experiment 1 as a function of age group and prism shift. Standard deviations appear in parentheses.
| Left Prism | |||
| Young | |||
| Left | Right | Center | |
| Trial 1 | 1.21 (0.47) | 1.52 (1.48) | 1.26 (0.65) |
| Trial 10 | 0.48 (0.54) | 0.59 (0.48) | 0.80 (0.63) |
| Aged | |||
| Left | Right | Center | |
| Trial 1 | 0.88 (0.67) | 1.52 (0.81) | 1.13 (0.54) |
| Trial 10 | 0.13 (0.53) | 0.90 (0.92) | 0.68 (0.60) |
| Right Prism | |||
| Young | |||
| Left | Right | Center | |
| Trial 1 | 1.07 (1.15) | 0.68 (0.56) | 0.55 (0.97) |
| Trial 10 | 0.08 (0.43) | 0.40 (0.45) | 0.02 (0.43) |
| Aged | |||
| Left | Right | Center | |
| Trial 1 | 0.82 (0.71) | 1.04 (0.89) | 1.03 (0.47) |
| Trial 10 | 0.88 (0.44) | 0.11 (0.58) | 0.65 (0.28) |
Aftereffects
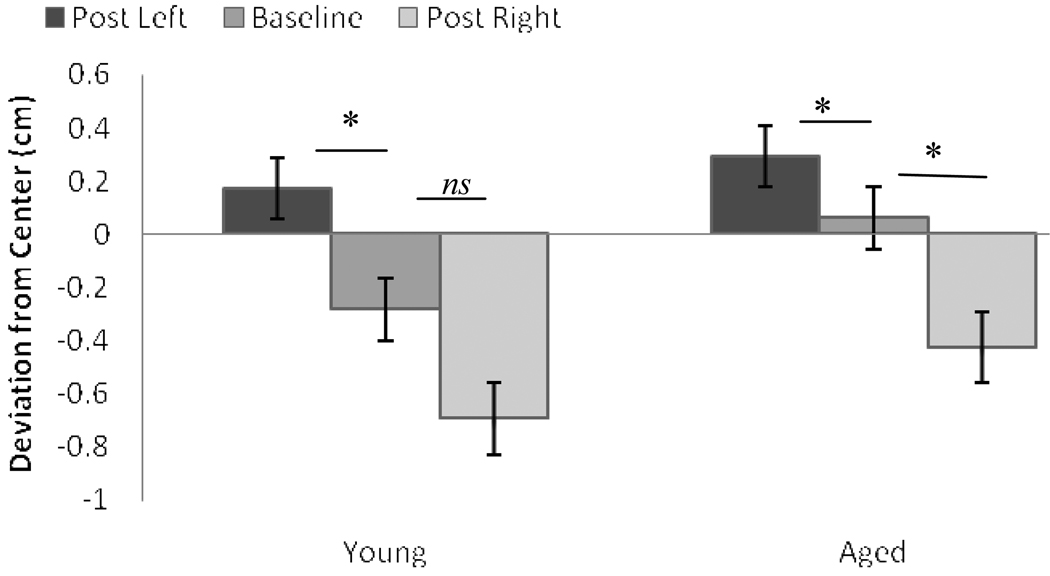
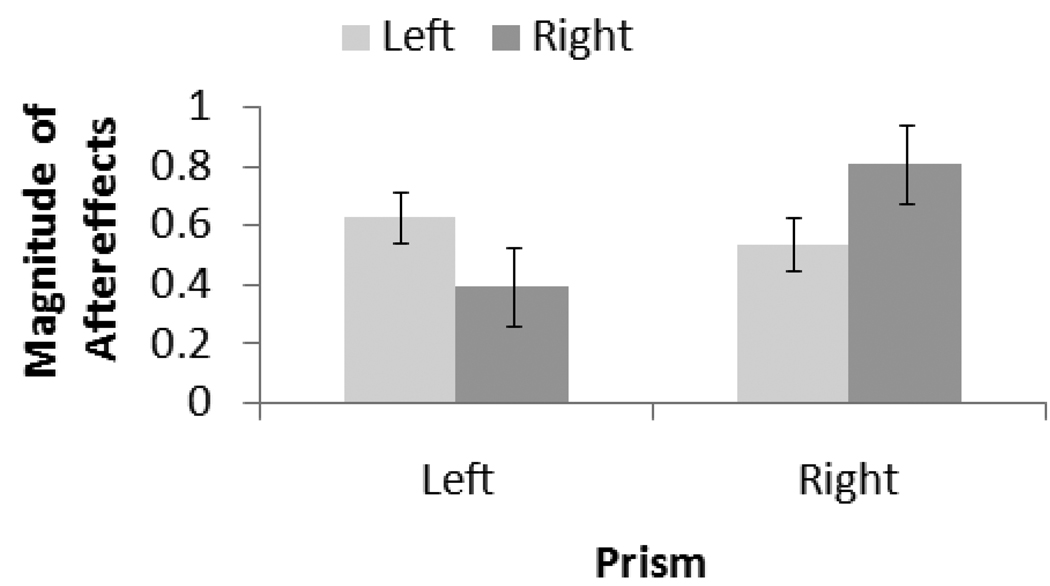
Preliminary analyses ruled out any interactions between line position and pre-post test. Because a full discussion of the effects of line position would be redundant with that discussed for baseline, we focus on the effects of age group and pre-post test. Aftereffect error appears in Figure 3. For the left prism, the MANOVA revealed a main effect of pre-post test, F(1, 22) = 14.2, p < .001, . Both young, t(11) = −2.8, p = .015, d = 1.08, and aged, t(11) = −2.5, p = .028, d = 0.59, erred significantly to the right of their baseline performance after training with the left prism. For the right prism, there was also a main effect of pre-post test, F(1, 22) = 12.4, p = .002, : aged participants erred significantly left of their baseline after training with the right prism, t(11) = 3.2, p = .008, d = 1.17. Leftward post-adaptation error in young participants after training with the right prism did not reach significance, t(11) = 2.0, p = .07, d = 0.89. Although the direction of the means was similar for both the young and aged groups, the patterns of significance and effect size are consistent with our hypothesis: young participants, who as a group were left biased, demonstrated significant aftereffects with the left, but not the right, prism. Conversely, aged participants, who as a group were not biased, demonstrated significant aftereffects for both prisms.
Figure 3.
Aftereffect error in Experiment 1 as a function of age group. Error bars = 1 SE.
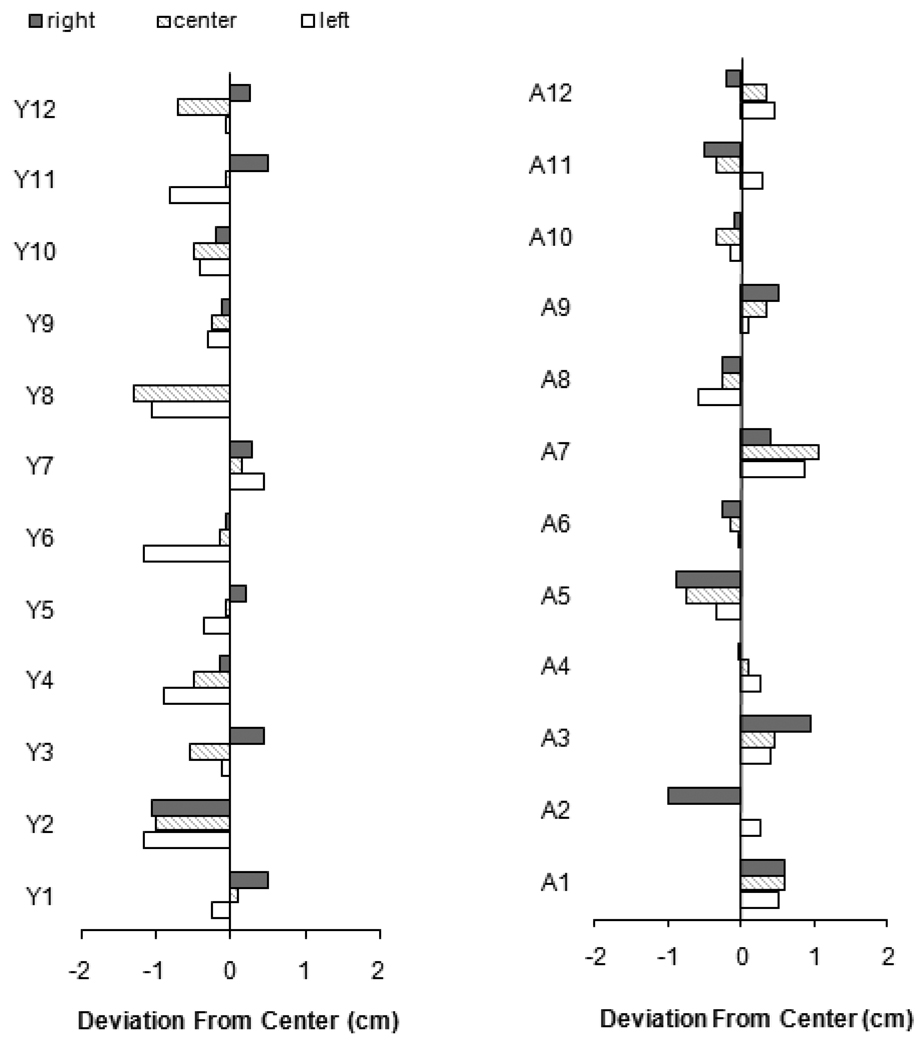
Although we found the expected baseline biases at the group level, we investigated individual bisection error to determine whether the group averages reflected biases at the individual level. Figure 4 depicts the bisection error for each participant across the three line positions. As is apparent in the figure, there was within-group heterogeneity in baseline bias. Therefore, we performed an additional non-parametric Wilcoxon signed ranks analysis on the aftereffects of sub-groups of participants with consistent biases. Ten participants (4 aged, 6 young) had a consistent leftward bias and five (4 aged, 1 young) had a consistent rightward bias.
Figure 4.
Raw deviations from center for individual participants in Experiment 1, with young participants on left (Y) and aged participants on right (A). Legend indicates line position.
Figure 5 depicts participants’ aftereffect error as a function of their baseline bias. The observation of aftereffects depended critically on participants’ baseline biases. For the left-shifting prism, left-biased individuals demonstrated a significant rightward shift, Z = −2.8, p = .005, but the right-biased individuals did not, Z = −0.41, p = 0.69. For the right-shifting prism, right-biased individuals demonstrated a significant leftward shift, Z = −2.0, p = .043, but left-biased individuals did not, Z = −0.76, p = .45. Collectively, these data suggest that the presence of baseline biases may severely limit the ability to either create or to detect significant aftereffects post prism adaptation.
Figure 5.
Aftereffect error in Experiment 1 as a function of baseline bias.
Although the results of Experiment 1 support the hypothesis that baseline biases limit the ability to observe aftereffects, we performed a second experiment to clarify two potential issues. In particular, in Experiment 1, participants adapted to the prisms while performing a line bisection task. However, in many studies, participants adapt to prisms by performing a pointing task (e.g., Berberovic & Mattingley, 2003; Colent et al., 2000). Because the observation of aftereffects may vary with the task performed during prism adaptation (Michel et al., 2008; Morton & Bastian, 2004; Redding & Wallace, 2006), it is possible that the effects observed in Experiment 1 were particular to a situation in which participants adapt to prisms by bisecting lines. Additionally, in Experiment 1, the clearest evidence for the role of baseline biases in limiting aftereffects was based on a non-parametric analysis of a subset (n = 15) of the participants. This relatively small sample size may lead to concerns about the generalizability of the results. Experiment 2 was designed to address these issues.
EXPERIMENT 2
In Experiment 2 participants performed one of three tasks during prism adaptation: dot-pointing, line bisection or line bisection with feedback. This design allowed for the direct comparison of the effects of adapting with the dot-pointing and line bisection tasks. Additionally, one primary difference between dot-pointing and line bisection is that dot-pointing allows participants to immediately see their error (i.e., the difference between the endpoint of their movement and the position of the dot), but the line bisection task does not. When bisecting lines, participants need to compare the endpoint of their movement with an internal representation of the center of the line to infer movement error. Thus, in Experiment 2, we added a training condition in which participants received visual feedback indicating the exact center of the line immediately after each line bisection trial.
Finally, to address the issue of small sample size, we recruited a large sample of young participants (N = 69) with the goal of identifying larger subsets of participants with left and right baseline biases. We restricted ourselves to a larger sample of the young, rather than once again recruiting aged participants, to avoid heterogeneity beyond that associated with differences in baseline bias (e.g., the aged are less likely than the young to use compensatory strategies while wearing prisms and this influences the extent of their aftereffects; Fernández-Ruiz, Hall, Vergara & Díaz, 2000).
Method
Participants
Sixty-nine right-handed, healthy individuals aged 18 to 25 (M = 19.07, 46 female) participated for a course requirement. All had normal or corrected-to-normal vision. All gave informed consent and were treated in accordance with the Declaration of Helsinki, and as approved by the IRB of Seton Hall University.
Procedure
Participants adapted using the same prisms described for Experiment 1. Unlike Experiment 1, participants performed all tasks on a touch screen monitor, making movements beneath a shelf that occluded the initial view of their hand-path.
Participants completed three baseline line bisection trials. On each trial a black line, 13.5 cm long, appeared centered on the monitor. Participants bisected the line by using their index finger to draw a vertical line directly on the monitor. The drawn line appeared in red and remained visible for 500 ms. Between each line bisection trial a random-dot visual mask appeared for 1 sec.
During adaptation participants wore either the left – or right-shifting prisms and performed either the dot-pointing, line bisection or bisection with feedback task. All participants completed 10 blocks of 6 training trials. During dot-pointing trials, a black dot, 1.5 cm in diameter, appeared at random locations on the monitor. Participants “pointed” at the dot, by touching the dot’s location. The dot then disappeared and a new dot appeared in a different location after a 500 ms delay. In the line bisection task, each adaptation trial proceeded exactly like those performed at baseline. Finally, in the line bisection with feedback task, training was similar to standard line bisection with the exception that immediately upon completion of participants’ bisection responses a black line appeared perpendicular to and at the exact center of the stimulus line, making it simultaneously visible with the bisection mark drawn by the participants. After 500 ms, these lines were replaced by the visual mask.
After adaptation, all participants donned the placebo goggles and performed three post-test line bisections exactly as described for baseline.
Results and Discussion
We measured horizontal error (in cm) as either distance from the dot or distance from the center of the line.
Baseline
As a group, participants exhibited the expected leftward bias in their average error at baseline [M = −0.086, SD = .19, t(68) = −3.8, p < .001]. However, similar to what we observed in Experiment 1, there were individual differences in baseline bias. We labeled individuals whose average baseline line bisection was less than zero as left-biased, and those for whom it was greater than zero as right-biased. Of the 35 participants training with the left prism, 24 were left-biased, 10 were right-biased and 1 was not biased. Of the 34 participants training with the right prism, 23 were left biased, 10 were right-biased and 1 was not biased. Preliminary analyses revealed that the factor of baseline bias did not interact with the factor of adaptation task. Thus, for the sake of clarity, we examine the effects of adaptation training task and that of baseline bias separately.
Effects of Adaptation Task
Adaptation
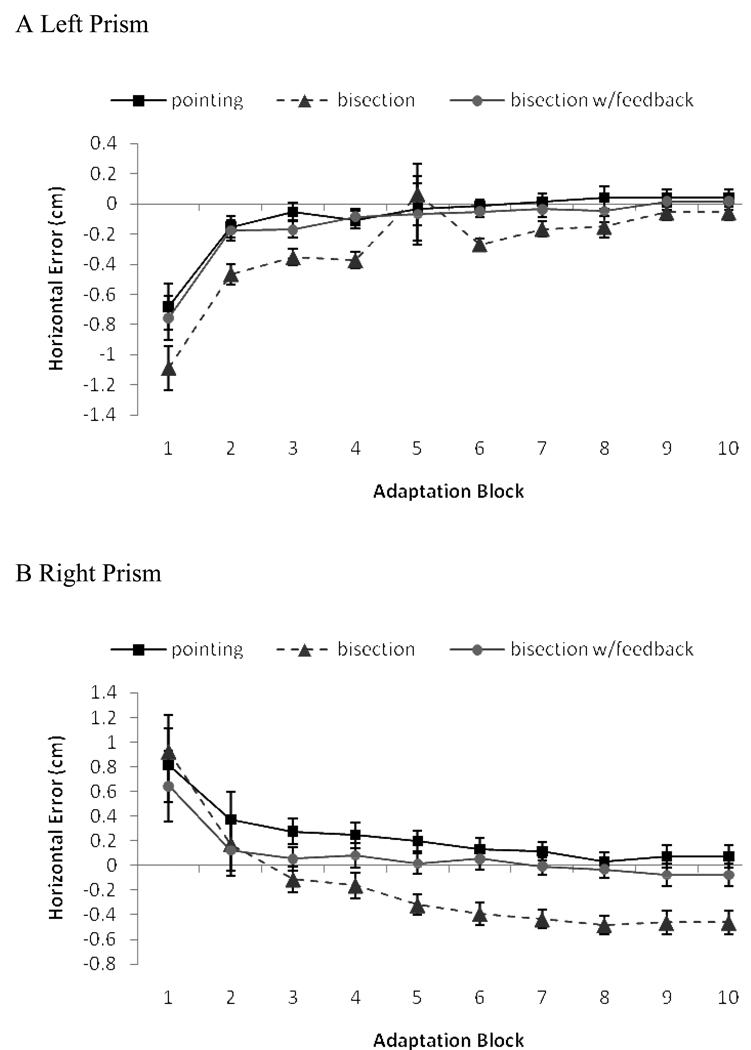
Figure 6 depicts the average error in blocks one through ten as a function of adaptation task and prism shift. Regardless of training task, all participants reduced their error between the first and last blocks of the adaptation training. For both prisms, separate block (one, ten) by task MANOVAs revealed only main effects of block: F(31) = 88.0, p < .001, , for the left prism and F(30) = 28.03, p < .001, , for the right. Paired samples t tests comparing error in block one to that in block ten yielded significant effects for all training tasks with both prisms (all ts > .26, all ps <.025). Thus, regardless of the training task, participants demonstrated the typical error reduction associated with adaptation to the prism goggles.
Figure 6.
Average error across adaptation blocks in Experiment 2 as a function of (A) left- and (B) right-shifting prisms. Legend indicates adaptation task. Error bars = 1 SE.
Aftereffects
All aftereffects comparisons were based on the first line bisection trials performed at baseline and immediately after adaptation training. Training with both left- and right-shifting prisms produced significant aftereffects that did not vary with the type of adaptation task. Participants trained with the left-shifting prism erred significantly right of their baseline after adaptation (M = 0.01, SD = .32 at baseline and M = .55, SD = .49 post-adaptation), F(1, 32) = 34.9, p < .001, . Participants trained with the right prism, erred significantly left of their baseline after adaptation (M = −.013, SD = .29 at baseline and M = −.61, SD = .48 post-adaptation), F(1,31) = 45.3, p < .001, . For both prisms, separate MANOVAs revealed only the main effects of pre-post session and no effects involving adaptation task, Fs < 1.5, ps > .22. Thus, our results demonstrate similar degrees of adaptation and aftereffects for the dot pointing and line bisection tasks.
Effects of Baseline Biases on Aftereffects
One goal of Experiment 2 was to yield more individuals exhibiting rightward baseline biases. We accomplished this goal, with 20 participants demonstrating a rightward bias (10 in each prism group), and 47 participants demonstrating a leftward bias (24 in the left and 23 in the right prism groups). Thus, we present the results of parametric analyses assessing the effects of baseline bias and direction of prism shift on the magnitude of participants’ aftereffects.1
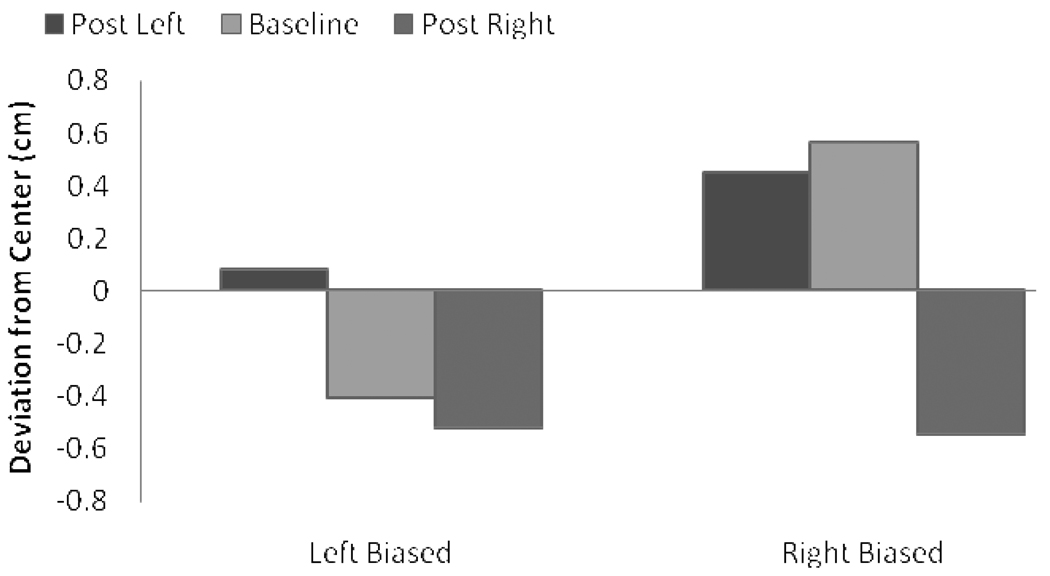
So that the magnitude of the aftereffects associated with training on left and right prisms could be directly compared, the measure of aftereffects performance was the absolute difference between pre- and post-adaptation line bisections. As is evident in Figure 7, we observed the predicted interaction between prism and baseline bias. The MANOVA with prism-shift and baseline bias as factors revealed only an interaction between the two, F(1, 63) = 5.08, p = .028, . Although participants with a leftward baseline bias demonstrated similar aftereffects regardless of prism, F < 1, participants with a rightward bias demonstrated greater aftereffects after training with the right as opposed to left prism, F(1, 18) = 7.2, p = .015, .
Figure 7.
Aftereffects performance in Experiment 2 as a function of baseline bias and prism. Error bars = 1 SE.
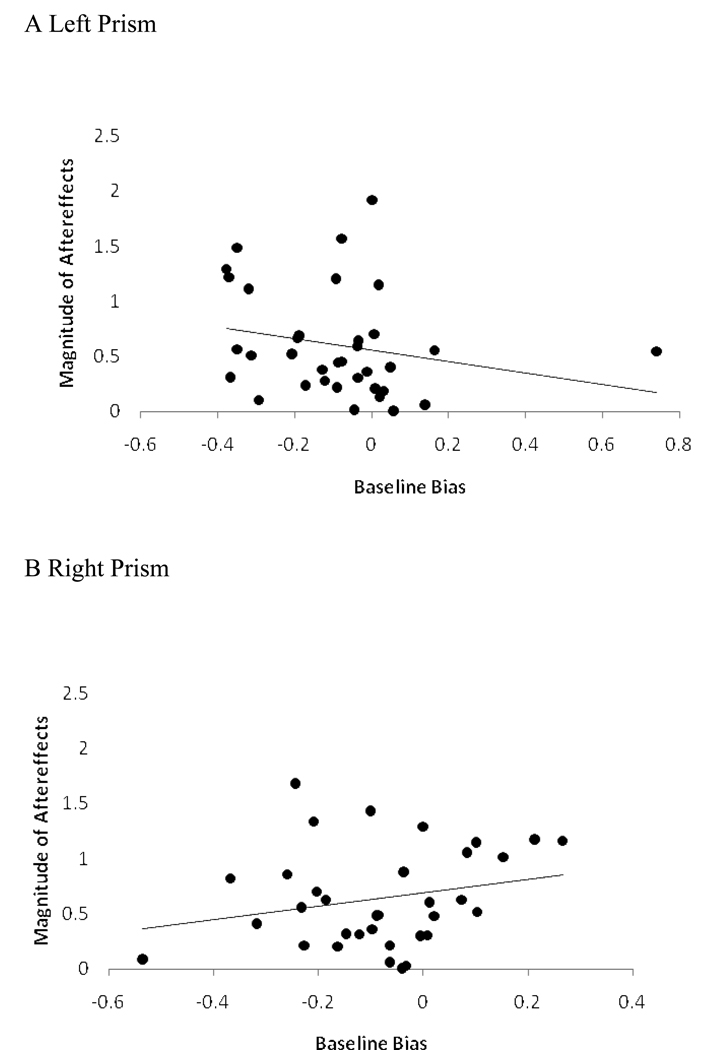
We confirmed and extended the results of the previous analysis by using participants’ baseline bias as a continuous predictor. A custom general linear model created to examine the main effect of baseline bias (as a continuous variable), the main effect of prism-shift, and the interaction of the two yielded only a prism-shift by baseline bias interaction, F(1, 63) = 4.2, p = .045, . Figure 8 depicts this interaction. For the left prism a greater magnitude of leftward bias (i.e., negative baseline error) was associated with greater aftereffects (β = −.513).2 Conversely, for the right prism, a greater magnitude of rightward bias (i.e., positive baseline error) was associated with greater aftereffects (β = .603). Thus, we continued to observe the interaction between baseline biases and direction of prism shift when using baseline bias as a quantitative, continuous predictor.
Figure 8.
Relation between baseline bias and magnitude of aftereffects in Experiment 2 for (A) left and (B) right prisms.
General Discussion
In two experiments we tested the hypothesis that previously observed dissociations in the aftereffects produced by left- and right-shifting prisms result from the leftward baseline biases of healthy young adults. Consistent with this hypothesis, we observed that the extent of participants’ aftereffects with left- and right-shifting prisms depended on the direction of their baseline biases. Participants exhibiting a leftward bias at baseline demonstrated reliable rightward aftereffects after training with a left-shifting prism, but demonstrated no or reduced leftward aftereffects after training with a right-shifting prism. However, participants exhibiting a rightward bias at baseline exhibited the opposite pattern: They demonstrated reliable leftward aftereffects after training with the right-shifting prism, but they demonstrated no or reduced rightward aftereffects after training with the left-shifting prism.
Large magnitude post-adaptation errors in healthy individuals exposed to left-shifting prisms have been taken as evidence for a special vulnerability of right hemisphere systems to prism-induced bias (Colent et al., 2000; Michel, 2006). By this logic, left prism adaptation training may temporarily activate right hemisphere attentional systems, while left hemisphere systems lack the attentional capacity to produce leftward error after right prism exposure. Our results suggest that a reinterpretation of the previously-observed dissociation is necessary. We suggest that previous reports were limited by ceiling effects on the ability to observe post-adaptation prism-induced errors. What our results point to as the phenomenon of interest is not left- versus right-shifting prisms, but left versus right baseline biases.
One may argue that participants with a rightward baseline bias are not right- lateralized for line bisection. Thus, they would not show the same pattern of asymmetry as left-biased individuals. We find this argument difficult to reconcile when considering the literatures on spatial neglect, line bisection, and prism adaptation as a whole. Our aged participants in Experiment 1 were not left-biased as a whole, but spatial neglect occurs more commonly in the aged (Gottesman et al., 2008). This suggests that an individual lacking left bias may still have strongly right-lateralized spatial attention. Additionally, recent neuroimaging results demonstrated bilateral activations, not preferential right hemisphere activations, in unimpaired individuals during adaptation to left-shifting prisms (Luauté et al., 2009). This finding makes sense, as left- and right-shifting prisms produce optical distortion of both visual fields. Lastly, much as current researchers are remarking on the similarities between the performance of neglect patients and that of healthy individuals adapted to left-shifting prisms, earlier researchers remarked on the similarities between the performance of neglect patients and the leftward bias of young individuals on the line bisection task, terming it pseudoneglect (Bowers & Heilman, 1980; McCourt & Jewell, 1999). Rather than revealing something special about how different prisms preferentially recruit different hemispheres of the brain, recent studies may actually elucidate more fundamental hemispheric dominance for attention and action, unrelated to the direction of prism displacement per se (i.e., many of these differences exist at baseline and are unrelated to the direction of prism shift).
Ours is not the first body of work to elucidate the importance of baseline biases in prism adaptation. Streimer et al. (2006) found that the effects of prism adaptation on reflexive and voluntary orienting varied systematically with participants’ baseline orienting performance. Collectively, these results suggest that researchers must seriously consider how differences in baseline performance may affect the observation of differences post-adaptation.
Why Individual Differences At Baseline?
One explanation for these biases and their potential for variability across individuals is the lateralization of visuospatial function. Visuospatial function is thought to be right-lateralized. Consistent with the notion that line bisection may be preferentially right-lateralized, functional imaging reveals greater activation of right than left hemisphere structures for line bisections performed in peripersonal space (Fink et al., 2000; Fink, Marshall, Weiss, & Zilles, 2001). Were visual attention disproportionately allotted contralateral to the more activated hemisphere (Reuter-Lorenz, Kinsbourne, & Moscovitch, 1990), it would result in an over-representation of the left side of space and thus a leftward bisection bias (e.g., Bultitude & Davies, 2006).
An increase in bilateral as opposed to lateralized recruitment of the cerebral hemispheres with age (see Cabeeza, 2002 for review) could explain why one would see a lack of bias in older adults. In addition, recent work demonstrates rightward shifts in attention with decreasing alertness (Manly, Dobler, Dodds, & George, 2005). A decline in alertness associated with age (Robinson & Kertzman, 1990) could potentially be a mechanism for a rightward shift in attention that produces either no bias or the rightward line bisection bias observed in some of our aged adults and in the aged adults of Fuji et al. (1995).
Furthermore, sex differences in line bisection performance have been reported. In particular, lifespan changes in spatial bias may differ for the sexes, potentially reflecting sex differences in the effects of aging on lateralization (Barrett & Craver-Lemley, 2008). For example, Varnava and Halligan (2007) found that both young and aged women made leftward errors in line bisections with their dominant hand, but that while young men also made leftward errors, aged men made rightward or no errors. Although the studies reported here were not designed to capture the effects of gender on baseline line bisection biases, sex differences may explain, at least in part, some of the heterogeneity we observed in the baseline biases of our participants (see Table 2 for a depiction of baseline line bisection performance as a function of age and gender).
Table 2.
Mean bisection error at baseline in Experiments 1 and 2 as a function of age group and gender. Standard deviations appear in parentheses.
| Experiment 1 | Experiment 2 | |||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Young | −0.33 (.32) | −0.24 (.46) | −0.01 (.23) | −0.12 (.16) |
| Aged | 0.11 (.52) | 0.01 (.39) | -- | -- |
Effects of Spatial Position on Bisection Error
Although not the focus of the current paper, in Experiment 1 we found that the baseline biases in young adults varied with the spatial position at which they bisected lines. In particular, at baseline they exhibited leftward bias for lines bisected in left and center space, but not for lines bisected in right space. This pattern of differing bias for different line positions is consistent with other observations that left hemi-space presentation leads to greater leftward error and that right hemi-space presentation leads to reduced error or to rightward error (e.g., Luh, 1995; McCourt & Jewell, 1999). Nevertheless, there are a number of demonstrations of the opposite pattern of bias based on hemi-space differences (i.e., greater leftward bias in right space; for a comprehensive review see Jewell & McCourt, 2000). Although a meta-analysis of line bisection studies reveals an average increased leftward deviation with lines presented in left hemi-space (Jewell & McCourt, 2000), it has been suggested that the variability in reported effects of spatial position on line bisection error indicates that this effect is very sensitive to minor differences in the line bisection task participants are asked to perform (Luh, 1995). However, of particular importance to the current investigation, we did not find an interaction between pre- to post-prism line bisection performance and the spatial position of the line. This lack of interaction suggests that the observed effects of prism adaptation were similar across all line positions regardless of the pre-existing baseline differences in line bisection error for lines in different spatial positions.
Conclusion
We demonstrated that inducing post-prism adaptation horizontal errors on line bisection depends critically on the baseline biases of individuals. In our study, individuals with an a priori leftward bias on the line bisection task demonstrated a rightward shift after training with a left prism, but demonstrated no or a reduced leftward shift after training with a right prism. Conversely, individuals with an a priori rightward bias on the line bisection task demonstrated a leftward shift after training with a right prism, but demonstrated not or a reduced rightward shift after training with a left prism. We suggest that asymmetries in post-adaptation aftereffects to left- and right-shifting prisms previously observed in young adults may be the result of ceiling effects generated by baseline spatial biases. Our results suggest that training healthy individuals with left-shifting prisms does not produce a model of spatial neglect. Whether an individual’s degree of leftward bias due to post-prism adaptation may reflect capacity of that person’s right hemispheric systems mediating spatial attention and action, independent of baseline bias, is not yet known and may deserve further investigation.
Acknowledgements
The authors thank Siby Varughese, Milda Woods, Elizabeth Murray, and Maeve O’Donnell for technical and administrative support. Study supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (K02 NS47099), and the Henry H. Kessler Foundation.
Footnotes
One concern with using unequal sample sizes is the potential for violating the homogeneity of variance assumption. For the analyses reported here, this assumption was not violated (Levene’s test ps > .38).
This relation still obtains when the participant with the large rightward baseline bias is removed from this analysis (β = −.915).
References
- Barrett AM, Burkholder S. Monocular patching in subjects with right-hemisphere stroke affects perceptual-attentional bias. Journal of Rehabilitation Research and Development. 2006;43:337–346. doi: 10.1682/jrrd.2005.01.0015. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Craver-Lemley CE. Is it what you see, or how you say it? Spatial bias in young and aged subjects. Journal of the International Neuropsychological Society. 2008;14:562–570. doi: 10.1017/S1355617708080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberovic N, Mattingley JB. Effects of prismatic adaptation on judgments of spatial extent in peripersonal and extrapersonal space. Neuropsychologia. 2003;41:493–503. doi: 10.1016/s0028-3932(02)00090-8. [DOI] [PubMed] [Google Scholar]
- Bowers D, Heilman KM. Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychologia. 1980;18:491–498. doi: 10.1016/0028-3932(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Bultitude JH, Davies AMA. Putting attention on the line: Investigating the activation-orientation hypothesis of pseudoneglect. Neuropsychologia. 2006;44:1849–1858. doi: 10.1016/j.neuropsychologia.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Charles J, Sahraie A, McGeorge P. Hemispatial asymmetries in judgment of stimulus size. Perception & Psychophysics. 2007;69:687–698. doi: 10.3758/bf03193771. [DOI] [PubMed] [Google Scholar]
- Colent C, Pisella L, Bernieri C, Rode G, Rossetti Y. Cognitive bias induced by visuo-motor adaptation to prisms: A simulation of unilateral neglect in normal individuals? Neuroreport. 2000;11:1899–1902. doi: 10.1097/00001756-200006260-00019. [DOI] [PubMed] [Google Scholar]
- Failla CV, Sheppard DM, Bradshaw JL. Age and responding-hand related changes in performance of neurologically normal subjects on the line-bisection and chimeric-faces tasks. Brain and cognition. 2003;52:353–363. doi: 10.1016/s0278-2626(03)00181-7. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Hall C, Vergara P, Diaz R. Prism adaptation in normal aging: Slower adaptation rate and longer aftereffect. Cognitive Brain Research. 2000;9:223–226. doi: 10.1016/s0926-6410(99)00057-9. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall J, Shah N, Weiss P, Halligan P, Grosse-Ruyken M, et al. Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology. 2000;54:1323–1331. doi: 10.1212/wnl.54.6.1324. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Zilles K. The neural basis of vertical and horizontal line bisection judgments: An fMRI study of normal volunteers. NeuroImage. 2001;14:S59–S67. doi: 10.1006/nimg.2001.0819. [DOI] [PubMed] [Google Scholar]
- Fujii T, Fukatsu R, Yamadori A, Kimura I. Effect of age on the line bisection test. Journal Of Clinical And Experimental Neuropsychology. 1995;17:941–944. doi: 10.1080/01688639508402443. [DOI] [PubMed] [Google Scholar]
- Girardi M, McIntosh RD, Michel C, Vallar G, Rossetti Y. Sensorimotor effects on central space representation: Prism adaptation influences haptic and visual representations in normal subjects. Neuropsychologia. 2004;42:1477–1487. doi: 10.1016/j.neuropsychologia.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Gottesman RF, Kleinman JT, Davis C, Heidler-Gary J, Newhart M, Kannan V, Hillis AE. Unilateral neglect is more severe and common in older patients with right hemispheric stroke. Neurology. 2008;71:1439–1444. doi: 10.1212/01.wnl.0000327888.48230.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. fourth edition. New York: Oxford University Press; 2003. pp. 296–346. [Google Scholar]
- Jewell G, McCourt ME. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38:93–110. doi: 10.1016/s0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Keane S, Turner C, Sherrington C, Beard JR. Use of Fresnel prism glasses to treat stroke patients with hemispatial neglect. Archives of Physical Medicine and Rehabilitation. 87:1668–1672. doi: 10.1016/j.apmr.2006.08.322. [DOI] [PubMed] [Google Scholar]
- Luauté J, Halligan P, Rode G, Jacquin-Courtois S, Boisson D. Prism adaptation first among equals in alleviating left neglect: A review. Restorative Neurology and Neuroscience. 2006;24:409–418. [PubMed] [Google Scholar]
- Luauté J, Schwartz S, Rossetti Y, Spiridon M, Rode G, Boisson D, Vuilleumier P. Dynamic changes in brain activity during prism adaptation. The Journal of Neuroscience. 2009;29:169–178. doi: 10.1523/JNEUROSCI.3054-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luh KE. Line bisection and perceptual asymmetries in normal individuals: What you see is not what you get. Neuropsychology. 1995;9:435–448. [Google Scholar]
- Manly T, Dobbler VB, Dodds CM, George MA. Rightward shift in spatial awareness with declining alertness. Neuropsychologia. 2005;43:1721–1728. doi: 10.1016/j.neuropsychologia.2005.02.009. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Jewell G. Visuospatial attention in line bisection: Stimulus modulation of pseudoneglect. Neuropsychologia. 1999;37:843–855. doi: 10.1016/s0028-3932(98)00140-7. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Attentional networks, confusional states and neglect syndromes. In: Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology, second edition. New York: Oxford University Press; 2000. pp. 174–256. [Google Scholar]
- Michel C. Simulating unilateral neglect in normals: Myth or reality? Restorative Neurology and Neuroscience. 2006;24:419–430. [PubMed] [Google Scholar]
- Michel C, Pisella L, Halligan PW, Luauté J, Rode G, Boisson D, et al. Simulating unilateral neglect in normals using prism adaptation: Implications for theory. Neuropsychologia. 2003;41:25–39. doi: 10.1016/s0028-3932(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Michel C, Rossetti Y, Rode G, Tilikete C. After-effects of visuo-manual adaptation to prisms on body posture in normal subjects. Experimental Brain Research. 2003;148:219–226. doi: 10.1007/s00221-002-1294-3. [DOI] [PubMed] [Google Scholar]
- Michel C, Vernet P, Courtine G, Ballay Y, Pozzo T. Asymmetrical after-effects of prism adaptation during goal oriented locomotion. Experimental Brain Research. 2008;185:259–268. doi: 10.1007/s00221-007-1152-4. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Prism adaptation during walking generalizes to reaching and requires the cerebellum. Journal of Neurphysiology. 2004;92:2497–2509. doi: 10.1152/jn.00129.2004. [DOI] [PubMed] [Google Scholar]
- Nicholls MER, Bradshaw JL, Mattingley JB. Free-viewing perceptual asymmetries for the judgment of shade, numerosity and size. Neuropsychologia. 1999;37:307–314. doi: 10.1016/s0028-3932(98)00074-8. [DOI] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Calibration and alignment are separable: Evidence from prism adaptation. Journal of Motor Behavior. 2001;33:401–412. doi: 10.1080/00222890109601923. [DOI] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Generalization of prism adaptation. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:1006–1022. doi: 10.1037/0096-1523.32.4.1006. [DOI] [PubMed] [Google Scholar]
- Redding GM, Rossetti Y, Wallace B. Application of prism adaptation: A tutorial in theory and method. Neuroscience and Biobehavioral Reviews. 2005;29:431–444. doi: 10.1016/j.neubiorev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Kinsbourne M, Moscovitch M. Hemispheric control of spatial attention. Brain and Cognition. 1990;12:240–266. doi: 10.1016/0278-2626(90)90018-j. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Saver JL, Woolson RF, Clarke WR, Adams HP. Frequency, risk factors, anatomy, and course of unilateral neglect in an acute stroke cohort. Neurology. 2004;63:468–474. doi: 10.1212/01.wnl.0000133011.10689.ce. [DOI] [PubMed] [Google Scholar]
- Robinson DC, Kertzman C. Visuospatial attention: Effects of age, gender, and spatial reference. Neuropsychologia. 1990;28:241–301. doi: 10.1016/0028-3932(90)90022-g. [DOI] [PubMed] [Google Scholar]
- Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, Perenin M-T. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature. 1998;395:166–169. doi: 10.1038/25988. [DOI] [PubMed] [Google Scholar]
- Striemer C, Sablatnig J, Danckert J. Differential influences of prism adaptation on reflexive and voluntary covert attention. Journal of the International Neuropsychological Society. 2006;12:337–349. doi: 10.1017/s1355617706060553. [DOI] [PubMed] [Google Scholar]
- Varnava A, Halligan PW. Influence of age and sex on line bisection: A study of normal performance with implications for visuospatial neglect. Aging Neuropsychology and Cognition. 2007;14(6):571–585. doi: 10.1080/13825580600826454. [DOI] [PubMed] [Google Scholar]