Abstract
Study Design
Utilizing a complete transection spinal cord injury (SCI) model at the fourth thoracic vertebral level in adult rats, we evaluated whether blocking noxious stimuli below the injury diminishes abnormal somatic and autonomic motor reflexes, manifested in muscular spasticity and hypertensive autonomic dysreflexia, respectively. Gabapentin (GBP) is well-tolerated and currently used to manage neuropathic pain in the SCI population; evidence suggests it acts to decrease presynaptic glutamate release. Since clinical evidence indicates that GBP may suppress muscular spasticity in the chronic SCI population, we hypothesized that preventing neurotransmission of noxious stimuli with GBP eliminates a critical physiological link to these distinct, debilitating SCI-induced secondary impairments.
Objectives
Behavioural assessments of tail muscle spasticity and mean arterial blood pressure responses to noxious somatic and/or visceral stimulation were used to test the effects of GBP on these abnormal reflexes.
Setting
Lexington, Kentucky
Methods
We employed femoral artery catheterization and radio-telemetric approaches to monitor blood pressure alterations in response to noxious colorectal distension (CRD) weeks after complete SCI.
Results
At 2-3 weeks post-SCI, acute GBP administration (50 mg/kg, i.p.) significantly attenuated both autonomic dysreflexia and tail spasticity induced by noxious stimuli compared to saline-treated cohorts.
Conclusion
These results demonstrate, for the first time, that a single pharmacological intervention, GBP, can effectively attenuate the manifestation of both muscular spasticity and autonomic dysreflexia in response to noxious stimuli.
Keywords: blood pressure, hypertension, pain, spasms, hyper-reflexia, Neurontin, spinal reflex
INTRODUCTION
Approximately 250,000 Americans are living with the typically devastating neurological deficits and debilitating somatic and autonomic reflexes that develop in chronic spinal cord injury (SCI) (see http://www.spinalcord.uab.edu). A common secondary impairment following SCI is muscular spasticity in response to stretch and noxious cutaneous stimulation, which is characterized by increased muscle tone (hypertonus), increased somatic reflexes (hyper-reflexia), clonus, and painful spasms. In the complete SCI rodent model, it is has been demonstrated that spasticity results, in part, due to increased glutamatergic signaling to the motoneurons below the level of the injury (1). Such aberrant somatic reflexes, if severe enough, can impact the ability of the SCI individual to perform routine activities of daily living such as transfers, independent dressing, management of bowel and bladder, along with the grave potential of falling out of a wheelchair.
Another common secondary impairment is the development of autonomic dysfunction, which occurs after complete as well as incomplete SCI, notably above high-thoracic levels which comprise the majority of SCI individuals. In particular, noxious somatic and/or visceral stimuli below the injury level often trigger a potentially life-threatening hypertensive condition termed autonomic dysreflexia (2). This syndrome is characterized by episodic, uninhibited sympathetically-driven hypertension which is usually accompanied by intact baroreflex-mediated bradycardia. Episodes of autonomic dysreflexia often cause debilitating symptoms including pounding headache, acute anxiety, shivering, flushing and profuse sweating (3).
Collectively, there appears to be a common pathway by which noxious stimuli are capable of inducing both spasticity and autonomic dysreflexia. Thus a pharmacological agent that can block the transmission of noxious stimuli could potentially decrease the manifestation of spasticity and autonomic dysreflexia. One such compound that is safe for clinical use is gabapentin (Neurontin®; Pfizer, New York, NY, USA), which is approved for the treatment of epilepsy and is widely used off-label for the treatment of neuropathic pain (4). Although gabapentin (GBP) possesses multiple cellular mechanisms, recent work has suggested that inhibition of glutamatergic transmission may be preeminent in mediating its therapeutic effects in epilepsy, neuropathic pain, and perhaps muscle spasticity (5). Specifically, GBP has been shown to inhibit presynaptic glutamate release (6, 7). When incorporated as an adjunct to standard pharmacological interventions, GBP demonstrates the potential to help decrease the manifestation of spasticity in SCI-individuals (8, 9). Importantly, we have demonstrated that blocking glutamate release with GBP effectively reduces the manifestation of spasticity in tail muscles in response to a noxious pinch (4).
While numerous individuals with SCI are using GBP for management of chronic neuropathic pain, to date there has been no evaluation of its potential effects on the incidence or severity of autonomic dysreflexia, either experimentally or clinically. Since there is no single clinical intervention which effectively attenuates the manifestation of both spasticity and autonomic dysreflexia, we chose to characterize GBP in managing the severity of both these secondary impairments since it is well-tolerated and acts to suppress neurotransmission of noxious stimuli. We theorized that blocking such neurotransmission into the injured spinal cord would eliminate a critical physiological link to the often debilitating somatic and autonomic motor reflexes. This study employed both physiological monitoring of the severity of dysreflexic hypertension and behavioral analysis of muscle spasticity in response to noxious visceral and/or somatic stimuli, respectively. Such a multidiscipline quantitative approach to evaluate debilitating somatic and autonomic motor reflexes with GBP is essential for clinical translation and implementation.
MATERIALS AND METHODS
Surgical methods and spinal cord injury
All surgical procedures were performed under aseptic conditions using sterilized instruments, following the University of Kentucky IACUC and NIH guidelines. Adult female Wistar rats (~225 g) were anaesthetized with a mixture of ketamine (80mg/kg, i.p.) and xylazine (10mg/kg, i.p.). All animals underwent a complete single level laminectomy of the T3 vertebra to expose the T4 spinal cord (n=15 for femoral artery catheterization; n=6 for telemetric monitoring). The exposed T4 spinal cord segment was then completely transected with a scalpel blade and hemostasis was achieved with gelfoam placed into the resection site, as previously detailed (10). Wounds were then irrigated with sterile saline, the muscles sutured using 3-0 vicryl and skin openings stapled with wound clips. Injured rats were treated with 33.3 mg/kg Cefazolin antibiotic (s.c., SoloPak Laboratories, New Gove, IL) and injected with 20 ml Lactated Ringer’s solution s.c. for fluid replacement before being returned to their cages, with food and water ad libitum, and placed on a heating pad during recovery. Animals were housed one per cage and required manual bladder evacuation twice a day.
Drug administration
Two and three-week injured rats were injected i.p. with either saline vehicle (n=6, femoral catheter; n=3, aortic catheter) or 50 mg/kg GBP (n=9, femoral catheter; n=3, aortic catheter) at 1 hr prior to cardiophysiological measurements in response to CRD, as well as behavioral assessments in response to tail manipulations (see below).
Blood pressure recordings via femoral artery during colorectal distension (CRD)
Two weeks after T4-transection, one group of injured rats (n=15) was re-anesthetized and a catheter was placed in the femoral artery for arterial pressure monitoring, as detailed previously (10). The following day, pulsatile arterial pressure (PAP), mean arterial pressure (MAP), and heart rate (HR) were monitored prior to and during CRD in gently restrained, un-anesthetized rats within individual cages using a pressure transducer connected to an amplifier (Grass model 7P1; Quincy, MA) and stored using a Powerlab system that digitized information from the amplifier and stored it on a computer hard drive. The PAP was measured before (30 sec baseline), during, and after (30 sec) a 1 min period of balloon catheter inflation with 2 ml of air to distend the distal colon designed to mimic noxious fecal impaction. Two trials were conducted per animal per day, with an inter-trial interval of approximately 30 min. A period of 15 minutes was allowed to pass to allow the gently restrained rats to become quiet after connecting the catheters to the measurement devices and gently inserting a latex balloon-tipped catheter (Swan-Ganz Paceport catheter; Baxter Healthcare Corporation, CA) 2 cm inside the rectum and securing it to the tail with tape. To initiate spinal viscero-sympathetic reflexes, the distal colon was distended by gradual (15 s) inflation of the 10 mm long balloon with 2.0 ml of air for 1 min. Such CRD expands the colon as would several large fecal boluses.
Implantation of blood pressure telemetry devices in descending aorta
Seven days prior to T4 transection, naïve anesthetized rats (n=6) were implanted with telemetric pressure transmitters (model TA11PA-C40, Data Sciences International, Inc., St. Paul, MN) into the descending aorta and secured to the abdominal wall with silk sutures. The skin was closed with surgical staples after rinsing abdominal cavity with sterile saline. The animals were then treated, as above, and monitored for blood pressure daily to ensure patency of the probes.
Telemetric monitoring of blood pressure via aorta during colorectal distension (CRD)
Three weeks following T4 transection, the Dataquest A.R.T. system (Data Sciences International, Inc., St. Paul, MN) was used for on-demand telemetric monitoring of arterial blood pressure prior to, during, and after CRD, identical to procedures with femoral artery catheterizations. During a recording period, gently restrained animals within individual cages were placed upon receiver plates and PAP readings were transmitted to a receiver as a radio frequency signal integrated by a data exchange matrix. Traces for each animal were saved and stored in a selected file for all subsequent analyses.
Behavioral assessment for spasticity in the tail muscles
In between CRD trials, spasticity in the tail was assessed behaviorally using an established scale (1, 4). Specifically, the response of the tail muscles to a quick stretch, light stroking (non-noxious stimulus) and a light pinch (noxious stimulus) applied approximately 10 cm from the tip of the tail were assessed. Tail manipulations were performed with the animals lightly restrained and the tail was free to move over its full length. In this study, the animals displayed either a stage-2, stage-3 or stage-4 spasticity prior to initiating pharmacological intervention. For the purpose of statistical analysis, responses to quick stretch and pinch were graded using a 5 point scale in which 0 = minimal (≤ 45° flexion) response to the stimulus, 1 = 45-90° flexion, 2 = >90°-180° flexion, 3= >180°-225° flexion, 4 = >225°- 360° flexion, and 5 = significant coiling of the tail and/or activation of flexors, extensors, and abductors (writhing) lasting > 2 seconds and the presence of clonus. The response to light touch was scored using a 3 point grading scale in which 0 = no response, 1 = minimal flexion of the tail away from the stimulus, and 2 = pronounced flexing of the tail away from the stimulus.
Spinal cord tissue processing and histology
After final analyses, all injured rats were overdosed with sodium pentobarbital and transcardially perfused with 4% paraformaldehyde in PBS. Dissected spinal cords from T4-transected rats were stored for long term storage at 4°C in 20% sucrose/PBS containing 0.02% sodium azide.
Statistical analysis
All analyses were performed by individuals blinded with respect to treatment. For comparisons of CRD-induced MAP and HR changes, unpaired Student’s t-tests, with bonferroni correction factor when appropriate, were used between saline- and GBP-treated groups. Each behavioral test (tail responses to stretch, noxious pinch, and non-noxious light touch, as well as presence of clonus) was compared using the Mann-Whitney U test for ordinal data. Statistical significance was set a prior at p<0.05 for all analyses.
We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research.
RESULTS
Efficacy of GBP to reduce severity of hypertension in response to CRD (femoral artery)
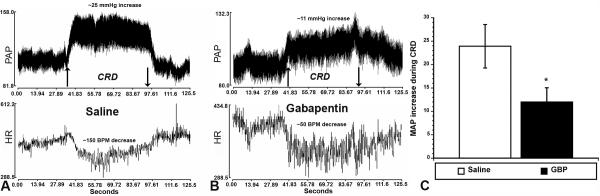
As illustrated in Figure 1A and B, after 14 days the injured rats injected with GBP versus saline one hour prior showed a marked decrease in CRD-evoked hypertension. Note that the rapid and sustained MAP increase and bradycardia during CRD with saline treatment were both attenuated with GBP treatment. Quantitative analysis (Figure 1C) established that GBP treatment significantly (p<0.05) reduced CRD-induced hypertension by more than two-fold compared to saline treatment. Notably, the MAP baselines (30 sec prior to CRD) were not significantly different (Saline = 99.4 mmHg ± 4.3; GBP = 96.6 mmHg ± 2.3), indicating GBP did not alter resting MAP within an hour post administration.
Figure 1.
Illustrative traces of pulsatile arterial pressure (PAP) and heart rate (HR), measured by femoral artery cannulation before, during and after one minute of painful colorectal distension (CRD) in (A) injured rats injected with saline versus (B) injured rats injected with gabapentin (GBP) 14 days post injury. Up and down arrows indicate rectal balloon catheter inflation and deflation, respectively. In GBP-treated rats, the increased MAP and decreased HR (bradycardia) are attenuated in response to CRD. Quantitative analysis (C) confirmed a significant reduction in CRD-induced MAP of more than two-fold with GBP treatment. *p<0.05 n=6 Saline; n=9 GBP (50 mg/kg) Bars represent mean ± SEM
Efficacy of GBP to reduce severity of muscle spasticity in response to tail stretch/pinch
At 14 days post-injury, in the same injured rats shown in Figure 1, GBP treatment produced significant (p<0.05) decreases in behavioral reflex responses of the tail musculature to both stretch (non-noxious) and pinch (noxious) stimuli, compared to saline-treated injured rats (Figure 2A and B). These results are similar to those reported for spastic animals that had received GBP administration following spinal transection at the S2 level (4).
Figure 2.
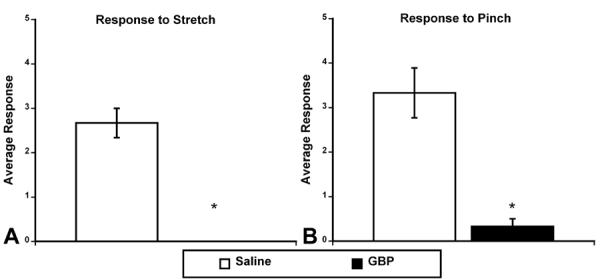
Behavioral responses of the tail musculature to different stimuli 2 weeks following T4 transection (same rats as in Figure 1). A. Average response to stretch 1 hour following the administration of saline (n=6) or gabapentin (GBP; 50 mg/kg; n=9). B. Average response to noxious (pinch) stimulus 1 hour following the administration of saline or GBP. Responses to stretch and pinch were scored on a 5 point scale in which 0 = no spasticity and 5 = severe spasticity. Notice that following administration of GBP there was no response to stretch (A) and a striking decrease in response to pinch stimulus (B). * p<0.05 Bars represent mean ± SEM for visualization purposes only.
Efficacy of GBP to reduce severity of hypertension in response to CRD (telemetric monitoring)
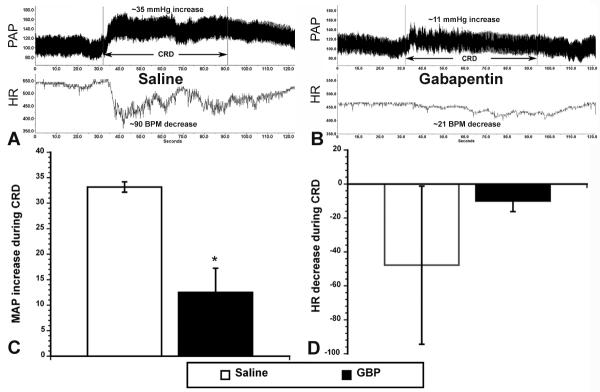
As illustrated in Figure 3A and B, at 3 weeks post-injury, the rats injected with GBP versus saline one hour prior showed a marked decrease in CRD-evoked hypertension. Note that the fidelity of the traces was markedly improved with the aortic probe telemetric system (compare to Fig. 1A and B) and that there was highly reduced variability in PAP and HR traces in the GBP-treated rats. This is likely due to reduced hind leg muscle spasms that occur during CRD (see below). Quantitative analysis (Figure 3C) established that GBP treatment significantly (p<0.05) reduced CRD-induced hypertension by almost three-fold compared to saline treatment. Although there was a trend for reduced bradycardia with GBP treatment versus saline (Figure 3D), variability in HR within the latter group obviated significant differences. Notably, the MAP baselines (30 sec prior to CRD) were not significantly different (Saline = 93.0 mmHg ± 5.9; GBP = 91.2 mmHg ± 1.1), again indicating GBP did not alter resting MAP within an hour post administration. Interestingly, the more refined telemetric monitoring resulted in lower basal MAP than femoral catheterization, though not significantly (Femoral = 97.7 mmHg ± 2.1; Telemetry = 92.1 mmHg ± 2.7).
Figure 3.
Illustrative traces of pulsatile arterial pressure (PAP) and heart rate (HR), measured by telemetric probes in descending aorta before, during and after one minute of noxious colorectal distension (CRD) in (A) injured rats injected with saline versus (B) injured rats injected with gabapentin (GBP) 21 days post injury. With GBP treatment, the increased MAP and decreased HR (bradycardia) appear strikingly attenuated in response to CRD. Quantitative analysis (C) confirmed a significant reduction in CRD-induced MAP of more than two-fold with GBP treatment. While bradycardia appeared to be reduced (D), the variability precluded significant differences. *p<0.05 n=3 Saline; n=3 GBP (50 mg/kg) Bars represent mean ± SEM
Efficacy of GBP to reduce severity of muscle spasticity in response to tail touch/stretch/pinch
At 3 weeks post-injury, in the same injured rats shown in Figure 3, GBP treatment produced significant (p<0.05) decreases in behavioral reflex responses of the tail musculature to light touch, stretch and pinch, compared to saline-treated injured rats (Figure 4A-C). As with the animals that had been assessed using the femoral artery recordings (see Fig. 2), GBP-treated injured rats demonstrated significantly reduced responsiveness of the tail musculature to both non-noxious and noxious stimuli by at least 80%.
Figure 4.
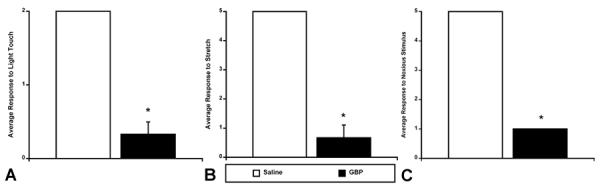
The average behavioral response of the tail musculature 3 weeks following T4 transection (same rats as in Figure 3). Panel A represents the average response to light touch 1 hour following the administration of saline (n=3) or GBP (50 mg/kg; n=3). Panel B represents the average response to tail stretch 1 hour following the administration of saline or GBP. Panel C represents the average response to noxious stimulus (tail pinch) 1 hour following the administration of saline or GBP. Response to light touch was scored on a 2 point scale in which 0 = no spasticity and 2 = severe spasticity. Responses to stretch and pinch were scored on a 5 point scale in which 0 = no spasticity and 5 = severe spasticity. Notice that following administration of GBP there was a significant decrease in response to light touch and stretch (A, B), and a striking decrease in response to noxious tail pinch (C). *p<0.05 Bars represent mean ± SEM for visualization purposes only.
DISCUSSION
There is currently no single clinical intervention which is known to effectively attenuate the manifestation of both muscular spasticity and autonomic dysreflexia, which are secondary complications of SCI that significantly impact the quality of daily living for affected individuals. Therefore, this study was designed to test the hypothesis that pharmacological suppression of the neurotransmission of noxious stimuli (somatic and/or visceral), following peripheral administration of a drug used for neuropathic pain, GBP, would alleviate both muscular spasticity and autonomic dysreflexia in response to noxious stimuli below the injury level in chronic stages of complete SCI. Here we report, for the first time, that there is striking efficacy for GBP to reduce both aberrant spinal motor reflexes when given to spinal rats within one hour prior to assessing tail spasticity and dysreflexic hypertension in response to noxious stimuli. Such efficacy was demonstrated in two separate SCI cohorts using either femoral artery catheterization or telemetric monitoring of blood pressure for measurements during noxious CRD; accompanied by tail spasticity assessments.
Clinical evidence indicates that GBP is well tolerated and may suppress muscular spasticity in the chronic SCI population (8).
Several research groups have focused on GBP as a potential inhibitor of spasticity in a number of upper motoneuron disorders. Its short term efficacy seems to be established (8, 9, 11). However, a closer look reveals that even for well-established drugs, such as Baclofen or Diazepam, the clinical evidence derived from good studies is infrequent, at best (12). Accordingly, we have confirmed such a beneficial effect of GBP experimentally employing a model to assess tail muscle spasticity in chronic SCI animals (4). Remarkably, for both quadriplegics and paraplegics surveyed, it was reported that alleviating autonomic dysfunction (bowel, bladder, autonomic dysreflexia) was of higher priority than even restoration of walking (13). The development of a rat model of noxious CRD-induced autonomic dysreflexia to mimic clinical manifestations of fecal impaction (14) has enabled the detailed analysis of temporal dynamics of CRD-induced hypertension (15). Notably, and germane to the present study, glutamatergic neurotransmission has been shown to contribute to spinal viscerosympathetic initiation of episodic hypertension (16).
For clinical purposes it will be very interesting to gain knowledge about the magnitude of the effect measured for GBP in relation to other drugs routinely used for the treatment of spasticity (e.g. Baclofen or Diazepam). Furthermore, it will be of great importance for future studies to examine whether the effects of GBP are subject to the same attenuation seen with other drugs after longer treatment. SCI-induced muscular spasticity below the injury level is characterized by intermittent or sustained involuntary activation of muscles in response to either a stretch or noxious stimulus which can dramatically reduce the person’s ability to participate in the activities of daily living that contribute to their well-being (17, 18). Concomitantly, aberrant autonomic reflexes also develop after SCI above high-thoracic levels that can lead to a potentially life-threatening hypertensive condition termed autonomic dysreflexia. One of the most common triggers of hypertensive autonomic dysreflexia is the distension of pelvic viscera (bladder and bowel) (3). Therefore, in addition to debilitating muscle spasms, an individual with SCI also suffers repeated unpleasant bouts of hypertension throughout the day and evening; notably for those employing an intermittent self-catheterization routine for emptying their bladder or during episodes of bowel/colorectal obstruction (constipation) and/or evacuation.
It is important to note that we employed a complete T4 transection model rather than what many deem as the more clinically relevant contusion SCI model. However, the problem lies in the reproducibility and fidelity of the data following incomplete SCI. Notably, seminal data published by (19) demonstrated that even marginal sub-pial tissue sparing after severe clip compression SCI altered the severity of CRD-induced AD compared to complete transection. Similarly, we have reported (20) that even following severe 350 kdyn T4 contusions with 20 second dwell times we could not demonstrate reproducible manifestation of AD (i.e., hypertention accompanied bradycardia during noxious CRD). As suggested by Mayorov et al. (2001), such discrepancies may be related to the preservation of certain descending vasomotor pathways maintaining excitatory supraspinal input to the sympathetic preganglionic neurons, resulting in persistent sympathetically-mediated cardiovascular reflexes.
In summary, we designed a preclinical study to develop a treatment for chronic SCI individuals who continually suffer from secondary complications, notably abnormal muscle spasms and autonomic spinal reflexes. There is no pharmaceutical intervention which is known to effectively attenuate neuropathic pain as well as the manifestation of both muscular spasticity and autonomic dysreflexia after chronic SCI. Consequently, this quantitative experimental study was requisite to establish proof-of-principle prior to direct clinical application employing a two-pronged approach designed to alleviate dissimilar aberrant neurologic reflexes with a single drug, GBP. Studies are underway to establish IND-enabling preclinical data supporting the novel indication of GBP for the effective treatment and maintenance of both spasticity and autonomic dysreflexia.
ACKNOWLEDGMENTS
Supported by NIH/NINDS R01 NS049901 and Kentucky Spinal Cord and Head Injury Research Trust (KSCHIRT) #3-11 (AGR), KSCHIRT #4-8 (PHK) and NIH/NINDS P30 NS051220.
Sponsorship: NIH/NINDS R01 NS049901 and (KSCHIRT) #3-11 (AGR), KSCHIRT #4-8 (PHK) and NIH/NINDS P30 NS051220.
Footnotes
CONFLICT OF INTEREST STATEMENT The authors declare no competing financial interests in relation to the work described in this report.
References
- 1.Kitzman P. Changes in vesicular glutamate transporter 2, vesicular GABA transporter and vesicular acetylcholine transporter labeling of sacrocaudal motoneurons in the spastic rat. Exp Neurol. 2006;197(2):407–19. doi: 10.1016/j.expneurol.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Karlsson AK. Autonomic dysreflexia. Spinal Cord. 1999;37(6):383–91. doi: 10.1038/sj.sc.3100867. [DOI] [PubMed] [Google Scholar]
- 3.Krassioukov AV, Furlan JC, Fehlings MG. Autonomic dysreflexia in acute spinal cord injury: an under-recognized clinical entity. J Neurotrauma. 2003;20(8):707–16. doi: 10.1089/089771503767869944. [DOI] [PubMed] [Google Scholar]
- 4.Kitzman PH, Uhl TL, Dwyer MK. Gabapentin suppresses spasticity in the spinal cord-injured rat. Neuroscience. 2007;149(4):813–21. doi: 10.1016/j.neuroscience.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler G. Gabapentin. Pfizer. Curr Opin Investig Drugs. 2002;3(3):470–7. [PubMed] [Google Scholar]
- 6.Coderre TJ, Kumar N, Lefebvre CD, Yu JS. Evidence that gabapentin reduces neuropathic pain by inhibiting the spinal release of glutamate. J Neurochem. 2005;94(4):1131–9. doi: 10.1111/j.1471-4159.2005.03263.x. [DOI] [PubMed] [Google Scholar]
- 7.Shimoyama M, Shimoyama N, Hori Y. Gabapentin affects glutamatergic excitatory neurotransmission in the rat dorsal horn. Pain. 2000;85(3):405–14. doi: 10.1016/S0304-3959(99)00283-3. [DOI] [PubMed] [Google Scholar]
- 8.Gruenthal M, Mueller M, Olson WL, Priebe MM, Sherwood AM, Olson WH. Gabapentin for the treatment of spasticity in patients with spinal cord injury. Spinal Cord. 1997;35(10):686–9. doi: 10.1038/sj.sc.3100481. [DOI] [PubMed] [Google Scholar]
- 9.Priebe MM, Sherwood AM, Graves DE, Mueller M, Olson WH. Effectiveness of gabapentin in controlling spasticity: a quantitative study. Spinal Cord. 1997;35(3):171–5. doi: 10.1038/sj.sc.3100366. [DOI] [PubMed] [Google Scholar]
- 10.Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J Neurosci. 2006;26(11):2923–32. doi: 10.1523/JNEUROSCI.4390-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevenson VL. Rehabilitation in practice: Spasticity management. Clin Rehabil. 24(4):293–304. doi: 10.1177/0269215509353254. [DOI] [PubMed] [Google Scholar]
- 12.Montane E, Vallano A, Laporte JR. Oral antispastic drugs in nonprogressive neurologic diseases: a systematic review. Neurology. 2004;63(8):1357–63. doi: 10.1212/01.wnl.0000141863.52691.44. [DOI] [PubMed] [Google Scholar]
- 13.Anderson KD. Targeting recovery: priorities of the spinal cord injured population. J Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 14.Krassioukov AV, Weaver LC. Episodic hypertension due to autonomic dysreflexia in acute and chronic spinal cord-injured rats. Am J Physiol. 1995;268(5 Pt 2):H2077–83. doi: 10.1152/ajpheart.1995.268.5.H2077. [DOI] [PubMed] [Google Scholar]
- 15.Maiorov DN, Weaver LC, Krassioukov AV. Relationship between sympathetic activity and arterial pressure in conscious spinal rats. Am J Physiol. 1997;272(2 Pt 2):H625–31. doi: 10.1152/ajpheart.1997.272.2.H625. [DOI] [PubMed] [Google Scholar]
- 16.Maiorov DN, Krenz NR, Krassioukov AV, Weaver LC. Role of spinal NMDA and AMPA receptors in episodic hypertension in conscious spinal rats. Am J Physiol. 1997;273(3 Pt 2):H1266–74. doi: 10.1152/ajpheart.1997.273.3.H1266. [DOI] [PubMed] [Google Scholar]
- 17.Mayer NH. Clinicophysiologic concepts of spasticity and motor dysfunction in adults with an upper motoneuron lesion. Muscle Nerve Suppl. 1997;6:S1–13. [PubMed] [Google Scholar]
- 18.Walter JS, Sacks J, Othman R, Rankin AZ, Nemchausky B, Chintam R, et al. A database of self-reported secondary medical problems among VA spinal cord injury patients: its role in clinical care and management. J Rehabil Res Dev. 2002;39(1):53–61. [PubMed] [Google Scholar]
- 19.Mayorov DN, Adams MA, Krassioukov AV. Telemetric blood pressure monitoring in conscious rats before and after compression injury of spinal cord. J Neurotrauma. 2001;18(7):727–36. doi: 10.1089/089771501750357663. [DOI] [PubMed] [Google Scholar]
- 20.Rabchevsky AG, Duale H, Lyttle TS, O’Dell CR, Kitzman PH. Gabapentin for spasticity and autonomic dysreflexia after severe spinal cord injury. J Neurotrauma. 2009;26(8):A-65. doi: 10.1038/sc.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]