Abstract
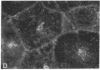
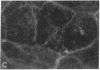
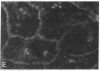
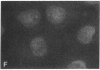
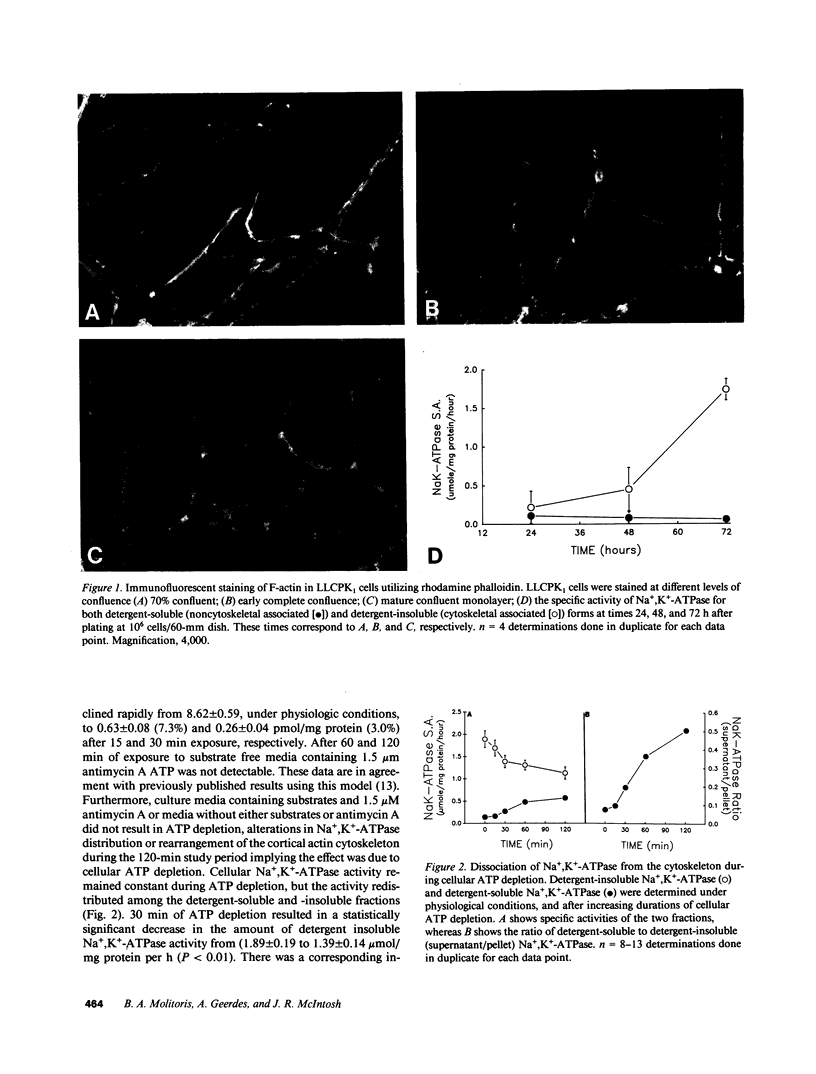
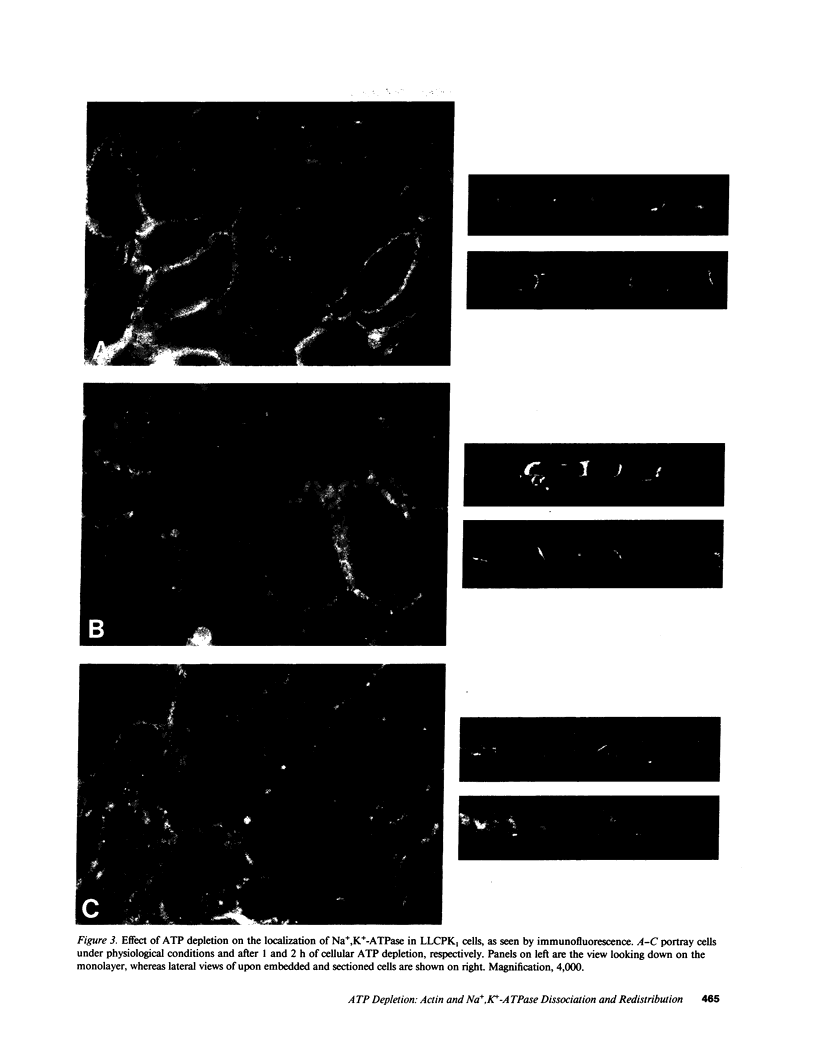
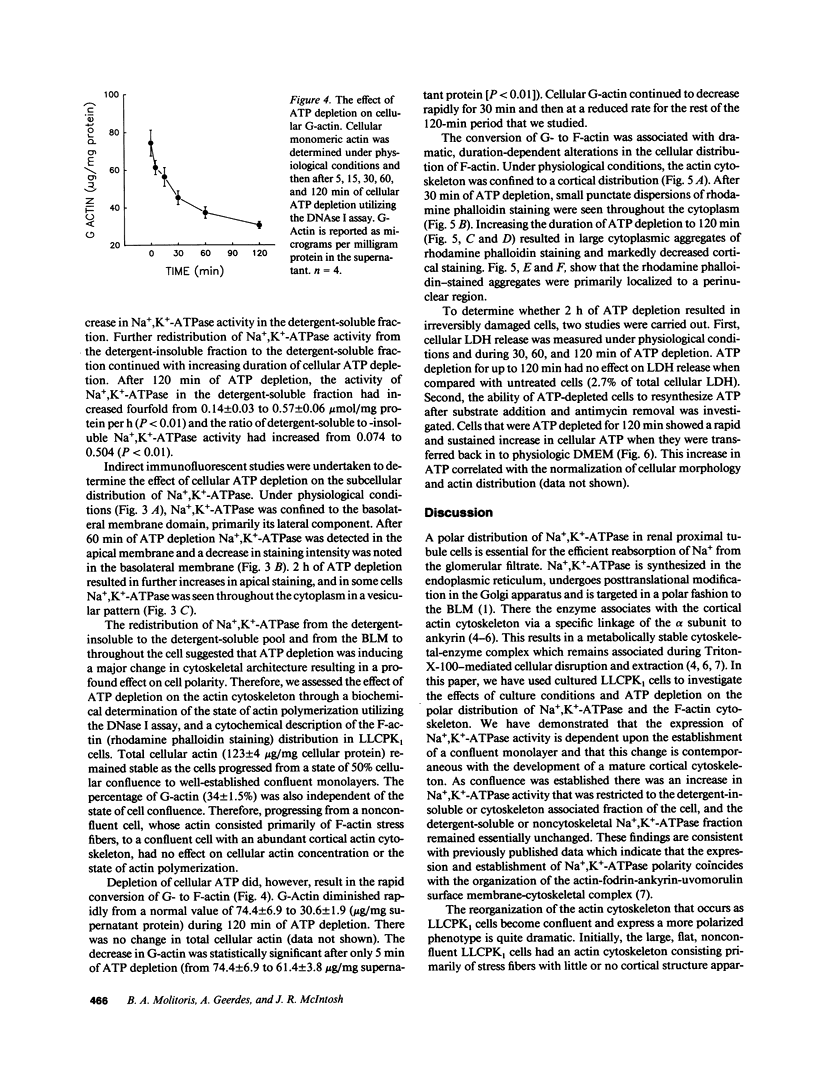
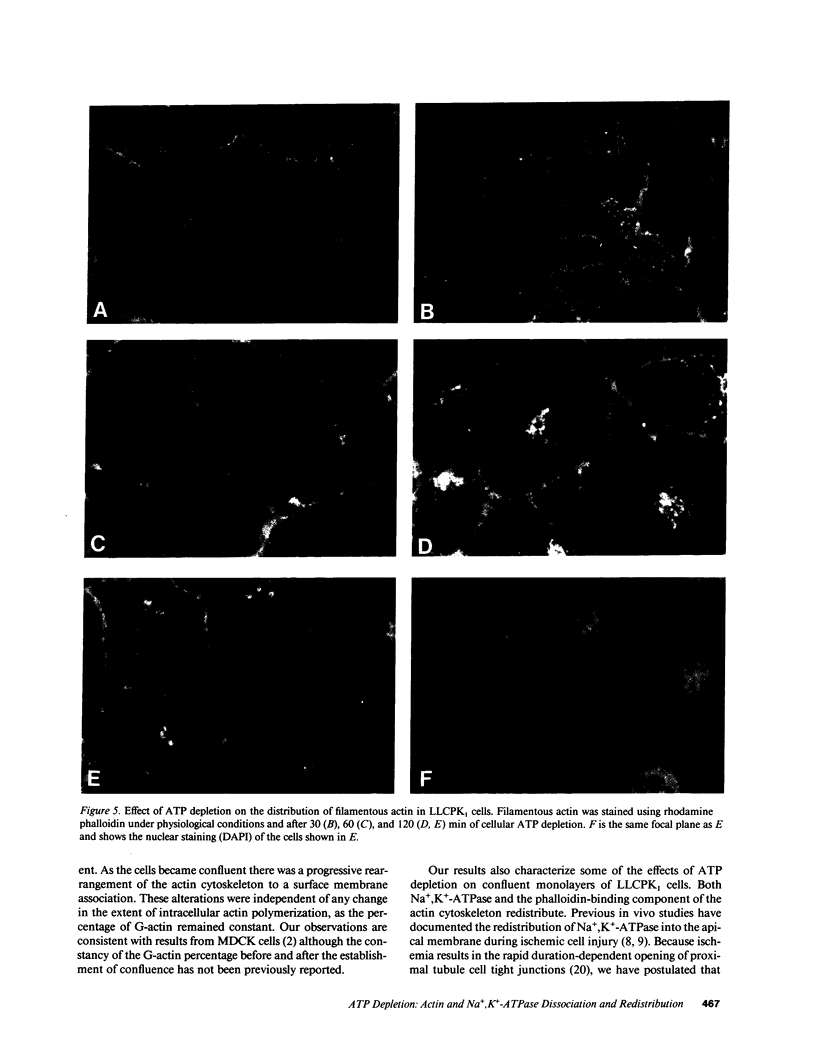
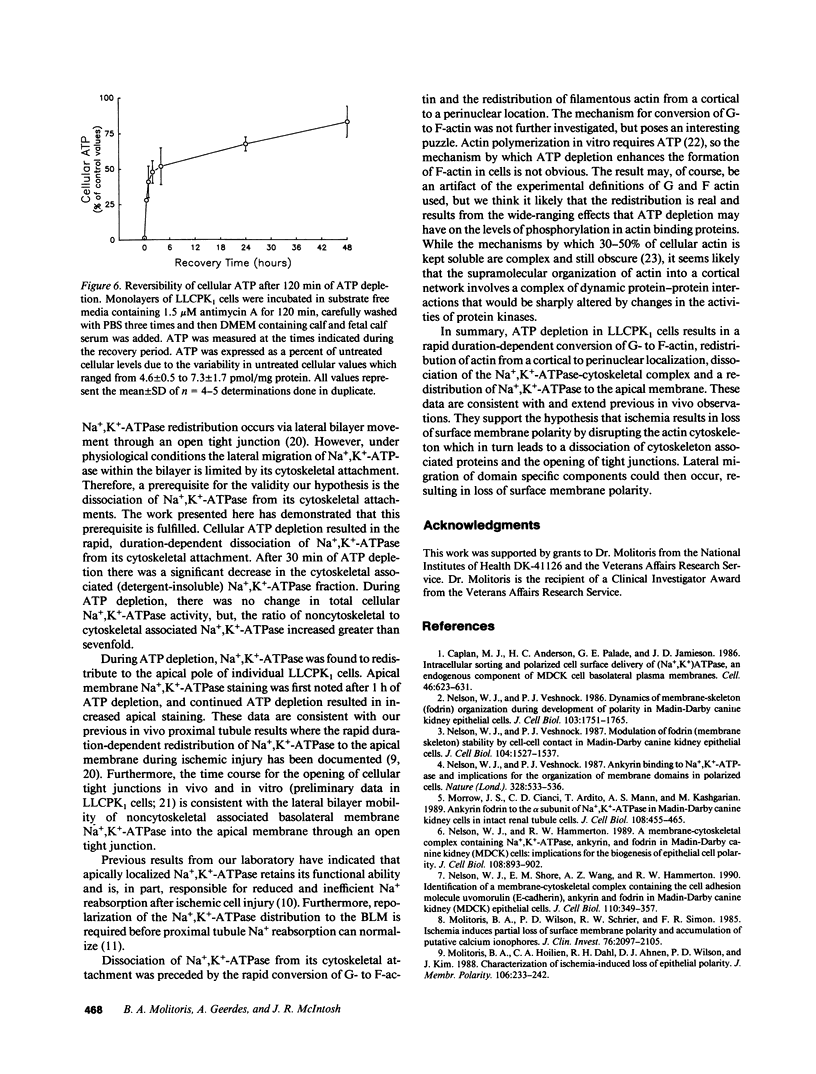
Establishment and maintenance of a polar distribution of Na+,K(+)-ATPase is essential for efficient Na+ reabsorption by proximal tubule cells and is dependent upon the formation of a metabolically stable, detergent-insoluble complex of Na+,K(+)-ATPase with the actin membrane cytoskeleton. The present studies show that cellular ATP depletion results in a rapid duration-dependent dissociation of Na+,K(+)-ATPase from the actin cytoskeleton and redistribution of Na+,K(+)-ATPase to the apical membrane. During ATP depletion, total cellular Na+,K(+)-ATPase activity was unaltered, but the Triton-X-100-insoluble fraction (cytoskeleton associated) of Na+,K(+)-ATPase activity decreased (P less than 0.01), with a corresponding increase in the detergent-soluble fraction of Na+,K(+)-ATPase (P less than 0.01). Indirect immunofluorescent studies of cells with depleted ATP revealed a redistribution of Na+,K(+)-ATPase from the basolateral membrane into the apical membrane and throughout the cytoplasm. ATP depletion also resulted in the redistribution of F-actin from a primarily cortical concentration to a perinuclear location. There was also a rapid, duration-dependent conversion of monomeric G-actin to F-actin starting during the first 5 min of ATP depletion. Taken together, these data suggest that ATP depletion causes profound alterations in cell polarity by inducing major changes in the actin cytoskeletal architecture.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Caplan M. J., Anderson H. C., Palade G. E., Jamieson J. D. Intracellular sorting and polarized cell surface delivery of (Na+,K+)ATPase, an endogenous component of MDCK cell basolateral plasma membranes. Cell. 1986 Aug 15;46(4):623–631. doi: 10.1016/0092-8674(86)90888-3. [DOI] [PubMed] [Google Scholar]
- Kellerman P. S., Clark R. A., Hoilien C. A., Linas S. L., Molitoris B. A. Role of microfilaments in maintenance of proximal tubule structural and functional integrity. Am J Physiol. 1990 Aug;259(2 Pt 2):F279–F285. doi: 10.1152/ajprenal.1990.259.2.F279. [DOI] [PubMed] [Google Scholar]
- Korn E. D., Carlier M. F., Pantaloni D. Actin polymerization and ATP hydrolysis. Science. 1987 Oct 30;238(4827):638–644. doi: 10.1126/science.3672117. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laub F., Kaplan M., Gitler C. Actin polymerization accompanies Thy-1-capping on mouse thymocytes. FEBS Lett. 1981 Feb 9;124(1):35–38. doi: 10.1016/0014-5793(81)80048-8. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Chan L. K., Shapiro J. I., Conger J. D., Falk S. A. Loss of epithelial polarity: a novel hypothesis for reduced proximal tubule Na+ transport following ischemic injury. J Membr Biol. 1989 Feb;107(2):119–127. doi: 10.1007/BF01871717. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Falk S. A., Dahl R. H. Ischemia-induced loss of epithelial polarity. Role of the tight junction. J Clin Invest. 1989 Oct;84(4):1334–1339. doi: 10.1172/JCI114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris B. A., Hoilien C. A., Dahl R., Ahnen D. J., Wilson P. D., Kim J. Characterization of ischemia-induced loss of epithelial polarity. J Membr Biol. 1988 Dec;106(3):233–242. doi: 10.1007/BF01872161. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Simon F. R. Renal cortical brush-border and basolateral membranes: cholesterol and phospholipid composition and relative turnover. J Membr Biol. 1985;83(3):207–215. doi: 10.1007/BF01868695. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Wilson P. D., Schrier R. W., Simon F. R. Ischemia induces partial loss of surface membrane polarity and accumulation of putative calcium ionophores. J Clin Invest. 1985 Dec;76(6):2097–2105. doi: 10.1172/JCI112214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. S., Cianci C. D., Ardito T., Mann A. S., Kashgarian M. Ankyrin links fodrin to the alpha subunit of Na,K-ATPase in Madin-Darby canine kidney cells and in intact renal tubule cells. J Cell Biol. 1989 Feb;108(2):455–465. doi: 10.1083/jcb.108.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Hammerton R. W. A membrane-cytoskeletal complex containing Na+,K+-ATPase, ankyrin, and fodrin in Madin-Darby canine kidney (MDCK) cells: implications for the biogenesis of epithelial cell polarity. J Cell Biol. 1989 Mar;108(3):893–902. doi: 10.1083/jcb.108.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Shore E. M., Wang A. Z., Hammerton R. W. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1990 Feb;110(2):349–357. doi: 10.1083/jcb.110.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987 Aug 6;328(6130):533–536. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Dynamics of membrane-skeleton (fodrin) organization during development of polarity in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1986 Nov;103(5):1751–1765. doi: 10.1083/jcb.103.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Modulation of fodrin (membrane skeleton) stability by cell-cell contact in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1987 Jun;104(6):1527–1537. doi: 10.1083/jcb.104.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolski J. L., Steck T. L. Length distribution of F-actin in Dictyostelium discoideum. J Biol Chem. 1990 Jan 25;265(3):1312–1318. [PubMed] [Google Scholar]
- Rieder C. L., Bowser S. S. Correlative immunofluorescence and electron microscopy on the same section of epon-embedded material. J Histochem Cytochem. 1985 Feb;33(2):165–171. doi: 10.1177/33.2.3881520. [DOI] [PubMed] [Google Scholar]
- Sanders M. C., Wang Y. L. Exogenous nucleation sites fail to induce detectable polymerization of actin in living cells. J Cell Biol. 1990 Feb;110(2):359–365. doi: 10.1083/jcb.110.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel D. M., Wilson P. D., Molitoris B. A. Epithelial polarity following ischemia: a requirement for normal cell function. Am J Physiol. 1989 Mar;256(3 Pt 2):F430–F436. doi: 10.1152/ajprenal.1989.256.3.F430. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Patel Y. J., Kreisberg J. I., Weinberg J. M. Energy thresholds that determine membrane integrity and injury in a renal epithelial cell line (LLC-PK1). Relationships to phospholipid degradation and unesterified fatty acid accumulation. J Clin Invest. 1988 Mar;81(3):745–758. doi: 10.1172/JCI113380. [DOI] [PMC free article] [PubMed] [Google Scholar]