Abstract
Purpose
Absence seizures cause transient impairment of consciousness. Typical absence seizures occur in children, and are accompanied by 3–4 Hz spike-wave discharges (SWD) on EEG. Prior EEG-fMRI studies of SWD have shown a network of cortical and subcortical changes during these electrical events. However, fMRI during typical childhood absence seizures with confirmed impaired consciousness has not been previously investigated.
Methods
We performed EEG-fMRI with simultaneous behavioral testing in 37 children with typical childhood absence epilepsy. Attentional vigilance was evaluated by a continuous performance task (CPT), and simpler motor performance was evaluated by a repetitive tapping task (RTT).
Results
SWD episodes were obtained during fMRI scanning from 9 patients among the 37 studied. fMRI signal increases during SWD were observed in the thalamus, frontal cortex, primary visual, auditory, somatosensory, and motor cortex, and fMRI decreases were seen in the lateral and medial parietal cortex, cingulate gyrus, and basal ganglia. Omission error rate (missed targets) with SWD during fMRI was 81% on CPT and 39% on RTT. For those seizure epochs during which CPT performance was impaired, fMRI changes were seen in cortical and subcortical structures typically involved in SWD, while minimal changes were observed for the few epochs during which performance was spared.
Discussion
These findings suggest that typical absence seizures involve a network of cortical-subcortical areas necessary for normal attention and primary information processing. Identification of this network may improve understanding of cognitive impairments in childhood absence epilepsy, and help guide development of new therapies for this disorder.
Keywords: epilepsy, attention, consciousness, thalamus, BOLD, spike-wave
Introduction
Absence seizures cause a temporary and involuntary arrest of consciousness. Patients, most often children, stare unresponsively for a few seconds, and then resume prior activities. The electrical events, behavioral changes, and more recently neuroimaging in absence seizures have been investigated thoroughly. Interestingly, however, not all electrical discharges resembling those of absence seizures cause complete behavioral unconsciousness. There is substantial variability in the degree of impairment depending on the task used, and even from one episode to the next within the same patient (reviewed in (Blumenfeld, 2005a)). Recent functional neuroimaging studies have revealed a network of cortical and subcortical regions involved during electrical absence-like episodes. However, the functional neuroimaging changes during typical absence seizures with behavioral impairment have not been previously determined.
Electroencephalogram (EEG) recordings in patients with CAE reveal large amplitude, bilateral 3–4 Hz spike-wave discharges (SWD). “Generalized” SWD can arise from the frontal regions (Tukel and Jasper, 1952) and in CAE show a clear frontal amplitude maximum, greatest in the midline (Holmes et al., 2004). Selective regional involvement is also found in animal models of absence seizures (Meeren et al., 2002; Nersesyan et al., 2004b; Nersesyan et al., 2004a), suggesting that these so called “generalized” seizures involve focal bilateral brain regions. Focal involvement of specific networks during absence seizures may have implications for the behavioral impairments seen in this disorder.
Behavioral testing during absence seizures has shown that tasks requiring decision making and verbal responses are most severely affected, while simpler tasks, such as repetitive tapping or simple reaction time tasks, are often relatively spared (Shimazono et al., 1953; Mirsky and Van Buren, 1965; Blumenfeld, 2005a). One attention task that has been studied previously in absence epilepsy is the continuous performance task (CPT) (Tizard and Margerison, 1963; Mirsky and Van Buren, 1965). In this task, a series of stimuli (e.g. letters) are presented, and patients are instructed to respond whenever the target stimulus (e.g. the letter X) appears. The CPT appears to be a relatively sensitive indicator of impaired consciousness during absence seizures since reported omission error rates (76%) are higher than, for example, simple repetitive tapping tasks (31%) (Mirsky and Van Buren, 1965).
Recent advances in functional magnetic resonance imaging (fMRI) provide a new window to study brain networks affected by SWD. Simultaneous EEG–fMRI with blood oxygen level dependent contrast (BOLD) has been used to image cortical and subcortical networks during SWD in adult (Archer et al., 2003; Salek-Haddadi et al., 2003; Aghakhani et al., 2004; Gotman et al., 2005; Hamandi et al., 2006; Laufs et al., 2006) and pediatric patients (Labate et al., 2005; Moeller et al., 2008b; Moeller et al., 2008a). Although some of these patients had SWD related to other idiopathic generalized epilepsy disorders and not CAE, the fMRI changes reported in these studies were generally similar, most often showing fMRI increases in the thalamus and variable cortical regions, and fMRI decreases in the lateral and medial parietal cortex, cingulate, lateral frontal cortex, and basal ganglia. Investigations in both humans (Carmichael et al., 2008; Hamandi et al., 2008) and animals models (Nersesyan et al., 2004b; Nersesyan et al., 2004a; Mishra et al., 2008) have suggested that BOLD fMRI changes during SWD are likely an accurate reflection of increases and decreases in underlying neuronal activity.
Since SWD can occur in the “absence of absence,” i.e. without clinical manifestations of impaired consciousness, one critical question is whether the fMRI changes reported in the studies above will be present when patients have true absence seizures with behavioral impairment. Addressing this question is challenging since typical absence seizures occur most commonly in young children, where it is difficult to perform EEG-fMRI, particularly with simultaneous behavioral testing. In addition to the difficulties of obtaining fMRI without significant movement artifact, behavioral tasks are known to reduce absence seizure frequency (Tizard and Margerison, 1963; Blumenfeld, 2005a), lowering the chances of obtaining seizures during fMRI.
To determine the pattern of fMRI changes during typical childhood absence seizures with behavioral impairment, we performed simultaneous EEG-fMRI and behavioral testing on a relatively homogenous population of children with CAE. We studied a large number of subjects to increase the chances of capturing seizures during fMRI, and all children underwent training sessions to reduce movement. During EEG-fMRI, we performed CPT and a repetitive tapping task, since these are known to be differentially impaired by true absence seizures (Mirsky and Van Buren, 1965). Using this approach, we were able to measure fMRI changes during typical childhood absence seizures. We found fMRI increases in the thalamus, frontal cortex, primary visual, auditory, somatosensory, and motor cortex, and fMRI decreases in medial and lateral parietal cortex, cingulate, and basal ganglia during absence seizures which caused impaired attention testing. These findings suggest a cortical-subcortical network of areas involved in attention and primary information processing, which may be crucial for altered consciousness during absence seizures.
Methods
Subjects
All human subject procedures were approved by the institutional review boards at Yale University School of Medicine, and by the Yale Magnetic Resonance Imaging Center. Thirty-seven children between 6–18 years of age with typical childhood absence epilepsy were referred by their pediatric neurologists and participated in this study after written informed consent. Patients fulfilling the following inclusion criteria participated: 1. Clinical diagnosis of childhood absence epilepsy based on International League Against Epilepsy criteria (ILAE, 1989); 2. EEG with typical 3–4 Hz bilateral SWD and normal background activity; 3. age 6–18 years. Exclusion criteria were: 1. additional seizure types such as myoclonic, tonic-clonic, or partial seizures; 2. known structural brain abnormality; 3. other neurological disorders. Of the 37 patients studied, 9 had absence seizures during EEG-fMRI (Table 1).
Table 1.
Patient CAE clinical information
| Pt | Gender | Age at onset (years) |
Age at scan (years) |
Medicationa | EEGb | Reported sz frequency at time of onset |
Reported sz frequency at time of scans |
# Szs during EEG/fMRId |
#CPT Runsc | #RTT Runsc | #Fixation Runsc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 8 | 14 | Lamotrigine/none | Normal 8–9 Hz background. Frequent generalized frontal predominant 1.5 to 3.5 Hz spike-wave. | 10/day | 10/day | 1 | 4 | 0 | 2 |
| 2 | F | 11 | 12 | none | Normal 10 Hz background. Frequent generalized frontal predominant 3 Hz spike-wave. | 240/day | 240/day | 9 | 10 | 0 | 8 |
| 3 | M | 6 | 10 | Lamotrigine/none | Normal 9 Hz background. Bursts of symmetrical large amplitude 3 Hz spike-wave. | 1/week | none | 3 | 4 | 3 | 0 |
| 4 | F | 4 | 13 | Ethosuximide/none | Normal 9 Hz background. Frequent generalized frontal predominant 3.5 to 4 Hz spike-wave. | 1–2/week | 1/week | 6 | 2 | 2 | 0 |
| 5 | M | 13 | 13 | none | Normal 9 Hz background. Frequent generalized frontal predominant 3 Hz spike-wave. | 15/day | 1/day | 7 | 4 | 4 | 5 |
| 6 | F | 9 | 12 | Valproate/none | Normal background of alpha activity with intermixed theta. Frequent generalized 3 Hz spike-wave. | 100/day | 5/day | 3 | 4 | 4 | 0 |
| 7 | M | 10 | 15 | none | Normal 9–10 Hz background. Frequent 3 Hz spike-waves lasing 3–5 sec. | none | none | 7 | 4 | 4 | 0 |
| 8 | F | 5 | 6 | none | Normal 7–8 Hz background. Frequent 3–3.5 Hz spike- waves lasing 5–12 sec. | none | 5/day | 18 | 5 | 1 | 4 |
| 9 | F | 6 | 8 | Ethosuximide/none | Normal 10 Hz background. Generalized 3 Hz spike-wave lasting 8 sec. | 10–50/day | 120/day | 6 | 0 | 0 | 4 |
Medication being taken before scan/medication at time of scan (see Methods).
EEG at time of recruitment obtained from patient clinical EEG records. Similar EEG recordings were obtained for each patient during EEG-fMRI.
Scans were repeated on different days for some patients. Several (up to 4) data runs were performed on each day.
Total number of seizures across all scans and runs.
Abbreviations: Pt = patients, sz = seizure
Subject training
Prior to participating in MRI scanning and behavioral testing, patients underwent a training session as a separate visit, where they were familiarized with the scanner environment and sounds in a mock-scanner, and also underwent a practice session with each of the behavioral tasks. Subjects were rated on movement during the practice session, and feedback was given to patients and parents to enhance cooperation during the actual MRI scans. They were also provided with a CD recording of the MRI sounds and instructed to practice lying still while listening to the MRI sounds in the days preceding the MRI scan, while parents rated their movement and provided feedback.
Seizure activation procedures
We employed medication withdrawal as the only means of increasing the probability of recording a seizure during simultaneous EEG-fMRI recording (hyperventilation, photic stimulation and sleep deprivation were not used). Patients who were on medication were tested after their medications were temporarily discontinued for up to 48 hours. This procedure was approved by our human studies institutional review board and was used successfully with no adverse side effects. Specifically, of the 37 patients that underwent the study, no patient has had any clinical worsening of their absence seizures, emergence of other seizures, or other adverse effects from the medication withdrawal. While holding medications, patients and parents were instructed to prohibit the patient from operating a bicycle, motor vehicle, or other dangerous machinery, and patients were under constant observation by a parent or other responsible adult. Additionally, clinical neurologists involved with the study were in frequent contact with the families during the medication withdrawal period. Medication withdrawal was usually done over a weekend or other school break in order to not interfere with school performance. Medications were resumed immediately after the testing session.
The mean half life of ethosuximide in children is approximately 30 hours, 32 hours for lamotrigine, and 10–11 hours for valproic acid (Levy et al., 2002). It is therefore possible that not all the medicine was eliminated during the 48 hour withdrawal period. However the period was chosen to balance efforts to reduce medication with the concern for patient safety.
Prior to scanning, patients and family members were asked to report their absence seizure frequency per day during medication withdrawal, as well as at the time of initial diagnosis (Table 1).
EEG recording and analysis
EEG recordings in patients 1–7 were performed with an EEG cap with silver/silver-chloride electrodes (modified from Quik-Cap 21 channel, Neuroscan Inc., North Carolina, U.S.A.), carbon fiber cables (in-house), a 125Hz analog low-pass Butterworth filter (in-house), and an EEG recorder (NuAmps, Neuroscan Inc.). EEG signal was digitized at 500Hz with 32-bit DC recording, utilizing a standard 10–20 montage. Signals were recorded with respect to a ground electrode (located anterior to Fz) and rereferenced at the time of review to Cz reference, linked ears reference, and to a sagittal bipolar montage. A combination of SCAN (Neuroscan) software and in-house temporal principal component analysis (PCA) software were used for filtering artifact (Negishi et al., 2004) (Supplementary Figure 1). EEG in patients 8–9 was recorded using a similar system, except that carbon wire EEG electrodes were used (in-house), 32 channels were obtained, a pre-amplifier was introduced (in-house) (Negishi et al., 2008), and the signal was digitized at 1000Hz with a newer EEG recorder (SynAmps2, Neuroscan Inc.). Adaptive noise cancellation software was used for filtering in patient 8 (Negishi et al., 2008).
The EEG was low-pass filtered at 25 Hz and visually inspected for spike and wave seizures showing a frequency of 3–4 Hz. Although EEG duration of 3 or 4s has been used as a criterion to separate ictal from interictal spike-wave discharges in CAE (Daly and Pedley, 1990; Van Luijtelaar et al., 1991; Sadleir et al., 2009), there exists evidence that upon careful testing, even SWD lasting less than 1s can cause behavioral impairment (Shimazono and Hirai, 1953; Tuvo, 1958; Browne and Penry, 1974). Consequently we chose to include all SWD in our analysis. An example of an EEG prior to, and following, artifact removal is shown in Supplementary Figure 1. After processing, the in-magnet EEG shows sufficient quality to clearly reveal SWD (Supplementary Figure 1b).
Prior to in-magnet EEG recording, five minutes of out-of-magnet EEG data were collected in a room adjacent to the scanner. Once the subject was inside the magnet, continuous EEG recording occurred throughout the session. Two minute intervals of no-scanning EEG collection were included between successive runs of fMRI data acquisition in order to collect artifact free (gradient and RF) in-magnet EEG data. The out-of-magnet EEG data and no-scanning EEG data were used for PCA-based ballistocardiogram and scanner noise removal from the in-magnet EEG data, respectively (Negishi et al., 2004). To ensure correct synchronization of EEG and MRI data, the initation of the stimulus presentation program was triggered by a TTL pulse from the MRI scanner. In addition, the behavioral stimulus presentations and button push responses were recorded in separate channels along with the EEG data, as well as by the stimulus presentation software.
fMRI acquisition
The subjects’ heads were secured in the head-coil with foam pads and a Velcro strip to minimize head movement. The subjects were instructed to lie with their arms at their sides supported by foam padding. Imaging was performed at 3 Tesla on a Magnetom Trio scanner (Siemens Medical Systems, Erlangen, Germany). Prior to the task, AC-PC aligned axial T1 anatomical images (spin echo, repetition time = 300 ms, echo time = 2.47 ms, matrix size = 256 × 256, 25 slices per image, slice thickness = 6 mm, field of view = 22cm) were acquired in the same image planes as the functional MRI data. Functional images were acquired with an EPI BOLD sequence (repetition time = 1550 ms, echo time = 30 ms, flip angle = 80, matrix size = 64 × 64, other parameters were the same as the T1 anatomical images). BOLD fMRI was obtained in 10 minute 40 sec runs.
Behavioral Tasks
We used two tasks that have been used previously to test patients during absence seizures (Mirsky and Van Buren, 1965).
Continuous performance task (CPT)
In this test of attentional vigilance, the subject viewed a series of random letters and was instructed to push a button each time they saw an X. During the CPT task, the following 16 letters were displayed, selected in random sequence: A B C D E F H I L M N O T X Y Z. 25% of all letters presented were the target X, so that an X appeared on average every 4 seconds. Each letter was displayed for 250 ms. The interval between consecutive letter onsets was 1000 ms, allowing 1000 ms for response. During fixation, subjects viewed a + sign. 32 second epochs of fixation (+) and CPT were alternated in a block design, repeated 10 times, for a total of 10 minutes and 40 seconds per run.
Repetitive Tapping Task (RTT)
This task was similar to repetitive motor tasks used for testing CAE patients in prior studies (Mirsky and Van Buren, 1965). The RTT was identical to the CPT except that subjects were instructed to push the button for every letter, and no letter X was presented. Like with CPT there were alternating 32 second epochs of fixation and random letter presentations at 1 letter per second. Multiple 10 minute, 40 second imaging runs were repeated up to 4 times alternating between the tasks.
Fixation Only
Patients performed 10 min 40 sec runs, during which they viewed the fixation (+) for the entire period of imaging. The purpose of fixation only was to determine fMRI changes in the same patients during SWD but without any task. Fixation only was performed at separate scanning sessions separated by at least two weeks from the tasks.
Of the 37 patients studied, CPT and/or RTT was performed during EEG-fMRI on 33; 12 of the 37 underwent fixation only runs. Of the 9 total patients who had seizures during EEG-fMRI, 8 underwent behavioral testing (CPT and/or RTT) and 5 underwent fixation only runs (Table 1). Behavioral tasks were programmed using E-Prime 1.1 (Psychology Software Tools, Inc. Pittsburgh, PA) projected onto a screen in the scanner room and viewed by patients via a mirror fixed to the head coil. Button pushes were recorded via a hand-held button box in the scanner. Stimuli and response timing were recorded both using the E-Prime program, as well as directly on the EEG tracing, (Supplementary Figure 1b) to ensure correct synchronization of data from the task, EEG, and fMRI.
Analysis of Behavioral Data
CPT and RTT data were analyzed for errors of omission (% of missed targets) for letters presented during seizures. Target for the CPT task was the letter X, and target for RTT was any letter. The cut-off time for correct responses was 1000 ms after stimulus presentation. In any case, none of the patient responses to the target stimuli occurred later than 1000 ms after targets during seizures.
fMRI Data Analysis
Imaging data were analyzed using SPM2 (http://www.fil.ion.ucl.ac.uk/SPM) on a MATLAB 6.5 platform (MathWorks, Natick, MA). Images were realigned to the first scan of each functional series and spatially normalized to the SPM EPI template, which is in MNI space. Images were spatially smoothed using a 10 mm full width at half maximum Gaussian kernel. Timing of all image acquisitions was determined in reference to EEG spike-wave discharges based on image synchronization signals acquired with the EEG. A standard double gamma hemodynamic response function (HRF) in SPM was used to model the fMRI signal changes during seizures. Default parameters were used as documented in the SPM_HRF.m file (delay of response relative to onset = 6s; delay of undershoot relative to onset = 16s; dispersion of response = 1; dispersion of undershoot = 1; ratio of response to undershoot = 6; onset = 0s; length of kernel = 32s), resulting in a peak time of 5.1s and undershoot nadir time of 15.8s. Seizure onset and duration were determined from EEG and used to define a box-car function which was then convolved with the HRF. For analysis of all seizures within a given subject, all seizure onset and offset times were modeled using one regressor in the SPM design matrix. For analysis of single seizures in a given subject, each seizure was modeled using a separate regressor in the SPM design matrix; thus with single-seizure analysis the number of seizure regressors was equal to the number of seizures. Single-seizure analysis enabled us to then relate the fMRI changes to performance on the behavioral task during the corresponding seizure. In patients who underwent behavioral testing (CPT or RTT) the 32 s block design intervals of task and fixation were included in the model, in order to remove effects of the behavioral task from the fMRI results. A t-test was applied to ascertain BOLD signal changes during seizures in each patient compared to non-seizure times. To correct for multiple comparisons, we used the false discovery rate (FDR) (Genovese et al., 2002; Schwartzman et al., 2008), which had been used in some prior fMRI studies of spike-wave (Labate et al., 2005; Moeller et al., 2008a), with an FDR-corrected P threshold = 0.05. This FDR-correction yielded the same general results as analysis of our data with uncorrected SPM voxel-level height threshold P=0.001 (data not shown). Extent threshold k was set at 3 voxels.
Additionally, all fMRI analyses took into account the effects of subject movement during scanning. For all seizures we found no rotation >1°, and we found that translation during seizure periods was 0–1mm for 53/60 seizures, 1–2mm for 3/60 seizures, and 2–3mm for 4/60 seizures. Movement did not correlate temporally with seizure onset. In order to fully account for the motion-related effects, however, we did include 6 rigid-body motion parameters in our general linear model (3 translation, 3 rotation) (Friston et al., 1996; Lund et al., 2005).
Results
During typical childhood absence seizures we found fMRI increases most commonly in the bilateral thalamus, lateral frontal, medial occipital, lateral temporal, and superior Rolandic cortex and decreases in the parietal cortex, cingulate gyrus, and basal ganglia. We obtained a total of 60 absence seizures in 9 patients, from a total of 37 patients with typical childhood absence epilepsy who underwent EEG/fMRI. Average seizure duration was 6.2 ± 4.0 s (mean ± SD). The patients with seizures included 6 females and 3 males between the ages of 6–15 years (Table 1). All patients had a clinical diagnosis of typical childhood absence epilepsy (ILAE, 1989). EEGs performed by referring pediatric neurologists demonstrated a normal 8–10 Hz background with typical 3–4 Hz bilateral SWD.
Of the 60 seizures, 42 were obtained from 8 patients (Patients 1–8) during behavioral testing with simultaneous EEG-fMRI. The remaining 18 seizures were acquired from 4 patients during fixation only without behavioral testing (Patients 2, 5, 8, 9).
fMRI changes during absence seizures
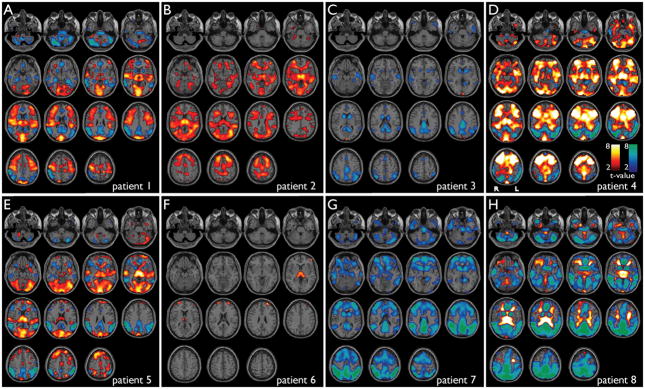
Eight patients had typical absence seizures during behavioral testing (Patients 1–8). We found mainly fMRI increases in the thalamus, and a combination of fMRI increases and decreases in the cortex (Figure 1; Table 2). In spike-wave seizures during behavioral tasks, we observed fMRI increases in the lateral frontal cortex (6/8 patients), occipital cortex (6/8), superior Rolandic cortex (4/8), superior temporal (Heschl’s) cortex (4/8), thalamus (6/8), cerebellum (5/8), lateral ventricle and white matter (5/8). Decreases were present in the lateral parietal cortex (6/8 patients), medial parietal cortex (precuneus, posterior cingulate) (6/8), anterior cingulate-medial frontal (5/8), basal ganglia (6/8), cerebellum (6/8), pons (4/8), and lateral frontal (3/8) (Figure 1; see also Table 2). Interestingly, in addition to the thalamus, many of the changes during seizures involved areas of primary sensory (visual, auditory, somatosensory) and motor (Rolandic) function, as well as the fronto-parietal association cortex.
Figure 1.
Cortical and subcortical fMRI changes during absence seizures in 8 patients while undergoing behavioral testing. Six motion-related parameters (3 translation, 3 rotation) are included in the modeling. Axial sections show BOLD fMRI increases (warm colors) and decreases (cool colors) during seizures compared to baseline analyzed in SPM. fMRI increases were seen most consistently in the thalamus, medial occipital, lateral temporal (Heschl’s), superior Rolandic, and lateral frontal cortex, and decreases were seen in lateral parietal, medial parietal, cingulate cortex and basal ganglia. a) Patient 1, seizure duration =12.0 s. b) Patient 2, seven seizures (mean duration = 4.9 s). c) Patient 3, three seizures (mean duration = 7.8 s). d) Patient 4, six seizures (mean duration = 6.0 sec). e) Patient 5, six seizures (mean duration = 6.8 s). f) Patient 6, three seizures (mean duration = 5.8 s). g) Patient 7, seven seizures (mean duration = 2.4 s). h) Patient 8, nine seizures (mean duration = 11.4 s). SPM analysis threshold P=0.05, FDR-corrected, and extent threshold k=3 voxels.
Table 2.
Summary of regions showing significant fMRI changes during seizures
| Pt | Lateral Frontal | Occipital | Superior Rolandic | Superior Temporal | Thalamus | Cerebellum | Ventricle/White Matter | Lateral Parietal | Medial Parietal | Anterior Cingulate | Basal Ganglia | Pons |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seizures during task conditions (Figure 1): | ||||||||||||
| 1 | + | + | + | + | + | +/− | + | − | − | − | − | − |
| 2 | + | + | + | + | + | + | + | |||||
| 3 | + | − | − | − | − | − | ||||||
| 4 | +/− | + | + | + | + | +/− | + | − | − | − | − | |
| 5 | + | + | + | + | + | +/− | + | − | − | − | − | |
| 6 | +/− | + | ||||||||||
| 7 | − | − | − | − | − | − | − | |||||
| 8 | + | + | + | +/− | + | − | − | − | − | − | ||
| Seizures during fixation (Figure 2): | ||||||||||||
| 2 | + | + | + | + | + | + | + | − | + | + | − | |
| 5 | − | + | − | − | − | |||||||
| 8 | +/− | + | + | + | +/− | + | − | − | − | − | − | |
| 9 | − | + | + | + | +/− | − | − | − | − | |||
+ significant fMRI increases. − significant fMRI decreases.
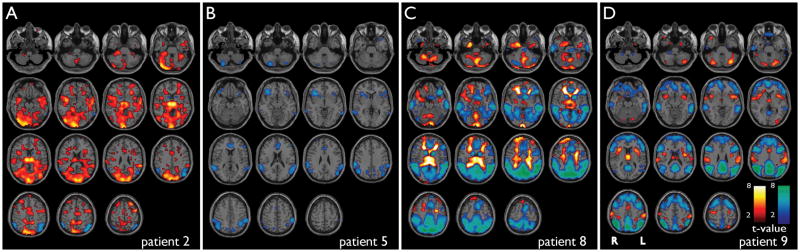
Four patients had seizures during the fixation-only paradigm (Patients 2, 5, 8, 9) (Figure 2; Table 2). Generally, the fMRI changes were very similar during seizures with fixation only and during tasks (Patients 2, 5 and 8 had seizures under both conditions). Once again, increases were present mainly in the thalamus, occipital, temporal, and frontal cortex, and decreases were seen mainly in the medial and lateral parietal cortex, cingulate, and basal ganglia.
Figure 2.
fMRI changes during absence seizures in 4 patients during fixation only (no task) with 6 motion related parameters (3 translation, 3 rotation) included in the modeling. Results were generally similar to fMRI changes in the same patients with seizures during behavioral tasks (Figure 1), showing increases in the thalamus, medial occipital, temporal and lateral frontal cortex, and decreases in the parietal cortex, cingulate gyrus and basal ganglia. Axial sections show BOLD fMRI increases (warm colors) and decreases (cool colors) during seizures compared to baseline analyzed in SPM. a) Patient 2, two seizures (mean duration = 2.3 s). Compare to same patient in Figure 1b. b) Patient 5, one seizure (duration = 7.5 s). Compare to same patient in Figure 1e. c) Patient 8, nine seizures (mean duration = 7.0 s). Compare to same patient in Figure 1h. d) Patient 9, 6 seizures (mean duration = 1.9 s). SPM analysis threshold P=0.05, FDR-corrected, and extent threshold k=3 voxels.
Performance on CPT and RTT behavioral testing during seizures
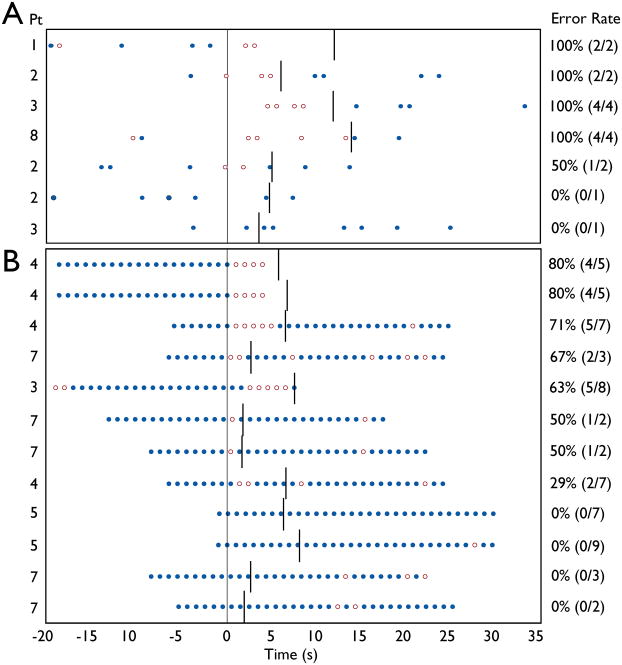
Prior fMRI studies of SWD have not evaluated behavior during the episodes to determine if attention was impaired, as is typically seen in absence seizures. We used the continuous performance task (CPT) to test attentional vigilance, and a simpler repetitive tapping task (RTT) to test ability to maintain repeated motor function. Tasks were administered in separate fMRI runs (CPT or RTT for the entire run) lasting 10 minutes, 40 seconds, with alternating blocks of task and fixation lasting 32 seconds (see Methods). As in prior studies (Tizard and Margerison, 1963; Blumenfeld, 2005a), the incidence of seizures appeared to be modulated by vigilance and attentional demand, since during CPT testing in Patients 1–8 there were an average of only 2.7 seizures per hour, while during RTT there were 6.8 seizures per hour (P = 0.05). Of the 42 seizures obtained during the task paradigms (Patients 1–8), 22 seizures were recorded during the task blocks; of these 10 were during CPT and 12 were during RTT. Of the 10 seizures obtained during CPT task blocks, only 7 contained target X presentations, while all 12 seizures during RTT task blocks contained target letters, yielding a total of 19 seizures for which behavioral data were obtained. Performance during these seizures is displayed in Figure 3. For the CPT attentional vigilance task, omission error rate (missed targets) during absence seizures was 81% (13/16). In contrast, omission error rate was significantly lower during RTT motor testing, at 39% (24/60) (χ2 = 8.60; p < 0.003). These error rates were similar to prior studies on absence seizures done without fMRI scanning, showing impaired attentional performance on CPT, and less severe impairment on simple repetitive motor testing (Mirsky and Van Buren, 1965). This confirms that the episodes investigated during fMRI were typical absence seizures with clinically significant impaired attention, and not just electrographic SWD without effects on behavior.
Figure 3.
Behavioral performance on CPT and RTT tasks during absence seizures. Behavioral tasks consisted of 32 s blocks of letter presentations, alternating with 32 s of fixation. Letters were presented once per second. a) For CPT, the target (X) occurred at random, at an average rate of once per 4 seconds. Mean omission error rate during seizures was 81%.(13/16). b) During RTT, patients were instructed to push the button for all letters (no Xs were presented). Mean omission error rate during seizures was 39% (24/60). ●= correct response to target. ○ = omission error. Symbols appear at time of letter presentation. Response was required within 1000 ms of letter presentation. Vertical line at t = 0 is seizure onset. Other vertical lines denote seizure end. 22 seizures were recorded during task blocks, but two seizures had no target letters occurring during the spike-wave discharge, and one could not be analyzed due to technical difficulty with button press recording, resulting in 19 seizures total.
BOLD fMRI correlates of impaired performance during seizures
Although seizures without behavioral impairment were obtained in a relatively small number of cases (Figure 3), we explored possible relationships between behavioral performance and fMRI changes by analyzing fMRI changes for single seizures. For comparison of behavioral performance to fMRI changes, we sorted individual seizures in descending order from worst performance (100% error rate) to best performance (0% error rate).
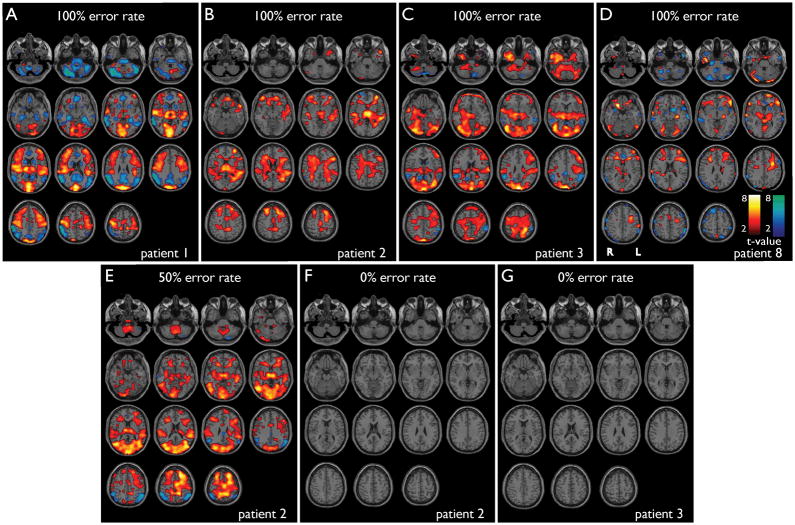
Impaired performance on CPT during seizures was associated with significant fMRI changes in the thalamus and cortical regions similar to those described above (Figure 4a–e). In contrast, during those two seizure for which there was a 0% error rate, the analyses revealed no significant fMRI changes (Figure 4f,g), despite the fact that these two seizures were obtained from patients that did show significant fMRI changes during other seizures with impaired performance (see Figure 4b, c). It should be noted that the seizures with 0% error rate on CPT had the shortest durations.
Figure 4.
fMRI changes in seizures with good vs. poor performance on CPT. SPM results for individual seizures are shown. (a–e) Seizures during impaired performance on CPT, showing cortical and subcortical fMRI changes. (f, g) Seizures during good performance on CPT (omission error rate 0%) show no significant cortical or subcortical changes on fMRI. a) Patient 1, seizure duration =12.0 s, omission error rate = 100%. b) Patient 2, seizure duration = 6.0 s, omission error rate = 100%. c) Patient 3, seizure duration = 11.9 s, omission error rate = 100%. d) Patient 8, seizure duration = 14.0 s, omission error rate = 100%. e) Patient 2, seizure duration = 5.0 s, omission error rate = 50%. f) Patient 2, seizure duration = 4.7 s, omission error rate = 0%. g) Patient 3, seizure duration = 3.8 s, omission error rate = 0%. Performance data on CPT during these same seizures is shown in Figure 3a. Warm colors represent fMRI increases, and cool colors decreases. SPM analysis threshold P=0.05, FDR-corrected, and extent threshold k=3 voxels, with 6 motion-related parameters included in the model.
A similar analyses of seizures during RTT testing did not reveal any consistent pattern (Supplementary Figure 2). Indeed, some patients were able to maintain a low error rate despite strong cortical and subcortical changes, while the reverse situation was true as well. These results may suggest that attentional performance on CPT is sensitive to regional changes during seizures, while simple motor tasks can continue in some cases despite robust involvement of corticothalamic networks. Without further testing in a larger sample size, however, it is difficult to establish a definitive trend.
Discussion
We found that during typical absence seizures in children with transient impaired consciousness there are distinct fMRI changes in cortical and subcortical structures important for attention and for primary information processing. To our knowledge, this is the first study investigating fMRI changes during SWD with confirmed behavioral impairment. We observed fMRI signal increases in the thalamus, frontal cortex, primary visual, auditory, somatosensory, and motor cortex, and fMRI decreases in the lateral and medial parietal cortex, cingulate gyrus, and basal ganglia during typical 3–4 Hz spike-wave discharges. Also during SWD and fMRI in the same patients, we found severely impaired performance on an attention task (CPT), while a simple motor task (RTT) had less severe impairments. Furthermore, patients with poor performance on CPT showed distinct corticothalamic changes, while those two patients with spared performance during seizures showed no significant fMRI changes despite SWD on EEG. These findings provide a potential link between behavioral unconsciousness during absence seizures and physiological changes in specific cortical and subcortical networks. We propose that impaired consciousness during SWD occurs when function is impaired in both networks important for attention (thalamus and fronto-parietal association cortex), and in primary processing regions (visual, auditory, somatosensory, and motor cortex).
The SWD episodes we observed during fMRI had several features typical of classic absence seizures. For example, as in prior studies (Tizard and Margerison, 1963; Blumenfeld, 2005a), the incidence of seizures appeared to be modulated by vigilance and attentional state, with a trend for fewer episodes during the more attentionally demanding CPT task compared to RTT. Another important feature reported previously (reviewed in (Blumenfeld, 2005a)), was the variability in attentional performance from one seizure to the next between patients, and even within the same patient. For example, during CPT some seizures caused all targets to be missed, while in other seizures targets were responded to, and similar variability was seen during RTT from one seizure to the next (Figure 3). These findings confirm that the fMRI measurements were performed during typical childhood absence seizures, with the classic pattern of ictal behavioral impairment seen in this disorder.
The fMRI changes that we observed during behaviorally confirmed absence seizures were generally similar to prior EEG-fMRI studies during SWD in both adults (Archer et al., 2003; Salek-Haddadi et al., 2003; Aghakhani et al., 2004; Gotman et al., 2005; Hamandi et al., 2006; Laufs et al., 2006) and in pediatric patients (Labate et al., 2005; Moeller et al., 2008b; Moeller et al., 2008a). The anatomical similarity of our results to those reported previously suggests that SWD in prior studies might also have been associated behavioral impairment if tested for. In addition, our results were in general agreement with a recent fMRI study which defined ictal vs. interictal SWD based on EEG duration and on parental report of behavioral changes in the scanner (Li et al., 2009).
One of the more consistent findings across studies has been thalamic fMRI increases during SWD. Thalamic increases have been reported in studies of SWD done in heterogeneous adult patients on various medications, as well as in a recent study done in drug naïve children (Moeller et al., 2008a). The important role of the thalamus in absence epilepsy has been investigated extensively in prior work (Avoli et al., 1990; Huguenard and Prince, 1997; Blumenfeld and McCormick, 2000; Blumenfeld, 2002, 2005b), and is supported by recent fMRI and other imaging studies in human patients.
Measurement of cortical fMRI changes during SWD have produced more inconsistent results. Human studies utilizing simultaneous EEG-fMRI have reported BOLD signal changes ranging from nearly the entire cortex (Salek-Haddadi et al., 2003) to more regional changes. fMRI signal decreases have been more consistently reported than increases during SWD. Descriptions of fMRI decreases in most studies have included bilateral lateral parietal cortex, precuneus, anterior cingulate, and frontal cortex. fMRI increases during SWD have been found previously in the bilateral precentral sulci (Archer et al., 2003), mesial frontal cortex, bilateral insula, lateral ventricles (Gotman et al., 2005), bilateral motor cortex (Labate et al., 2005), occipital cortex, and inferior parietal cortex (Laufs et al., 2006). We found fMRI increases mainly in the occipital, superior temporal, superior Rolandic, and lateral frontal cortex. The variation in reports of cortical fMRI increases during SWD may be related to different medications, epilepsy syndromes, and age across patients studied. It has been suggested that the variability in cortical signal might have less to do with SWD generation and more to do with the normal baseline activity of each individual (Laufs et al., 2006). If SWD leads to an interruption of normal activity, then baseline activity would dictate which cortical areas showed increases and decreases (Gotman et al., 2005). It is important to also recognize that seizures in one brain region can also have remote network effects leading to decreased activity in other brain areas without seizure activity in those locations (Blumenfeld et al., 2004b; Blumenfeld et al., 2004a; Englot et al., 2008).
In agreement with previous reports (Salek-Haddadi et al., 2003; Hamandi et al., 2006; Moeller et al., 2008a) we also found BOLD signal decreases in the basal ganglia. The importance of the basal ganglia in absence epilepsy has been investigated in animal models (Slaght et al., 2004; Mishra et al., 2008). There is some evidence of a role for the brainstem as well, in absence epilepsy (Kohsaka et al., 2001). We also found both increases (5/8) and decreases (6/8) in the cerebellum, which has been noted in prior studies (Hamandi et al., 2006; Laufs et al., 2006), and decreases in the pons in some (4/8) patients.
The selective network involvement during SWD suggest that impaired consciousness during absence seizures is not a global phenomenon, but rather can be decomposed into specific deficits in a number of different cognitive functions. Neuroimaging studies of the CPT in normal subjects have shown fMRI activations in the medial frontal cortex, thalamus, and other regions (Riccio CA, 2002), consistent with a role in sustained attention. Variable impaired performance during seizures may depend on whether or not seizures affect these regions important for attention. Furthermore, the CPT was also shown to be highly sensitive to attention dysfunction and has been linked to decreased prefrontal activity in other patient populations (Strakowski et al., 2005).
Some caveats are in order. For example, the CPT does not differentiate among particular attention networks, including orienting, alerting, and executive functions, as previously described (Fan et al., 2005). Future studies using tasks that probe various aspects of attention networks will prove useful in determining particular aspects of attention which are affected by SWD. Performing EEG-fMRI and behavioral studies on young children is technically challenging and although we included 6 motion related regressors in our analyses it is possible that the results could be improved further by inclusion of better models of motion effects (Lund et al., 2005). Moreover, because this is an exploratory study we felt it appropriate to correct our p-values using the false discovery rate rather than family-wise error, but this choice may affect the reliability and reproducibility of our maps. Also, our study is limited by relatively low sample size. Despite testing 37 subjects, we obtained seizures in only 8 during behavioral testing, because attentionally demanding tasks are known to affect the state of vigilance, and to therefore reduce the incidence of absence seizures. Our current sample size was not sufficient to permit group analysis of the data in a second-order random effects model. In addition, numerous prior studies have shown variable behavioral impairment during SWD from one patient to the next, and even from one SWD episode to the next in the same patient (reviewed in (Blumenfeld, 2005a)), similar to our observations here. While our results establish a tentative link between behavioral performance and involvement of corticothalamic networks on fMRI, studies of this kind are inherently limited by their correlative nature and by the indirect relationship between fMRI signals and underlying neuronal activity. Bearing these limitations in mind, a greater sample size may allow us to more confidently relate variations in performance with specific regional changes in fMRI. Time course analysis of seizures has recently yielded interesting results regarding possible changes before and after seizures, and regional variations in the hemodynamic response function in SWD (Aghakhani et al., 2004; Moeller et al., 2008b; Moeller et al., 2008a). Additional analyses taking these factors into account may demonstrate a complex sequence of fMRI changes in absence seizures, not detectible using conventional HRF modeling, and are the subject of a separate ongoing study in our laboratory (Bai et al., 2010). Time course analysis may be important for understanding mechanisms of seizure initiation and termination, and may also provide further insights into subtle changes in behavior which precede and follow absence seizures.
Conclusions
We show that in a group of pediatric patients off medication, SWD were associated with BOLD fMRI signal increases in the bilateral thalamus, occipital cortex, superior temporal gyrus, superior Rolandic cortex, and lateral frontal cortex. In addition, SWD in these patients were associated with fMRI signal decreases in lateral and medial parietal cortex, cingulate gyrus, and basal ganglia. Our results support the hypothesis that abnormal function in specific brain regions important for attention as well as in regions crucial for primary sensory-motor processing cause impaired consciousness in absence seizures.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institutes of Health [R01 NS055829, CTSA UL1 RR0249139, MSTP TG 5T32GM07205] and by the Betsy and Jonathan Blattmachr family.
We thank Allan F. Mirsky for originally developing the CPT task for use in CAE, and for helpful discussions on this project. We also thank Marvin Chun, Marcia Johnson, and Charles Greer for helpful comments. Iris Maldonado and Joanne Caprio-Adams provided training for children to undergo MRI scan. We are especially grateful to the patients and families who participated, and to the following clinicians who referred patients for the study: A Bhargava, H Blumenfeld, B Bourgeois, W Brown, G Castaneda, RL Cerciello, R Cheng, F DiMario, RB Duckrow, M Engel, J Gaitanis, J Gibbons, L Kan, SR Levy, D Mandelbaum, G Miller, S Moshe S Nallainathan, EJ Novotny, P Overby, S Rothman, R Smith, Y Sogawa, F Testa, S Wolf, and R Young. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
None of the authors has any conflict of interest to disclose.
References
- Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, Gotman J. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127:1127–1144. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI “deactivation” of the posterior cingulate during generalized spike and wave. Neuroimage. 2003;20:1915–1922. doi: 10.1016/s1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- Avoli M, Gloor P, Kostopoulos G, Naquet T, editors. Generalized Epilepsy. Boston: Birkhauser; 1990. [Google Scholar]
- Bai X, Vestal M, Berman R, Negishi M, Spann M, Vega C, DeSalvo MN, Novotny E, Constable RT, Blumenfeld H. Dynamic timecourse of typical childhood absence seizures: EEG, behavior and fMRI. J Neurosci. 2010 doi: 10.1523/JNEUROSCI.5101-09.2010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. The thalamus and seizures. Arch Neurol. 2002;59:135–137. doi: 10.1001/archneur.59.1.135. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H. Consciousness and epilepsy: why are patients with absence seizures absent? Prog Brain Res. 2005a;150:271–286. doi: 10.1016/S0079-6123(05)50020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005b;46(Suppl 9):21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, McCormick DA. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J Neurosci. 2000;20:5153–5162. doi: 10.1523/JNEUROSCI.20-13-05153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, Rivera M, McNally KA, Davis K, Spencer DD, Spencer SS. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 2004a;63:1015–1021. doi: 10.1212/01.wnl.0000141086.91077.cd. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, Norden AD, Stokking R, Studholme C, Novotny EJ, Zubal IG, Spencer SS. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 2004b;14:892–902. doi: 10.1093/cercor/bhh048. [DOI] [PubMed] [Google Scholar]
- Browne TR, Penry JK, et al. Responsiveness before, during and after spike-wave paroxysms. Neurology. 1974;24:659–665. doi: 10.1212/wnl.24.7.659. [DOI] [PubMed] [Google Scholar]
- Carmichael DW, Hamandi K, Laufs H, Duncan JS, Thomas DL, Lemieux L. An investigation of the relationship between BOLD and perfusion signal changes during epileptic generalised spike wave activity. Magn Reson Imaging. 2008 doi: 10.1016/j.mri.2008.01.041. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly D, Pedley TA. Current Practice of Clinical Electroencephalography. 2. New York: Raven Press; 1990. [Google Scholar]
- Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 2008;28(36):9066–9081. doi: 10.1523/JNEUROSCI.2014-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci U S A. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamandi K, Laufs H, Nöth U, Carmichael DW, Duncan JS, Lemieux L. BOLD and perfusion changes during epileptic generalised spike wave activity. Neuroimage. 2008;39:608–618. doi: 10.1016/j.neuroimage.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Hamandi K, Salek-Haddadi A, Laufs H, Liston A, Friston K, Fish DR, Duncan JS, Lemieux L. EEG-fMRI of idiopathic and secondarily generalized epilepsies. Neuroimage. 2006;31:1700–1710. doi: 10.1016/j.neuroimage.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Brown M, Tucker DM. Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia. 2004;45:1568–1579. doi: 10.1111/j.0013-9580.2004.23204.x. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Basic mechanisms of epileptic discharges in the thalamus. In: Steriade M, Jones EG, McCormick DA, editors. Thalamus. Oxford: Elsevier; 1997. pp. 295–330. [Google Scholar]
- ILAE. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Kohsaka S, Kohsaka M, Mizukami S, Sakai T, Kobayashi K. Brainstem activates paroxysmal discharge in human generalized epilepsy. Brain Res. 2001;903:53–61. doi: 10.1016/s0006-8993(01)02381-2. [DOI] [PubMed] [Google Scholar]
- Labate A, Briellmann RS, Abbott DF, Waites AB, Jackson GD. Typical childhood absence seizures are associated with thalamic activation. Epileptic Disord. 2005;7:373–377. [PubMed] [Google Scholar]
- Laufs H, Lengler U, Hamandi K, Kleinschmidt A, Krakow K. Linking generalized spike-and-wave discharges and resting state brain activity by using EEG/fMRI in a patient with absence seizures. Epilepsia. 2006;47:444–448. doi: 10.1111/j.1528-1167.2006.00443.x. [DOI] [PubMed] [Google Scholar]
- Levy RH, Meldrum BS, Mattson RH, Perucca E. Antiepileptic Drugs. 5. Lippincott Williams & Wilkins; Philadephia, PA: 2002. [Google Scholar]
- Li Q, Luo C, Yang T, Yao Z, He L, Liu L, Xu H, Gong Q, Yao D, Zhou D. EEG-fMRI study on the interictal and ictal generalized spike-wave discharges in patients with childhood absence epilepsy. Epilepsy Res. 2009 doi: 10.1016/j.eplepsyres.2009.08.018. In Press. [DOI] [PubMed] [Google Scholar]
- Lund TE, Norgaard MD, Rostrup E, Rowe JB, Paulson OB. Motion or activity: Their role in intra- and inter-subject variation in fMRI. Neuroimage. 2005;26:960–964. doi: 10.1016/j.neuroimage.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Meeren HK, Pijn JP, Van Luijtelaar EL, Coenen AM, Lopes da Silva FH. Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J Neurosci. 2002;22:1480–1495. doi: 10.1523/JNEUROSCI.22-04-01480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky AF, Van Buren JM. On the Nature of the “Absence” in Centrencephalic Epilepsy: A Study of some Behavioral, Electroencephalographic, and Autonomic Factors. Electroencephalogr Clin Neurophysiol. 1965;18:334–348. doi: 10.1016/0013-4694(65)90053-2. [DOI] [PubMed] [Google Scholar]
- Mishra AM, Ellens DJ, Schridde U, Motelow JM, Purcaro M, Hyder F, Blumenfeld H. Simultaneous fMRI/CBV and EEG during spike-wave seizures in WAG/Rij rat. Proc Intl Soc Mag Reson Med; Toronto Canada. 2008. p. 582. [Google Scholar]
- Moeller F, Siebner HR, Wolff S, Muhle H, Granert O, Jansen O, Stephani U, Siniatchkin M. Simultaneous EEG-fMRI in drug-naive children with newly diagnosed absence epilepsy. Epilepsia. 2008a;49(9):1510–1519. doi: 10.1111/j.1528-1167.2008.01626.x. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Wolff S, Muhle H, Boor R, Granert O, Jansen O, Stephani U, Siniatchkin M. Changes in activity of striato-thalamo-cortical network precede generalized spike wave discharges. Neuroimage. 2008b;39(4):1839–1849. doi: 10.1016/j.neuroimage.2007.10.058. [DOI] [PubMed] [Google Scholar]
- Negishi M, Abildgaard M, Nixon T, Constable RT. Removal of time-varying gradient artifacts from EEG data acquired during continuous fMRI. Clinical Neurophysiol. 2004;115:2181–2192. doi: 10.1016/j.clinph.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Negishi M, Abildgaard M, Laufer I, Nixon T, Constable RT. An EEG (electroencephalogram) recording system with carbon wire electrodes for simultaneous EEG-fMRI (functional magnetic resonance imaging) recording. J Neurosci Methods. 2008 doi: 10.1016/j.jneumeth.2008.05.024. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nersesyan H, Hyder F, Rothman D, Blumenfeld H. Dynamic fMRI and EEG recordings during spike-wave seizures and generalized tonic-clonic seizures in WAG/Rij rats. J Cereb Blood Flow Metab. 2004a;24:589–599. doi: 10.1097/01.WCB.0000117688.98763.23. [DOI] [PubMed] [Google Scholar]
- Nersesyan H, Herman P, Erdogan E, Hyder F, Blumenfeld H. Relative changes in cerebral blood flow and neuronal activity in local microdomains during generalized seizures. J Cereb Blood Flow Metab. 2004b;24:1057–1068. doi: 10.1097/01.WCB.0000131669.02027.3E. [DOI] [PubMed] [Google Scholar]
- Riccio CARC, Lowe P, Moore JJ. The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol. 2002:235–272. [PubMed] [Google Scholar]
- Sadleir LG, Scheffer IE, Smith S, Carstensen B, Farrell K, Connolly MB. EEG features of absence seizures in idiopathic generalized epilepsy: impact of syndrome, age, and state. Epilepsia. 2009;50:1572–1578. doi: 10.1111/j.1528-1167.2008.02001.x. [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR. Functional magnetic resonance imaging of human absence seizures. Ann Neurol. 2003;53:663–667. doi: 10.1002/ana.10586. [DOI] [PubMed] [Google Scholar]
- Schwartzman A, Dougherty RF, Lee J, Ghahremani D, Taylor JE. Empirical null and false discovery rate analysis in neuroimaging. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.04.182. Epub ahead of print Apr 24, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazono Y, Hirai T, Okuma T, Fukuda T, Yamamasu E. Disturbance of consciousness in petit mal epilepsy. Epilepsia. 1953;2:49–55. [Google Scholar]
- Slaght SJ, Paz T, Chavez M, Deniau JM, Mahon S, Charpier S. On the activity of the corticostriatal networks during spike-and-wave discharges in a genetic model of absence epilepsy. J Neurosci. 2004;24:6816–6825. doi: 10.1523/JNEUROSCI.1449-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski S, DelBello M, Adler C. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Tizard B, Margerison JH. The relationship between generalized and paroxysmal EEG discharges and various test situations in two epileptic patients. J Neurol Neurosurg Psychiatry. 1963:26. doi: 10.1136/jnnp.26.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukel K, Jasper H. The electroencephalogram in parasagittal lesions. Electroencephalogr Clin Neurophysiol. 1952;4:481–494. doi: 10.1016/0013-4694(52)90079-5. [DOI] [PubMed] [Google Scholar]
- Tuvo F. Contribution a l’etude des niveaux de conscience au cours des paroxysmes epileptiques infraclinique. Electroencephalogr Clin Neurophysiol. 1958;10:715–718. doi: 10.1016/0013-4694(58)90076-2. [DOI] [PubMed] [Google Scholar]
- Van Luijtelaar EL, de Bruijn SF, Declerck AC, Renier WO, Vossen JM, Coenen A. Disturbances in time estimation during absence seizures in children. Epilepsy Res. 1991;9:148–153. doi: 10.1016/0920-1211(91)90027-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.