Abstract
Genetic diseases known as ciliopathies have recently entered the limelight, placing new importance on a previously mysterious organelle: the primary cilium. Mutations affecting the primary cilium in both humans and animal models can lead to a plethora of distinct phenotypes including retinal degeneration, kidney cysts, and brain malformations. New findings are quickly lending insight into the functions of this cellular extension that seems to be especially important in modulation of subcellular signaling cascades at various stages of development and adult homeostasis.
Introduction
The terms ‘cilia’ and ‘flagella’ often evoke images of microscopic algae gliding through pond water, shifting about with incredible deftness, much as Leeuwenhoek first observed in 1675. Or perhaps instead, images emerge of millions of tiny sperm with lengthy whips projecting toward their destination: the ovum. But cilia and flagella are not only pistons of unicellular locomotion. From the ciliated epithelium of the oviduct, pushing the ovum toward the uterus, to the epithelial cells of the lung with their millions of tiny bristles brushing fluid and debris along like tiny broom-bearing maids, cilia are ubiquitous. From cleaning up our inside messes to their recently recognized signaling functions, cilia are involved in a myriad of biological processes, and research is beginning to reveal the importance of these tiny hair-like projections in a variety of disorders known as ‘ciliopathies’ [1].
It has been a long road to recognizing the importance of cilia in disease pathogenesis and vertebrate physiology. Until recently, vertebrate cilia were mainly recognized for their roles in clearing mucus from the lungs and generating flow. True, cilia had been described ubiquitously in other organs but they were viewed as ‘vestigial’ organelles, nothing more than a mere oddity. These cilia, known as primary cilia, are unlike their motile cousins that line the trachea. Primary cilia instead are generally nonmotile (with the exception of nodal cilia) and are normally present as a single cilium per cell. The primary cilium is made up of nine outer microtubule doublets with a modified centrosome at its base, known as the basal body (Figure 1) [2]. Primary cilia have been described on a multitude of cell types, from kidney tubules to neurons to the modified cilium of the retinal photoreceptor. The role of motile epithelial cilia has always been fairly intuitive: to help direct fluid and debris. But what could be the function of the more ubiquitous yet far more mysterious nonmotile cilium? That is precisely the question that has recently captivated the attention of multiple fields of biomedical research.
Figure 1.
Intraflagellar transport within the primary cilium. Kinesin 2 (with its major component Kif3a) transports cargo in an anterograde direction toward the tip of the cilium, while dynein heavy chain 2 (Dnchc2) travels in the retrograde direction toward the base of the cilium. Membrane cargo, like rhodopsin, is first loaded into a vesicle and transported to the basal body from the golgi by dynein 1. Vesicles then fuse with the cilia membrane and membrane bound cargo is transported along the ciliary length by Kif3a and dynein 2. Major components of this process include Rab8 as well as several ciliopathy genes, particularly the BBS proteins. Inset: schematic of a primary cilium cross-section revealing 9+0 architecture.
The list of disorders categorized as ciliopathies is constantly expanding as borders are blurred between what were previously considered distinct disorders. Overlapping phenotypes and genetic causes have revealed a continuum of disorders that all have one crucial thing in common: evidence to suggest a defect of the primary cilium. The primary cilium is at the heart of these disorders and its ubiquity can be blamed for the diversity of phenotypes. The ciliopathies therefore encompass a variety of seemingly distinct disorders depending upon the organs most severely affected (Table 1) [3]. For example, nephronophthisis (NPHP) and polycystic kidney disease (PKD) are both ciliopathies defined by cystic kidney pathologies, whereas Leber congenital amaurosis (LCA) is defined by its early onset retinal degeneration phenotype similar to retinitis pigmentosa (RP). Bardet– Biedl syndrome (BBS) is a compound phenotype disorder exhibiting obesity, cystic kidneys, and RP while Meckel–Gruber (MKS) and Joubert syndromes (JS) both exhibit brain malformations. These are some of the examples of cilium-associated phenotypes that can be associated with a plethora of other defects in a variety of affected organs. However, genetic and phenotypic overlap between these disorders has revealed that they are not as distinct as was once suspected. CEP290, for example, is mutated in several previously distinct disorders: NPHP, LCA, JS, and MKS. Additionally, an individual may present with several related disorders such as occurs between JS, NPHP, and RP leading to new classifications (cerebello-oculo-renal syndrome, CORS) and a new appreciation for potential similar molecular mechanisms.
Table 1.
Ciliopathy genes and their subcellular functions.
| Primary phenotypes | Gene/protein | Localization | Putative subcellular functions |
|---|---|---|---|
| Kartagener’s/primary ciliary diskinesia (PCD) | |||
| Situs inversus, chronic sinusitis, bronchiectasis |
DNAH5 | Outer dynein arms | Cilia motility |
| DNAL1 | Outer dynein arms | Cilia motility | |
| DNAH11/LRD | Axonemal | Cilia motility | |
| KTU | Cytoplasm | Dynein arm preassembly, cilia motility |
|
| DNAI2 | Outer dynein arms | Cilia motility | |
| RSPH9 | Radial spokes | Central pair/motility | |
| RSPH4A | Radial spokes | Central pair/motility | |
| Bardet–Biedl syndrome (BBS) | |||
| Obesity, cystic kidneys, retinal degeneration |
BBS1 | Basal bodies | BBsome, Wnt/PCP, IFT/trafficking |
| BBS2 | Basal bodies | BBsome, IFT/trafficking | |
| BBS3; ARL6 | Basal bodies | Vesicle trafficking | |
| BBS4 | Basal body, primary cilia | BBsome, Wnt/PCP, IFT/ trafficking |
|
| BBS5 | Basal bodies | BBsome, IFT/trafficking | |
| BBS6; MKKS | Basal bodies | IFT/trafficking, Wnt/PCP, cytokinesis, chaperonin |
|
| BBS7 | Basal bodies | BBsome, IFT/trafficking | |
| BBS8; TTC8 | Basal body, primary cilia | BBsome, IFT/trafficking | |
| BBS9; PTHB1 | Basal bodies | BBsome | |
| BBS10 | Basal bodies | Ciliogenesis, Wnt, chaperonin | |
| BBS11; TRIM32 | Basal bodies | E3 ubiquitin ligase | |
| BBS12 | Basal bodies | Ciliogenesis, Wnt, chaperonin | |
| Alstrom syndrome | |||
| Obesity, retinal degeneration, hearing loss |
ALMS1 | Basal bodies | Cilia maintenance |
| Leber congenital amaurosis (LCA) | |||
| Congenital retinal blindness | TULP1 | Connecting cilia | Rhodopsin trafficking |
| LCA5 | Connecting cilia | Unknown | |
| CEP290; NPHP6 | Basal bodies | RPGR complex, trafficking | |
| RPGRIP1 | Connecting cilia | RPGR complex | |
| Retinitis pigmentosa | |||
| Retinal degeneration | RPGR | Connecting cilia | IFT/trafficking, Disc morphogenesis |
| RP1 | Connecting cilia | IFT/trafficking, Disc morphogenesis | |
| Polycystic kidney disease (PKD) | |||
| Cystic kidneys | PKD1/PC1 | Primary cilia, cell–cell junction | Mechanosensation, Wnt, cell adhesion |
| PKD2/PC2 | Primary cilia, cell–cell junction | Calcium channel | |
| PKHD1/FPC | Primary cilia | PC2 modulation | |
| Nephronophthisis | |||
| Fibrocystic kidneys | NPHP1/Nephrocystin | Basal bodies, primary cilia, cell–cell contacts |
Cilia structure |
| NPHP2; INVS | Basal bodies, primary cilia | Wnt/PCP | |
| NPHP3 | Undetermined | Wnt/PCP | |
| NPHP4/Nephroretinin | Basal bodies, primary cilia | Cilia structure, IFT | |
| NPHP5; IQCB1 | Primary cilia | RPGR/calmodulin complex | |
| NPHP6; CEP290 | Basal bodies | RPGR complex, trafficking | |
| NPHP7; GLIS2 | Nucleus | Transcription factor, Wnt | |
| NPHP8; RPGRIP1L | Basal bodies | Shh signaling | |
| NPHP9; NEK8 | Basal bodies, primary cilia | Modulation of PC1 and PC2 | |
| Meckel–Gruber syndrome (MKS) | |||
| Brain malformation, cystic kidneys, polydactyly |
MKS1 | Basal bodies | Ciliogenesis |
| MKS3; TMEM67 | Primary cilia, membrane | Ciliogenesis | |
| CEP290; NPHP6 | Basal bodies | RPGR complex, trafficking | |
| CC2D2A | Basal bodies | Unknown | |
| Joubert syndrome (JS) | |||
| Brain malformation | AHI1/Jbn | Basal bodies, primary cilia | Wnt signaling, oncogene |
| NPHP1/Nephrocystin | Basal bodies, primary cilia, cell–cell contacts |
Cilia structure | |
| CEP290; NPHP6 | Basal bodies | RPGR complex, trafficking | |
| MKS3; TMEM67 | Primary cilia, membrane | Ciliogenesis | |
| RPGRIP1L | Basal bodies | Shh signaling | |
| ARL13B | Primary cilia | Cilia structure | |
| CC2D2A | Basal bodies | Unknown | |
| INPP5E | Primary cilia | PI signaling, cilia stability | |
| Oral-facial-digital syndrome | |||
| Craniofacial abnormalities, polydactyly, cystic kidneys |
OFD1 | Basal bodies, nucleus | Ciliogenesis, Wnt/PCP |
Information available on OMIM (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM).
In light of recent genetic findings it is now somewhat puzzling that these disorders have any phenotypic distinctions at all given their mutual underlying causes. Possible explanations for the variable phenotypes seen may be due to genetic background or differences in expression profiles as well as the complexity of the organelle involved: the cilia proteome database at present contains over 2500 entries! Thus, depending on the genetic makeup of an affected individual, some phenotypes may be more prominent, defining a unique syndromic entity. With recent advances we can now focus on understanding the commonalities of the molecular mechanisms of pathogenesis of the ciliopathies and the normal functions of primary cilia in the various organs affected. With that said, this review will focus on the recent advances in our understanding of this perplexing organelle particularly in three major organs affected in the ciliopathies. Recent findings of the functions of ciliopathy proteins will be examined in this context.
The retinal connecting cilium
The modified cilium of the photoreceptor is not a typical example of a vertebrate primary cilium for it is the only known primary cilium with membrane-enclosed vesicles, perhaps making it seem an odd place to begin our examination. However, the outer segment of the rods and cones of the eye make up a majority of the cell’s surface area and consume more energy than any other region of the eye. This modified structure is therefore especially sensitive to disruptions of cilia function particularly in terms of basic architecture and transport. Without an intact connecting cilium, the photoreceptor is prone to apoptosis leading to retinal degeneration. Such degeneration is known as retinitis pigmentosa (RP) [4].
RP can manifest through various mechanisms since it is defined by photoreceptor cell death, a process that may occur because of any number of defects. The congenital form of RP, defined as LCA, currently has 14 known causative genes, 4 of which are localized to the connecting cilium and/or basal body [5]. These are TULP1, CEP290, RPGRIP1, and LCA5. Autosomal dominant RP can be caused by mutations in the cilia associated gene RP1 while the majority of X-linked RP cases are caused by mutations in a gene known as RPGR, also localized to the connecting cilium. Thus, subtypes of LCA and RP can be considered ciliopathies. Similar RP phenotypes have been identified in several related ciliopathies that include but are not limited to NPHP, Usher syndrome, BBS, and CORS.
Insights into the role of the primary cilium in RP can be gained from understanding the normal functions of the cilium-associated causative genes within the photoreceptor. The strongest findings have come from various protein interaction studies that have identified complexes between several of the proteins involved in RP. RPGR and RPGRIP1 directly interact [6,7], and this interaction is disrupted with certain disease associated missense mutations suggesting it is relevant to disease pathogenesis [6]. Studies from mutant mice indicate a potential scaffolding role for RPGRIP1 at the connecting cilium whereby RPGR localization depends upon the presence of RPGRIP1 [8]. In addition, CEP290 has been identified to interact with RPGR and RPGRIP1 [9] supporting the presence of a multi-protein complex at the connecting cilium. Further interaction studies have revealed a variety of other proteins associated with this complex including NPHP genes 4 [10] and 5 [59] both of which are associated with RP in conjunction with NPHP (known as Senior-Loken syndrome, SLSN). Thus, the ‘interactome’ of the cilium probably consists of large multi-subunit complexes each mainly constituted by proteins clustered based upon the disease they share in common.
So what might be the role of this cilia-associated multi-protein complex? One attractive possibility that is gaining momentum suggests that RP proteins are necessary for transport of photoreceptor components into the outer segment (OS) through the connecting cilium. Transport within the primary cilium, known as intraflagellar transport (IFT), utilizes a specific set of motor proteins to move cargo up and down the cilium (Figure 1). The unicellular flagellate Chlamydomonas reinhardtii has been particularly instrumental in identifying the underlying mechanisms of IFT and the specific proteins involved [2]. Many of the IFT components identified in C. reinhardtii have mammalian orthologs that similarly function in IFT, indicating the high level of conservation of this process in general. Disruption of IFT components can perturb cilium architecture, from a complete loss of the cilium or flagellum to defects in length and overall shape. RPGR and CEP290 have both been found to interact with various microtubule transport proteins and IFT components including IFT88 [11] and the anterograde transport motor Kif3A [9], supporting a trafficking role for RP proteins. In fact, we and others have recently identified a role for CEP290 in cilia localization of Rab8 [12,13], a protein involved in docking vesicles to the base of cilia. Additionally, evidence from TULP1 mutant mice has revealed abnormal rhodopsin trafficking into the OS [14], and TULP1 has recently been shown to interact with dynamin-1, a GTPase that functions in vesicle trafficking [15]. This process is necessary for transport of membraneassociated proteins, like rhodopsin, into the OS.
These results are particularly intriguing given the strong support for roles of other ciliopathy proteins in IFT and vesicle transport. Many BBS proteins have been shown to interact as a large multi-protein complex termed the BBsome [16•], much like that between RPGR and RPGRIP1, which may serve as a scaffold for IFT components. Moreover, BBS4 mutant mice exhibit defects in opsin vesicle transport with mislocalization of rhodopsin [17]. These results increasingly point to pathological mechanisms involving disrupted IFT and defective localization of OS components.
Renal cilia in NPHP and PKD
NPHP and PKD are related cystic kidney disorders defined as ciliopathies given that nearly all of the known causative genes localize to the primary cilium [1]. Importantly, cystic kidney phenotypes have also been identified in several other related ciliopathies including BBS, oral-facial-digital syndrome (OFD) and CORS. NPHP can be caused by mutations in any of the NPHP1–9 genes, while PKD occurs with mutations in PKD1 or 2 (encoding polycystin-1, PC1, or polycystin-2, PC2) or PKHD1 (Table 1). Although the precise mechanisms of cystogenesis are not well understood, cysts seem to arise owing to a combination of abnormal proliferation, apoptosis, cell polarity, and cell fate disruptions.
Much as described in the retinal connecting cilium, there is some indication that basic cilium architecture or transport may be partly to blame for pathogenesis of cystic kidney disorders. NPHP1 and 4 have both been identified as necessary for precise formation of axoneme architecture in C. elegans [18] and, as described above, NPHP6 (alternatively known as CEP290) is required for Rab8 targeting and ciliogenesis [12,13]. Results from IFT animal mutants, such as IFT88 and Kif3A mouse mutants, underscore the importance of the cilium in pathogenesis of cystic kidney defects [19,20]. Despite this, however, none of the intrinsic IFT components have been identified as mutated in the human ciliopathies. This is probably owing to their central roles in overall cilia function and therefore may result in more severe phenotypes than NPHP and PKD.
With this in mind, emphasis has recently been placed on the function of ciliopathy proteins as potential key modulators of specific signaling cascades, which may depend upon the presence of the primary cilium. In fact, increasing evidence accentuates the role for the primary cilium in at least five different signaling cascades (Figure 2). The primary cilium has been most well characterized in Shh signaling in which Smo translocates into the cilium and activates Gli transcription factors within the cilium [21]. PDGFRα similarly translocates into the primary cilium to allow for its activation [22]. Additionally, increasing evidence is suggesting that the primary cilium is necessary for noncanonical Wnt signaling [23,24•], while in contrast to this positive regulatory role, it is instead inhibitory to the canonical Wnt pathway [25•,26]. In the kidney, calcium signaling, the Shh pathway and both canonical and noncanonical Wnt signaling have been implicated in cystic kidney pathogenesis (Table 1).
Figure 2.
Various signaling cascades converge at the cilium. (a) Sonic hedgehog (Shh) signaling depends upon translocation of smoothened (Smo) into the primary cilium (a process involving β-arrestins [55]) following binding of Shh to its receptor patched (Ptch) in order to activate Gli transcription factors from the repressor form (GliR) to the activator form (GliA). (b) PDGF signaling through PDGFRαα requires localization of the receptor within the primary cilium to activate MEK/ERK signaling. MEK itself is also localized to the primary cilium. (c) Although not well understood, noncanonical Wnt signaling (planar cell polarity, PCP) also requires the primary cilium and the basal body. Dishevelled (Dvl) activation through Frizzled 3 or 6 (Fzd3/6) leads to cell polarity determination as well as cytoskeletal rearrangements associated with PCP through interaction with Inversin (Inv, NPHP2) and the PCP component Vangl2. Vangl2 itself has also been localized to the primary cilium. (d) Canonical Wnt signaling is inhibited by the primary cilium through regulation of Dvl by CK1δ as well as proteasomal regulation of β-catenin at the basal body. Additional potential regulatory mechanisms are likely to exist as well. (e) Calcium signaling is activated by bending of the cilium in response to fluid flow through mechanosensation by polycystin-1 (PC1) and TRPV4 that form a complex with the calcium ion channel polycystin-2 (PC2). Calcium influx leads to further stimulation of calcium release from intracellular pools and activation of downstream signaling including cAMP-dependent MEK/ERK signaling [56]. (f) Additional signaling cascades implicated at the cilium include G-protein-coupled receptor (GPCR) signaling [57] and extra-cellular matrix (ECM) receptors [58].
The primary cilium is a particularly unique organelle because of its position extending away from the cell body, into the renal tubule lumen. This affords it the distinct ability to sense extracellular molecules as well as mechanostimulation such as fluid flow within the tubule lumen. Bending of the cilium in response to flow has been shown to generate a transient calcium influx [27] that depends upon the formation of a PC1/PC2 cation permeable channel within the primary cilium [28]. Disruption of either of these components or the related PKHD1 interrupts calcium signaling [29] potentially leading to a multitude of defects, from apoptosis to proliferation. Thus, a prevailing model has emerged known as the flow hypothesis of cystic kidney disease that suggests that abnormal response to tubular lumen fluid flow leads to pathogenesis of PKD and NPHP.
A similar flow hypothesis has been suggested by Simons et al. in regard to Wnt signaling in renal cystic disease [30]. NPHP2 as well as NPHP3 [31] have been shown to downregulate canonical Wnt signaling and upregulate noncanonical Wnt signaling, potentially in a flow-dependent manner. In support of a role for Wnt signaling, several noncanonical Wnt factors have recently been shown to lead to cystogenesis when mutated in animal models [32,33], while abnormal activation of the canonical pathway seems to lead to cystogenesis [34,35]. While the data seem to point to abnormal flow response in cystic kidney disease, it is not yet clear whether disrupted mechanosensation is sufficient to lead to cysts.
Recently, adult conditional mutant studies of Kif3a and Pkd1 mutant mice revealed that loss of the cilium or its mechanosensing components does not trigger cyst pathology [36•,37•], at least not for several months, suggesting that abnormal flow sensing is not sufficient to cause disease pathology. Additionally, Kottgen et al. recently identified a necessary component of the mechanosensation machinery, known as TRPV4, which when disrupted abolished flow triggered calcium transients, yet its loss did not lead to cystic kidney pathology [38]. These results suggest cilium-mediated flow sensing may not represent the primary pathogenic mechanism of cyst formation in PKD and NPHP.
Wnt signaling in cystic kidney disease likewise seems to be more complex than the initial switch mechanism model proposed by Simons et al. Although not as well appreciated, cysts have been documented both in mutants associated with reduced canonical Wnt signaling [39] as well as those that lead to hyperactivation of the pathway. Finally, BBS10 and BBS12 have recently been shown to be required for GSK3β inhibition and β-catenin cytosolic accumulation in the Wnt pathway [40]. Overall, cystic phenotypes seem to occur with disruption of canonical Wnt signaling in either direction or with disrupted noncanonical/PCP signaling.
Although these results at first seem contradictory, there have been several reports of similar canonical Wnt and PCP roles in developing renal tubules [41]. Thus, one possible model is that cystogenesis represents disruption of similar developmental processes. In line with this, Patel et al. described a particularly key set of experiments in which Kif3a conditional mutant mice were subjected to acute renal injury [42••]. Although loss of the cilium alone in this model is not sufficient to trigger cystic kidney disease in the adult, cyst pathology was evident with a specific time course following injury. Although not directly tested yet, this supports a model in which cilium-mediated developmental pathways may be reactivated upon injury. Disruption of any cilia signaling components or the cilium itself would be expected to disturb the processes of proliferation and differentiation of new tubules, potentially leading to cystogenesis. According to this model, it is developmental signaling rather than basal flow sensing that is involved in cystogenesis, although one cannot exclude the likely possibility that flow sensing may also occur in the context of these reactivated developmental pathways.
The neuronal primary cilium
Although cilia were identified throughout mammalian brain as early as the 1960s, their function is only just beginning to be understood. Neuronal cilia are ubiquitous, and are especially abundant in the hippocampus, developing cerebellum, olfactory bulb, and hypothalamus [43]. The largely accepted idea of the primary cilium as an antenna acting to bend in response to flow and receive external signals may apply to a subset of neurons in the ventricles, but the larger majority of ciliated neurons lie buried in a sea of dendrites and other cell bodies. In this context, the cilium does not extend nearly as far as other processes such as dendrites and axons. So what might be the function of this relatively tiny neuronal extension?
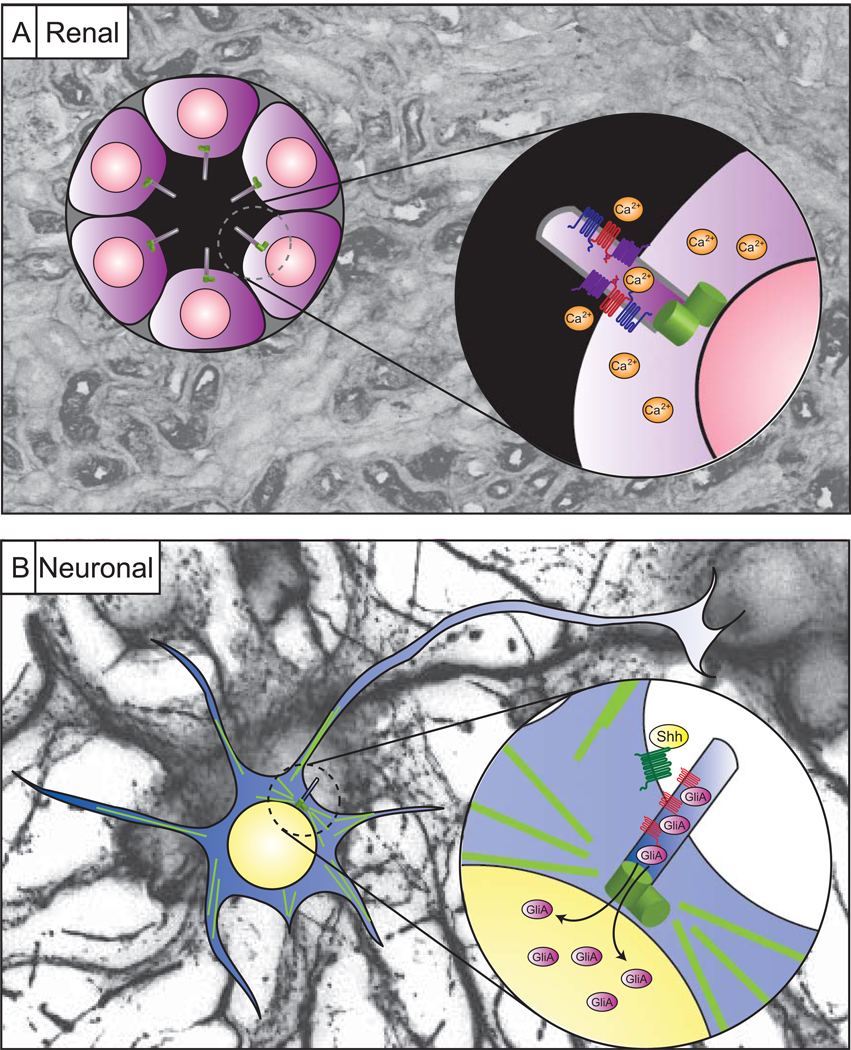
Several recent findings have begun to pinpoint specific roles for these mysterious organelles in the nervous system. Han et al. and Breunig et al. identified roles for primary cilia in stem cell maintenance of the hippocampus [44,45••] while Spassky et al. and Chizhikov et al. [46,47•] defined a similar role in cerebellar granule neuron proliferation, both in response to Shh. These findings point to a key role in signaling and a model in which the primary cilium may act as a signaling hub capable of concentrating signaling components in order to integrate or amplify specific cascades (Figure 3). Although this model of a concentrator remains to be specifically tested, it may explain the necessity for such an organelle on a cell that otherwise has thousands of longer extensions potentially capable themselves of acting as ‘antennae.’
Figure 3.
The primary cilium as a signaling hub. (a) Cilia projecting into fluid-filled luminal spaces are positioned to respond to signals and fluid flow within the tubule lumen in order to activate signaling cascades such as calcium signaling, as shown in the inset. (b) The neuronal cilium however appears buried among the dendrites and axons of surrounding neurons implicating an alternative rationale for its presence. One model is that the cilium is positioned next to the nucleus such that components of signaling cascades like the Shh pathway (inset) may be concentrated within the cilium in order to faithfully relay messages. Thus, the cilium acts as a concentrator of signaling components, or a signaling hub. Background images are immunostaining within renal tubules or cultured hippocampal neurons.
The findings by Spassky et al. and Chizhikov et al. that primary cilia of the developing cerebellum are required for CGN proliferation is particularly notable in light of the cerebellar phenotypes associated with MKS and JS, which are reminiscent of potentially similar proliferation defects. Additional insight into the roles of neuronal cilia has also come from Davenport et al. who described a striking obesity in mice with hypothalamic cilia disruption [37•] that is particularly applicable to the obesity phenotype seen in BBS. Although increasing evidence from IFT mutants reveals a vital role for the neuronal primary cilium in brain development, the adult neuronal functions of the ciliopathy proteins themselves are less well understood. Perhaps, as in the kidney, ciliopathy genes play more specific roles in neuronal cilium-based signaling. If the past is any judge, there may be many more well established signaling pathways influenced by the primary cilium.
We are still far from understanding the full complexity of the neuronal primary cilium, but these recent findings point to roles for the primary cilium and ciliopathy proteins in processes long thought to depend upon more traditional neuronal cell architecture. For example, AHI1 has now been identified as associated with both autism and schizophrenia [48,49], pointing to potential involvement of ciliopathy genes and the cilium itself in behavior. Finally, mental retardation is a fairly common component of several ciliopathies suggesting a role in learning and memory. Thus, cilia in regions of the brain involved in learning and behavior such as the cerebral cortex and hippocampus may play vital roles in neuronal function and these will need to be examined further.
Conclusion
We have focused on the role of ciliopathy genes within the context of ciliary function, yet other subcellular functions seem likely as well. For example, the polycystins and NPHP1 have been localized to cell–cell and cell–matrix contacts and PC1, in particular, is necessary for cell–matrix interactions [50]. The BBS proteins additionally seem to exhibit extra-ciliary functions such as potential chaperonin roles (BBS6, 10, 12) while BBS11 seems to be an E3 ubiquitin ligase [51]. Even integral IFT components such as Kif3a exhibit alternate subcellular roles: Kif3a also functions in axonal transport [52]. Thus, although the ciliopathies appear to share in common a localization to the primary cilium, there are additional possibilities that must be considered.
This review has focused on the primary cilium in the eye, kidney, and brain, but increasingly more pathologies are also being associated with the function of primary cilia. For example, liver cysts and fibrosis are also associated phenotypes within the ciliopathy spectrum and cystogenesis within the context of the liver may exhibit potentially similar pathologic mechanisms as seen in the kidney [1]. Other phenotypes include bone malformations, pancreatic cysts, Polydactyly, and vascular abnormalities suggesting unique roles for primary cilia within these organs. One exciting possibility is that cilia within the vasculature may function to measure flow similar to within the renal tubule, and calcium signaling in response to cilium bending in this context seems to lead to NO production [53•]. Thus, cilia may be vital for cardiovascular regulation. Finally, cilia may even play a role in cancer biology given their fundamental function in several developmental signaling pathways that are often misregulated in cancer. Indeed, HEF1 and Aurora A, two proteins involved in cancer cell proliferation and metastasis, have been found to regulate cilia stability [54]. The primary cilium is increasingly being identified as a novel regulator of a variety of cell biological processes, from development to homeostasis to cancer progression. This previously ‘vestigial’ organelle, it seems, does a lot more than we initially guessed.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen LB, Veland IR, Schroder JM, Christensen ST. Assembly of primary cilia. Dev Dyn. 2008;237:1993–2006. doi: 10.1002/dvdy.21521. [DOI] [PubMed] [Google Scholar]
- 3.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 4.Adams NA, Awadein A, Toma HS. The retinal ciliopathies. Ophthalmic Genet. 2007;28:113–125. doi: 10.1080/13816810701537424. [DOI] [PubMed] [Google Scholar]
- 5.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Roepman R, Bernoud-Hubac N, Schick DE, Maugeri A, Berger W, Ropers HH, Cremers FP, Ferreira PA. The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Hum Mol Genet. 2000;9:2095–2105. doi: 10.1093/hmg/9.14.2095. [DOI] [PubMed] [Google Scholar]
- 7.Boylan JP, Wright AF. Identification of a novel protein interacting with RPGR. Hum Mol Genet. 2000;9:2085–2093. doi: 10.1093/hmg/9.14.2085. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Hong DH, Pawlyk B, Yue G, Adamian M, Grynberg M, Godzik A, Li T. The retinitis pigmentosa GTPase regulator (RPGR)-interacting protein: subserving RPGR function and participating in disk morphogenesis. Proc Natl Acad Sci U S A. 2003;100:3965–3970. doi: 10.1073/pnas.0637349100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roepman R, Letteboer SJ, Arts HH, van Beersum SE, Lu X, Krieger E, Ferreira PA, Cremers FP. Interaction of nephrocystin-4 and RPGRIP1 is disrupted by nephronophthisis or Leber congenital amaurosis-associated mutations. Proc Natl Acad Sci U S A. 2005;102:18520–18525. doi: 10.1073/pnas.0505774102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna H, Hurd TW, Lillo C, Shu X, Parapuram SK, He S, Akimoto M, Wright AF, Margolis B, Williams DS, et al. RPGR-ORF15, which is mutated in retinitis pigmentosa, associates with SMC1, SMC3, and microtubule transport proteins. J Biol Chem. 2005;280:33580–33587. doi: 10.1074/jbc.M505827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet. 2008;17:3796–3805. doi: 10.1093/hmg/ddn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsang WY, Bossard C, Khanna H, Peranen J, Swaroop A, Malhotra V, Dynlacht BD. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell. 2008;15:187–197. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagstrom SA, Adamian M, Scimeca M, Pawlyk BS, Yue G, Li T. A role for the Tubby-like protein 1 in rhodopsin transport. Invest Ophthalmol Vis Sci. 2001;42:1955–1962. [PubMed] [Google Scholar]
- 15.Xi Q, Pauer GJ, Ball SL, Rayborn M, Hollyfield JG, Peachey NS, Crabb JW, Hagstrom SA. Interaction between the photoreceptor-specific tubby-like protein 1 and the neuronal-specific GTPase dynamin-1. Invest Ophthalmol Vis Sci. 2007;48:2837–2844. doi: 10.1167/iovs.06-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. A very thorough identification of a protein complex consisting of 7 BBS proteins that is necessary for ciliogenesis potentially through Rab8 function.
- 17.Abd-El-Barr MM, Sykoudis K, Andrabi S, Eichers ER, Pennesi ME, Tan PL, Wilson JH, Katsanis N, Lupski JR, Wu SM. Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet-Biedl syndrome. Vision Res. 2007;47:3394–3407. doi: 10.1016/j.visres.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jauregui AR, Nguyen KC, Hall DH, Barr MM. The Caenorhabditis elegans nephrocystins act as global modifiers of cilium structure. J Cell Biol. 2008;180:973–988. doi: 10.1083/jcb.200707090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr, Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol. 2002;282:F541–F552. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]
- 20.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 22.Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 24. Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. A study identifying a role for IFT88 in basal body positioning and PCP within the mouse cochlea. The authors identify a unique mechanism downstream of PCP component partitioning.
- 25. Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. A nice investigation of cilia function in canonical Wnt signaling making use of several cilia mutants and cell lines to begin to tease out a mechanism involving Dvl.
- 26.Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, Kato M, Beachy PA, Beales PL, DeMartino GN, Fisher S, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- 27.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 28.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol. 2007;27:3241–3252. doi: 10.1128/MCB.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergmann C, Fliegauf M, Bruchle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kranzlin B, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am J Hum Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 33.Kishimoto N, Cao Y, Park A, Sun Z. Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev Cell. 2008;14:954–961. doi: 10.1016/j.devcel.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Qian CN, Knol J, Igarashi P, Lin F, Zylstra U, Teh BT, Williams BO. Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem. 2005;280:3938–3945. doi: 10.1074/jbc.M410697200. [DOI] [PubMed] [Google Scholar]
- 35.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene. 2001;20:5972–5981. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- 36. Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007;13:1490–1495. doi: 10.1038/nm1675. An excellent set of findings that define a very narrow time window of cyst development in PKD1 mutant mice suggesting a unique dependence on the developmental state of the kidney.
- 37. Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. The first indication that primary cilia within the brain regulate obesity in adult mice as well as a primary study that challenges the flow hypothesis.
- 38.Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marose TD, Merkel CE, McMahon AP, Carroll TJ. Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol. 2008;314:112–126. doi: 10.1016/j.ydbio.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marion V, Stoetzel C, Schlicht D, Messaddeq N, Koch M, Flori E, Danse JM, Mandel JL, Dollfus H. Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc Natl Acad Sci U S A. 2009;106:1820–1825. doi: 10.1073/pnas.0812518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt-Ott KM, Barasch J. WNT/beta-catenin signaling in nephron progenitors and their epithelial progeny. Kidney Int. 2008;74:1004–1008. doi: 10.1038/ki.2008.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. The first indication that cysts may be triggered in adult cilia mutant mice with injury suggesting proliferation must be activated and potentially developmental pathways reactivated.
- 43.Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505:562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- 44.Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, Town T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signalling. Proc Natl Acad Sci U S A. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. The first indication that Shh signaling in hippocampal stem cells requires the primary cilium for maintenance of the stem cell pool.
- 46.Spassky N, Han YG, Aguilar A, Strehl L, Besse L, Laclef C, Ros MR, Garcia-Verdugo JM, Alvarez-Buylla A. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chizhikov VV, Davenport J, Zhang Q, Shih EK, Cabello OA, Fuchs JL, Yoder BK, Millen KJ. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. The first connection between Shh dependent cerebellar granule neuron proliferation and the primary cilium that is necessary for normal cerebellar development.
- 48.Amann-Zalcenstein D, Avidan N, Kanyas K, Ebstein RP, Kohn Y, Hamdan A, Ben-Asher E, Karni O, Mujaheed M, Segman RH, et al. AHI1, a pivotal neurodevelopmental gene, and C6orf217 are associated with susceptibility to schizophrenia. Eur J Hum Genet. 2006;14:1111–1119. doi: 10.1038/sj.ejhg.5201675. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez Retuerto AI, Cantor RM, Gleeson JG, Ustaszewska A, Schackwitz WS, Pennacchio LA, Geschwind DH. Association of common variants in the Joubert syndrome gene (AHI1) with autism. Hum Mol Genet. 2008;17:3887–3896. doi: 10.1093/hmg/ddn291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ibraghimov-Beskrovnaya O, Bukanov N. Polycystic kidney diseases: from molecular discoveries to targeted therapeutic strategies. Cell Mol Life Sci. 2008;65:605–619. doi: 10.1007/s00018-007-7362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tobin JL, Beales PL. Bardet-Biedl syndrome: beyond the cilium. Pediatr Nephrol. 2007;22:926–936. doi: 10.1007/s00467-007-0435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kondo S, Sato-Yoshitake R, Noda Y, Aizawa H, Nakata T, Matsuura Y, Hirokawa N. KIF3A is a new microtubule-based anterograde motor in the nerve axon. J Cell Biol. 1994;125:1095–1107. doi: 10.1083/jcb.125.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. A unique study identifying a potential mechanism of fluid sheer stress in endothelial cells: bending of the primary cilium that leads to NO production.
- 54.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ. Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int. 2003;63:1983–1994. doi: 10.1046/j.1523-1755.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 57.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGlashan SR, Jensen CG, Poole CA. Localization of extracellular matrix receptors on the chondrocyte primary cilium. J Histochem Cytochem. 2006;54:1005–1014. doi: 10.1369/jhc.5A6866.2006. [DOI] [PubMed] [Google Scholar]
- 59.Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O’Toole JF, Helou J, Attanasio M, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet. 2005;37:282–288. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]