Abstract
The “ciliopathies” are a newly defined group of disorders characterized by defects in the structure or function of the cellular primary cilium. Patients with these disorders display variably expressive fibrocystic renal disease, retinal blindness, polydactyly, obesity, and brain dysgenesis as well as neurocognitive impairments. Joubert syndrome is a ciliopathy defined by cerebellar vermis hypoplasia, oculomotor apraxia, intermittent hyperventilation, and mental retardation. Recent evidence suggests important roles for the primary cilium in mediating a host of extracellular signaling events such as morphogen, mitogen, homeostatic and polarity signals. Based upon the clinical features of ciliopathies and cilia mediated signaling pathways, the data support a role for the primary cilium in modulating neurogenesis, cell polarity, axonal guidance and possibly adult neuronal function.
Keywords: Ciliopathy, Joubert syndrome, Neuronal function, Primary cilia
Signaling function of cilia
Cilia are microtubule-based organelles that project from the surface of many cell types. These organelles can be divided into two types; motile cilia, which generate movement or flow, and nonmotile primary cilia, which move only passively. Both types contain nine parallel microtubules along their length, but only the motile cilia contain outer dynein arms, which are necessary for generating ciliary “strokes”. Nonmotile cilia play well-established roles in specialized sensory cells such as photoreceptors and cochlear hair cells, where they receive special sensory signals such as light and sound. The identification of non-motile cilia on the vast majority of non-mitotic cells throughout the body (Wheatley et al., 1996), initially suggested a non-functional vestigial role, since no known biological role could otherwise be ascribed. However, we now know that many receptors and ion channels are expressed on the membrane of primary cilia and they modulate various signaling pathways including sonic hedgehog (Shh), Wnt, and platelet-derived growth factor (PDGFα) that carry out diverse processes in tissue development and homeostasis. For example, binding of Shh ligand to Patched on the ciliary membrane regulates the concerted movement of Patched and Smoothened to activate downstream Shh signaling, which controls neural tube patterning and proliferation (Huangfu et al., 2003; Wechsler-Reya and Scott, 1999). The primary cilium is also involved in Wnt signaling through Wnt ligand, Frizzled receptor, and disheveled that regulates cell proliferation, fate determination, and migration (Simons et al., 2005; Wallingford and Habas, 2005). Additionally, it was shown that PDGFα signaling is coordinated by the PDGFRα in primary cilia and it plays a key role in cell survival, growth, and migration (Schneider et al., 2005). The recognition that cilia modulate many important signaling pathways has captured the imagination of many studying disease mechanisms in neuroscience, cell biology, and signaling.
Key discoveries in the cilia field
It had long been established that cilia completely lack vesicles, and instead use a unique set of motor and motor-adaptor proteins, termed intraflagellar transport (IFT). Despite these findings, ciliary biology remained a sleepy field for many years. However, this landscape began to change when it was recognized that mutations in IFT genes (identified in lower organisms such as Chlamydomonas) lead to defects in development in mammals. Four recent discoveries have opened the field of ciliary biology in mammalian systems. (1) The discovery that IFT molecules are required for the signaling of some pathways, such as the Shh pathway (Haycraft et al., 2005; Huangfu et al., 2003). (2) The discovery that polycystic kidney disease can be caused by mutations in IFT molecules (Qin et al., 2001; Yoder et al., 1995). (3) The identification of a host of disorders that share partially overlapping phenotypic features, which have helped define the “ciliopathies” (Adams et al., 2007; Badano et al., 2006; Hildebrandt et al., 2009). (4) The application of bioinformatics and proteomics approaches to determine the protein constituents of the “ciliome” (Andersen et al., 2003; Avidor-Reiss et al., 2004; Blacque et al., 2005; Broadhead et al., 2006; Efimenko et al., 2005; Li et al., 2004; Stolc et al., 2005). These findings have provided the framework for more recent studies, which have further established additional IFT molecular components that function within primary cilia and that can modulate a host of other signaling pathways, including Shh, Wnt, platelet-derived growth factor, and others (Fig. 1) (Corbit et al., 2008; Ross et al., 2005; Schneider et al., 2005; Simons et al., 2005). The emerging theme is that primary cilia can act to modulate cellular responses to various environmental or signaling cues, by helping the cell recognize the context of the particular cue.
Fig. 1.
Schematic diagram of primary cilium. Primary cilia lack the central microtubule pair and their cross-section show ‘9+0’ arrangement (inset). Cilia structure and function are maintained by intraflagellar transport (IFT) within primary cilia. The protein cargo is produced in the Golgi apparatus, moves towards the cilium, and binds to the IFT complex. IFT complex/cargo assembly can be transported along microtubules. The anterograde movement (toward the tip of the cilium) of IFT complex/cargo assembly relies on kinesin 2, while dynein 2 transports in the retrograde direction (toward the basal body of the cilium). Functionally, primary cilia are involved in various signaling pathways including Sonic hedgehog (Shh), Wnt, platelet-derived growth factor (PDGF), and G-protein coupled receptor (GPCR). These signaling pathways play important roles in regulating various neuronal functions including cerebellar development, hippocampal neurogenesis, and obesity. Abbreviations: PTCH, Patched 1; PDGFR, Platelet-derived growth factor receptor.
Characteristics of Joubert syndrome, ciliary dysfunctions and related signaling pathways
Joubert syndrome (JS) is a constituent of the newly emerging group of ciliopathy disorders that trace their pathophysiology back to defects either in the structure or function of primary cilia. JS is characterized by the triad of cerebellar vermis hypoplasia, oculomotor apraxia (jerky eye movements with inability to visually fixate) and intermittent hyperventilation (Joubert et al., 1968). The radiographic hallmark of JS is the presence of a unique feature on brain MRI known as the “molar tooth” sign, seen on axial image at the level of the midbrain–hindbrain junction, which has helped to narrow the phenotypic spectrum of the disorder, and led to the identification of genes mutated in these patients. Like the majority of ciliopathy diseases, the inheritance of JS is recessive, but there is tremendous phenotypic heterogeneity, even within single families, which is probably the result of multiple genetic or environmental modifier effects. The genes identified to date in JS appear to function at or near the primary cilium, either to regulate its structure or signaling events. For example, mutations in CEP290, a centrosomal protein, lead to JS with co-existent cystic kidneys and retinal degeneration (Valente et al., 2006). CEP290 is implicated in the ciliary targeting of Rab8, a small GTPase to promote ciliogenesis through its interaction with the centriolar satellite component PCM-1 (Kim et al., 2008) and the function of CEP290 is antagonized by the centrosomal protein CP110 (Tsang et al., 2008).
Mutations of the neuronal cilia localized ARL13B, a member of the Ras GTPase family, were also found in two families with the classical form of JS (Cantagrel et al., 2008). ARL13B mutant zebrafish displays curved tail and cystic kidney phenotypes (Cantagrel et al., 2008), the hennin (hnn) mutant mice, a null allele of ARL13B exhibit defects in neural tube patterning, the structure of cilia and display aberrant Shh signaling (Caspary et al., 2007), suggesting the disruption of ARL13B causes JS phenotypes in human through abnormal ciliary structure and Shh signaling. Additionally, recently identified hypomorphic loss of function mutations in the cilium localized inositol polyphosphate-5-phosphatase E (INPP5E) were shown to lead to JS (Bielas et al., 2009). These mutations in INPP5E altered phosphotidylinositol (Ptdlns) signaling and the stability of cilia in response to serum stimulation. Furthermore, blocking ciliary PDGFRα or PI3-kinase signaling restored ciliary stability, suggesting a link between PDGFα and downstream signaling components with the primary cilium in JS (Bielas et al., 2009; Jacoby et al., 2009). Finally, Jouberin, the protein product of the AHI1 gene that is mutated in JS (Dixon-Salazar et al., 2004), plays a key role as a positive modulator of the Wnt/β-catenin signaling pathway in primary cilia and its disruption leads to cystic kidney, an additional feature in JS (Lancaster et al., 2009). Although the direct signaling pathways altered in JS are not well understood, these data suggest that like other ciliopathy diseases, the pathogenic mechanisms of JS will implicate defective transport or signaling of well-established pathways at or near the cilium (Fig. 2).
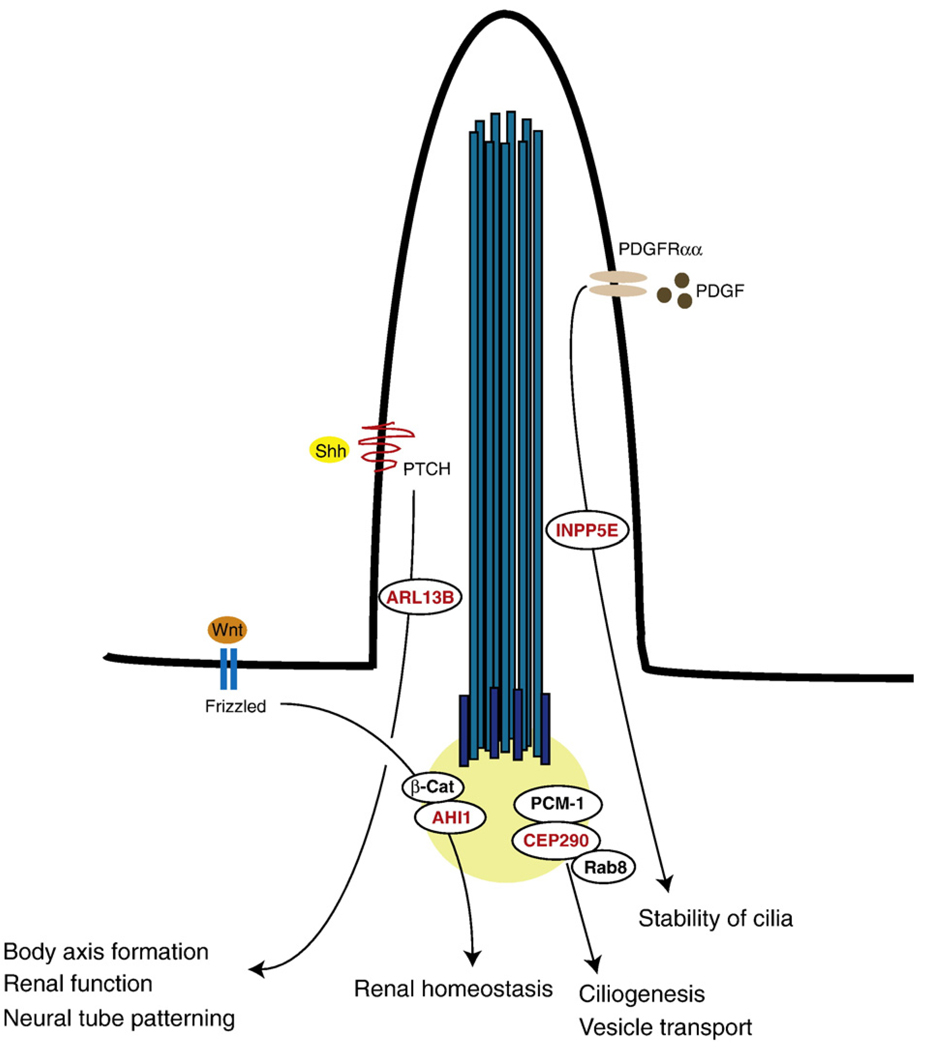
Fig. 2.
JS genes implicated in the Shh, Wnt, and PDGFα signaling pathway. ARL13B and INPP5E localize to the cilium, whereas AHI1 and CEP290 localize to the basal body. CEP290, a centrosomal protein, is involved in Rab8 contained vesicle transport and ciliogenesis through its interaction with PCM-1. The disruption of ARL13B causes defects in ciliary structure and Shh signaling. ARL13B is required for body axis formation, renal function, and neural tube patterning. INPP5E plays an essential role in stabilizing cilia in response to serum stimulation, possibly by controlling PDGFα signaling. AHI1 interacts with and facilitates β-catenin (β-Cat) accumulation in the nucleus to positively modulate Wnt signaling. Wnt/β-catenin signaling via AHI1 is essential for maintaining renal homeostasis.
Phenotypic variability in Joubert syndrome and other ciliopathies
In addition to JS, other ciliopathies may include features of retinal blindness and sensorineural deafness. This likely represents a requirement for the unique structure or signaling capability of the cilium in multiple types of specialized sensory cells. Additional features of ciliopathies include progressive renal fibrocystic disease (nephronophthisis), obesity, polydactyly, short stature, and encephalocele. Clinical geneticists have attempted to categorize these features into specific syndromes, such as Usher, Senior-Loken, Meckel-Gruber, Bardet-Biedl, Dekaban-Arima, Oro-facial-digital, Jeune, Ellis-van Cre-veld among other syndromes (Tobin and Beales, 2009), but the recent observations that mutations in a single gene can lead to a broad array of phenotypes in these diseases has blurred these boundaries, and led to the emerging concept of a spectrum disease (Bergmann et al., 2008; Brancati et al., 2009; Frank et al., 2008; Leitch et al., 2008). Furthermore, there is increasing awareness that a single patient may carry deleterious or potentially deleterious genetic alterations in more than one “cilia” gene (Tory et al., 2007). The data suggests a network of ciliary proteins functioning to mediate partially overlapping and non-redundant functions.
Ciliary function in neurons
Many of these syndromes and conditions share some important neurological disturbances (Table 1), most consistently mental retardation (Gitten et al., 1998), which raises the question: What are the potential functions of primary cilia in neurons, beyond their unique roles in specialized sensory cells? In which developmental stages do neurons display primary cilia, and is there evidence that neuronal cilia perform important functions during development or in adulthood?
Table 1.
Clinical features of the ciliopathies.
| Ciliopathies | Neurological deficits | Other deficits |
|---|---|---|
| Joubert syndrome (JBTS) | cognitive impairment cerebellar malformation oculomotor apraxia encephalocele polymicrogyria |
cystic kidney polydactyly retinal degeneration |
| Bardet-Biedl syndrome (BBS) |
cognitive impairment obesity |
retinal degeneration cystic kidneys polydactyly |
| Meckel syndrome (MKS) | cognitive impairment encephalocele hydrocephalous |
cystic kidney polydactyly |
| Ellis Van Creveld syndrome (EVC) |
cognitive impairment | short statue polydactyly cardiac defects |
| Oro-facial-digital syndrome type 1 (OFD1) |
cognitive impairment cerebellar malformation |
malformation of the face and oral cavity |
| Jeune syndrome (JATD) | cognitive impairment | skeletal dysplasia cystic kidneys |
Cerebellar development
When we consider the striking cerebellar hypoplasia in JS and other ciliopathies, the central role of Shh in proliferation of cerebellar granule neurons (Wechsler-Reya and Scott, 1999), and the critical role for the cilia in transducing Shh signals (Corbit et al., 2005; Liu et al., 2005; Rohatgi et al., 2007), one might speculate that defects in responsiveness to Shh might underlie the cerebellar defect in ciliopathy conditions. Using conditional alleles for murine Ift88 (orthologue of the Chlamydomonas Ift88 gene, which is necessary for IFT) and Kif3a (necessary for plus-end directed IFT movement), recent work has demonstrated defective expansion of the Shh-dependent cerebellar granule neuron progenitor pools in cilia deficient mutants (Chizhikov et al., 2007; Spassky et al., 2008). While the cerebellar dysgenesis in ciliopathy disorders likely accounts for the ataxic phenotypes, the degree to which these relates to the neurocognitive disease in these patients is not understood.
Hippocampal neurogenesis
Since both Shh and Wnt signaling play key roles in regulating progenitor cell maintenance in telencephalic stem cell niches (Pozniak and Pleasure, 2006), one might similarly speculate that mutations in ciliary proteins may lead to defective formation of adult neuronal stem cell regions such as the hippocampus. When directly examined in the Kif3a conditional mutant crossed with Gfap::Cre, as well as mutants of Ift88 and Ftm, all showed defective adult hippocampal neurogenesis and altered Shh signaling. However, the defect was not rescued by constitutive activation of the Shh pathway, suggesting that pathways other than Shh may be influenced by these genetic mutations (Han et al., 2008). A mutation in a new gene known as Stumpy also leads to ciliary biogenesis defects, reduction in hippocampal neural precursors and abrogated Shh activity (Breunig et al., 2008). In addition, blocking adult hippocampal neurogenesis can lead to deficits in learning and memory cognitive functions (Shors et al., 2001). Therefore, although no direct behavioral defects were evaluated in these mice, the possibility remains that at least some of the cognitive defects associated with ciliary defects are mediated by the essential role of cilia in neurogenesis within the hippocampus.
Polymicrogyria and other structural brain defects
There are many reports of defective neocortical development associated with ciliopathy diseases, most notably in the case of JS and related disorders, where generalized polymicrogyria and other structural brain lesions have been noted (Dixon-Salazar et al., 2004; Giordano et al., 2009; Gleeson et al., 2004). Similarly, patients with Meckel-Gruber or Bardet-Biedl syndrome can display occipital encephalocele, microcephaly, holoprosencephaly, and other structural brain defects (Ahdab-Barmada and Claassen, 1990; Rooryck et al., 2007). Thus, ciliary function is likely required for some aspects of neuronal development within the cerebral cortex, although mechanisms remain to be determined.
Axonal guidance
Early studies have shown that there are obvious defects in decussation of some of the major afferent and efferent axonal tracts within the brain of patients with JS (Friede and Boltshauser, 1978; ten Donkelaar et al., 2000; Yachnis and Rorke, 1999). These include most notably the finding that at least some patients with JS are completely missing the pyramidal decussation, which is the major site of crossing of the corticospinal tract (CST). Diffusion tensor imaging, an MRI-based method to label the orientation of tracts, identified failed CST decussation as well as failed decussation of the superior cerebellar peduncle (SCP) tract (the outflow tract of the cerebellum) in 6 of 6 JS subjects (Poretti et al., 2007). Absent SCP decussation was identified in an additional 6 JS patients, and not in 16 aged-matched controls (Spampinato et al., 2008). Further work demonstrated bilateral functional MRI (fMRI)-based activation of sensorimotor and cerebellar cortical activation upon volitional finger tapping, suggesting defective functional brain organization resulting from these defects (Parisi et al., 2004). The preponderance of evidence suggests that at least in some patients with ciliopathies, there is defective axonal tract decussation, which may underlie some of the observed cognitive and motor defects. Despite these interesting findings, many questions remain. First, since many genes for JS are known, are there genotype–phenotype correlations that would predict which patients display altered axonal guidance? Second, how could the cellular defects resulting from these mutations, which predominantly affect the function of the primary cilia (located adjacent to the cell body), lead to defects in guidance of the axon (usually located quite distal from the cell body)? Third, how do these axonal defects contribute to the cognitive or motor defects in the ciliopathies?
Potential functions of primary cilia in cognition
A surprising finding in the field of neurobiology was that most or all neurons display a primary cilium. Early electron microscopy studies demonstrated evidence of primary cilia in developing and adult neural tissue (Del Cerro and Snider, 1969; Mandl and Megele, 1989), as well as more recent studies using a host of cilia-specific antibodies (Bishop et al., 2007; Handel et al., 1999). In C. elegans olfactory epithelium, sensory signaling is required to maintain the architecture of the specialized neuronal cilia (Moussaif and Sze, 2009; Mukhopadhyay et al., 2008), suggesting that extracellular signals may regulate the size and shape of neuronal cilia.
What function could these organelles serve in neurons? Future studies aimed at removing cilia or disturbing ciliary function in subregions of adult brain will be required to address this question. For instance, Bardet-Biedl syndrome (BBS) and Alström (AS) patients frequently display hyperphagia-induced obesity, but the origins have remained elusive. Recent studies have pointed to a role for these proteins in mediating leptin signals, possibly by mediating ciliary function. Disruption of Ift88 or Kif3a in brain has been shown to result in obesity, an effect that was narrowed to the pro-opiomelanocortin (POMC)-expressing cells in the hypothalamus (Davenport et al., 2007). Disruption of Bbs2, Bbs4 or Bbs6 genes in mice also results in obesity, which was traced to a defect in leptin receptor trafficking (Seo et al., 2009). It remains to be determined whether leptin receptors are clustered in or near the cilium and to what extent leptin signaling is modulated by the cilium.
This work also raises the question of which other receptors might localize to primary cilia, and thus might be under regulatory control. For example, BBS2 and BBS4 are required for localization of some G protein-coupled receptors such as somatostatin receptor type 3 (SSRT3) and melanin-concentrating hormone receptor 1 (MCHR1) to primary cilia in neurons, which suggests that these signaling pathways may be modified by cilia function (Berbari et al., 2008b). Moreover, ciliary localization sequences have recently been identified within the third intracellular loop of several G-protein coupled receptors including serotonin receptor 6 (HTR6) and melanin-concentrating hormone receptor 1 (MCHR1) (Berbari et al., 2008a). This and other findings suggest that the number of signaling pathways that are potentially influenced by the cilium has the potential to grow as we gain a deeper appreciation of this key signaling organelle in neuronal function.
Acknowledgments
The authors wish to acknowledge financial support from National Institute of Neurological Diseases and Stroke (grant NS048453 and NS052455), the Burroughs Wellcome Fund and the Howard Hughes Medical Institute.
References
- Adams NA, et al. The retinal ciliopathies. Ophthalmic Genet. 2007;28:113–125. doi: 10.1080/13816810701537424. [DOI] [PubMed] [Google Scholar]
- Ahdab-Barmada M, Claassen D. A distinctive triad of malformations of the central nervous system in the Meckel-Gruber syndrome. J. Neuropathol. Exp. Neurol. 1990;49:610–620. doi: 10.1097/00005072-199011000-00007. [DOI] [PubMed] [Google Scholar]
- Andersen JS, et al. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- Avidor-Reiss T, et al. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- Badano JL, et al. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Berbari NF, et al. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol. Biol. Cell. 2008a;19:1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, et al. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl. Acad. Sci. U. S. A. 2008b;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am. J. Hum. Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielas SL, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat. Genet. 2009;41:1032–1036. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GA, et al. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J. Comp. Neurol. 2007;505:562–571. doi: 10.1002/cne.21510. [DOI] [PubMed] [Google Scholar]
- Blacque OE, et al. Functional genomics of the cilium, a sensory organelle. Curr. Biol. 2005;15:935–941. doi: 10.1016/j.cub.2005.04.059. [DOI] [PubMed] [Google Scholar]
- Brancati F, et al. MKS3/TMEM67 mutations are a major cause of COACH Syndrome, a Joubert Syndrome related disorder with liver involvement. Hum. Mutat. 2009;30:E432–E442. doi: 10.1002/humu.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig JJ, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhead R, et al. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440:224–227. doi: 10.1038/nature04541. [DOI] [PubMed] [Google Scholar]
- Cantagrel V, et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am. J. Hum. Genet. 2008;83:170–179. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary T, et al. The graded response to Sonic Hedgehog depends on cilia architecture. Dev. Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, et al. Cilia proteins control cerebellar morphogenesis by promoting expansion of the granule progenitor pool. J. Neurosci. 2007;27:9780–9789. doi: 10.1523/JNEUROSCI.5586-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, et al. Vertebrate smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Corbit KC, et al. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- Davenport JR, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr. Biol. 2007;17:1586–1594. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Cerro MP, Snider RS. The Purkinje cell cilium. Anat. Rec. 1969;165:127–130. doi: 10.1002/ar.1091650202. [DOI] [PubMed] [Google Scholar]
- Dixon-Salazar T, et al. Mutations in the AHI1 gene, encoding Jouberin, cause Joubert syndrome with cortical polymicrogyria. Am. J. Hum. Genet. 2004;75:979–987. doi: 10.1086/425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimenko E, et al. Analysis of xbx genes in C. elegans. Development. 2005;132:1923–1934. doi: 10.1242/dev.01775. [DOI] [PubMed] [Google Scholar]
- Frank V, et al. Mutations of the CEP290 gene encoding a centrosomal protein cause Meckel-Gruber syndrome. Hum. Mutat. 2008;29:45–52. doi: 10.1002/humu.20614. [DOI] [PubMed] [Google Scholar]
- Friede RL, Boltshauser E. Uncommon syndromes of cerebellar vermis aplasia: I. Joubert syndrome. Dev. Med. Child Neurol. 1978;20:758–763. doi: 10.1111/j.1469-8749.1978.tb15307.x. [DOI] [PubMed] [Google Scholar]
- Giordano L, et al. Joubert syndrome with bilateral polymicrogyria: clinical and neuropathological findings in two brothers. Am. J. Med. Genet., A. 2009;149A:1511–1515. doi: 10.1002/ajmg.a.32936. [DOI] [PubMed] [Google Scholar]
- Gitten J, et al. Neurobehavioral development in Joubert syndrome. J. Child Neurol. 1998;13:391–397. doi: 10.1177/088307389801300806. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, et al. Molar tooth sign of the midbrain-hindbrain junction: occurrence in multiple distinct syndromes. Am. J. Med. Genet., A. 2004;125A:125–134. doi: 10.1002/ajmg.a.20437. discussion 117. [DOI] [PubMed] [Google Scholar]
- Han YG, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- Handel M, et al. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;e53:1. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt F, et al. Nephronophthisis: disease mechanisms of a ciliopathy. J. Am. Soc. Nephrol. 2009;20:23–35. doi: 10.1681/ASN.2008050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Jacoby M, et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat. Genet. 2009;41:1027–1031. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- Joubert M, et al. Familial dysgenesis of the vermis: a syndrome of hyperventilation, abnormal eye movements and retardation. Neurology. 1968;18:302–303. [PubMed] [Google Scholar]
- Kim J, et al. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum. Mol. Genet. 2008;17:3796–3805. doi: 10.1093/hmg/ddn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, et al. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat. Med. 2009;15:1046–1054. doi: 10.1038/nm.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch CC, et al. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nat. Genet. 2008;40:443–448. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- Li JB, et al. Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell. 2004;117:541–552. doi: 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- Liu A, et al. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Mandl L, Megele R. Primary cilia in normal human neocortical neurons. Z. Mikrosk. Anat. Forsch. 1989;103:425–430. [PubMed] [Google Scholar]
- Moussaif M, Sze JY. Intraflagellar transport/Hedgehog-related signaling components couple sensory cilium morphology and serotonin biosynthesis in Caenorhabditis elegans. J. Neurosci. 2009;29:4065–4075. doi: 10.1523/JNEUROSCI.0044-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, et al. Sensory signaling-dependent remodeling of olfactory cilia architecture in C. elegans. Dev. Cell. 2008;14:762–774. doi: 10.1016/j.devcel.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi MA, et al. Cerebral and cerebellar motor activation abnormalities in a subject with Joubert syndrome: functional magnetic resonance imaging (MRI) study. J. Child Neurol. 2004;19:214–218. [PubMed] [Google Scholar]
- Poretti A, et al. Diffusion tensor imaging in Joubert syndrome. AJNR Am. J. Neuroradiol. 2007;28:1929–1933. doi: 10.3174/ajnr.A0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozniak CD, Pleasure SJ. A tale of two signals: Wnt and Hedgehog in dentate neurogenesis. Sci. STKE. 2006;2006:5. doi: 10.1126/stke.3192006pe5. [DOI] [PubMed] [Google Scholar]
- Qin H, et al. An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Curr. Biol. 2001;11:457–461. doi: 10.1016/s0960-9822(01)00122-1. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, et al. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Rooryck C, et al. Bardet-Biedl syndrome and brain abnormalities. Neuropediatrics. 2007;38:5–9. doi: 10.1055/s-2007-981466. [DOI] [PubMed] [Google Scholar]
- Ross AJ, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- Schneider L, et al. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr. Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Seo S, et al. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum. Mol. Genet. 2009;18:1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Simons M, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat. Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato MV, et al. Absence of decussation of the superior cerebellar peduncles in patients with Joubert syndrome. Am. J. Med. Genet., A. 2008;146A:1389–1394. doi: 10.1002/ajmg.a.32282. [DOI] [PubMed] [Google Scholar]
- Spassky N, et al. Primary cilia are required for cerebellar development and Shh-dependent expansion of progenitor pool. Dev. Biol. 2008;317:246–259. doi: 10.1016/j.ydbio.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolc V, et al. Genome-wide transcriptional analysis of flagellar regeneration in Chlamydomonas reinhardtii identifies orthologs of ciliary disease genes. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3703–3707. doi: 10.1073/pnas.0408358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Donkelaar HJ, et al. A case of Joubert's syndrome with extensive cerebral malformations. Clin. Neuropathol. 2000;19:85–93. [PubMed] [Google Scholar]
- Tobin JL, Beales PL. The nonmotile ciliopathies. Genet. Med. 2009;11:386–402. doi: 10.1097/GIM.0b013e3181a02882. [DOI] [PubMed] [Google Scholar]
- Tory K, et al. High NPHP1 and NPHP6 mutation rate in patients with Joubert syndrome and nephronophthisis: potential epistatic effect of NPHP6 and AHI1 mutations in patients with NPHP1 mutations. J. Am. Soc. Nephrol. 2007;18:1566–1575. doi: 10.1681/ASN.2006101164. [DOI] [PubMed] [Google Scholar]
- Tsang WY, et al. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev. Cell. 2008;15:187–197. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat. Genet. 2006;38:623–625. doi: 10.1038/ng1805. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Wheatley DN, et al. Expression of primary cilia in mammalian cells. Cell Biol. Int. 1996;20:73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- Yachnis AT, Rorke LB. Cerebellar and brainstem development: an overview in relation to Joubert syndrome. J. Child Neurol. 1999;14:570–573. doi: 10.1177/088307389901400904. [DOI] [PubMed] [Google Scholar]
- Yoder BK, et al. Insertional mutagenesis and molecular analysis of a new gene associated with polycystic kidney disease. Proc. Assoc. Am. Phys. 1995;107:314–323. [PubMed] [Google Scholar]